Abstract
Research on photocatalytic degradation rate of azo dyes using TiO2/SrTiO3 nanocomposite in the photocatalysis process was the main goal of the present study. The TiO2/SrTiO3 nanocomposite was first synthesized under ultraviolet (UV) irradiation and sonication, and then, its photocatalytic activity has been examined under UV irradiation. The absorbance of samples was measured using a UV-vis spectrophotometer. The structure and morphology of the nanocomposite were investigated by scanning electron microscopy and the crystalline structure by X-ray diffraction spectroscopy. The results reveal that adding nano-SrTiO3 to nano-TiO2 showed the most promising photocatalytic activity toward dye degradation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Environmental problem of toxic wastewater and infected waters is one of the main subjects that researchers work on. Due to this, organic dyes are one of the main industrial wastewater pollutions. More than 50% of textile dyes are azoic dyes which are recognized by their nitrogen π-bonds [1, 2]. Textile and industrial dyes contain large groups of organic compounds that are produced more than 700,000 tons per year. About 1% to 20% of the world's dye products are included in the textile wastewater during the dyeing process [3–5].
The use of semiconductors such as TiO2, ZnO, Fe2O3, and CdS as photocatalysts is interesting for the degradation of organic pollution. Due to the optical and electrical properties, low cost, high photocatalytic activity, chemical stability, and non-toxicity of nano-titanium dioxide, it is used as a common photocatalyst [6, 7].
Although there are numerous advantages in utilizing the TiO2, there are some disadvantages for the pure one, some of which are listed here: first, the bandgap larger than 3.2 eV causes low efficiency of nano-TiO2. Furthermore, the electron-hole recombination rate is too high, resulting in low photocatalytic efficiency [8–11]. One of the research fields for performance improvement of TiO2 photocatalysts is the retard of a charge recombination between excited electrons in the conduction band and holes in the valence band, which are formed by UV irradiation. Supporting the nanoparticle of a semiconductor or a metal, into which excited electrons in the conduction band of TiO2 can be injected, is one of the methods to increase the charge separation efficiency of TiO2[12–15].
The perovskite oxides recognized as photocatalysts have ABO3 formulas, where A is a rare earth metal with a large ionic radius or alkaline earth metal, and B is a transition metal with a small ionic radius. The alkali metal acts as the ionic balance (place in A) and the titanate framework plays the main role in the structure and properties with Ti in the B sites [16–19]. The presence of Sr in SrTiO3 gives more ionic properties in comparison with SrO, and Ti is more covalent in SrTiO3 than in TiO2; this causes the reduction of the acidity of Ti ions and the increase of covalent property. Thus, bound formation properties might be different with titania and can lead to different photocatalitical reaction [20]. In comparison with other oxidants, the multi-cation oxide of SrTiO3 is more capable of tuning the chemical and physical properties by altering the compositions and also has a larger number of photocatalytic sites [21]. By adding SrTiO3 on the TiO2, its demerits can be reduced. Zhang et al. produced TiO2-SrTiO3 composite heterostructures by hydrothermal treatment and proved the significant improvement in photoelectrochemical performance of TiO2 nanotube [22].
In this study, we dedicated our efforts to synthesize photocatalysts with a better quality. The photocatalytic degradation of dark green 6 and reactive orange 72 with TiO2/SrTiO3 nanocomposite under 20- and 400-W UV irradiations is investigated. Finally, for the investigation of the TiO2/SrTiO3 nanocomposite photocatalytic efficiency, its photocatalytic activity will be compared with nano-titanium dioxide and nano-strontium titanate.
Results and discussion
Photocatalyst performance
The results acquired indicate that adding nano-SrTiO3 to nano-TiO2 particles had a tangible effect on its photocatalytic activity (Figure 1C), and its photocatalytic degradation efficiency was higher than either SrTiO3 or TiO2. When the photocatalyst is illuminated by a light with energy higher than its bandgap energy, electron-hole pairs diffuse out to the surface of the photocatalyst. At the surface, the electrons and holes can either take part in the chemical reaction with electron donors and acceptors or recombine. In the absence of suitable electron and hole scavengers, the recombination of electron-hole pairs will occur within a very short time. Therefore, observed enhancement on the photocatalytic activity of TiO2 by supporting the SrTiO3 is thought to be explained on the basis of the increase in charge separation efficiency and the excited electrons in the conduction band of TiO2 by UV irradiation being injected to the conduction band of SrTiO3 to suppress the recombination between the electrons and holes in TiO2. In order to realize an effective suppression of the recombination, it is reasonably essential to have a contact of SrTiO3 particle with TiO2.
Particle size and surface area are the effective parameters of photocatalytic activity of nanomaterials. In our previous work, we revealed that the nano-TiO2 has smaller particle size and higher surface area than the nano-SrTiO3[23]. Therefore, photocatalytic degradation efficiency of the nano-TiO2 is more than the nano-SrTiO3 (Figure 1A,B).
For both dark and reactive dyes, the percentage of photocatalyst degradation with a 400-W UV lamp was higher than that with 20 W. This occurs for two reasons: first of all, there is more radiation intensity, and second, there is a lower wavelength that resulted in the electron excitation of nanomaterials.
The higher degree of degradation of the reactive dye compared with the direct dye is due to the chromophore structures of the dyes, which are monoazo and trisazo, respectively [24].
SEM-EDS measurement
A scanning electron microscopy (SEM) image (Figure 2A) provides morphological information of the TiO2/SrTiO3 nanocomposite. It is thoroughly possible to recognize the nanoparticles on the composite. It may be clearly seen that particle distribution is uniform in the TiO2/SrTiO3 nanocomposite.
Energy-dispersive X-ray spectrometry (EDS) is an analytical technique used for the elemental analysis or chemical characterization of a sample and detects the elements using X-rays that are emitted from the specimen. Energy peaks correspond to the various elements in the sample. The presence of strontium and titanium particles on the TiO2/SrTiO3 nanocomposite was proved using EDS (Figure 2B).
XRD spectroscopy
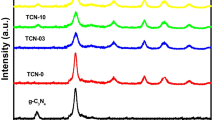
The crystalline status of nanoparticles was studied by X-ray powder diffraction (XRD). The XRD pattern of the TiO2/SrTiO3 nanocomposite is shown in Figure 3. In the XRD pattern, the peak is around 2Ɵ = 25.3° related to the anatase structure of nano-TiO2, and in 2Ɵ = 37.9°, the peaks of the rutile structure of TiO2 exist. One of the components of the fertilizing bath was SrTiO3/TiO2, as a result when the peaks of the tausonite structure of SrTiO3 appeared around 2Ɵ = 32.3°. Also, the crystal size was calculated, and for the TiO2/SrTiO3, it was 201.77 Å.
Conclusion
In the present work, effective enhancement in photocatalytic activity of TiO2 by supporting SrTiO3 is experimentally confirmed. Through the XRD pattern, SEM image, and EDS spectrum, the presence of nanomaterials on the nanocomposite sample was verified. The produced nanocomposite provides a higher photocatalytic degradation efficiency in comparison with nano-strontium titanate or nano-titanium dioxide. On the composite system, strontium titanate plays the role of electron acceptor in order to accelerate photodecomposition.
Methods
Materials and equipment
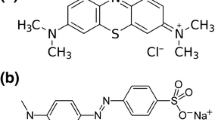
Nano-strontium titanate powder (P.N.517011) with an average particle size of less than 100 nm and nano-titania (P-25) with an average particle size of about 21 nm were provided by Sigma-Aldrich (St. Louis, MO, USA) and Degussa Companies (Dusseldorf, Germany), respectively. Commercially available dark green 6 (CI 30295) and reactive orange 72 (CI 17754) were obtained from Alvan Sabet Company, Hamedan, Iran, and their structures are shown in Figure 4.
The dye solution was mixed with a magnetic stirrer during reaction (MR Hei, Heidolf, Germany). Samples after photocatalytic treatment were filtered through a Millipore filter (0.45 μm) membrane (Millipore Filter Corporation, Bedford, Massachusetts). The pH of the solution was measured using a LI 120 pH meter (ELICO, Hyderabad, India), and concentrations of dyes were determined using a Carry 100 UV-vis spectrophotometer (Varian, Australia).
A UV-A 20W lamp (Sylvania, Belgium), with 365-nm wavelength radiation and light intensity of 0.2 to 0.4 μW · cm−2, and a UV400 W lamp (Philips, HPA 400 s, Guildford, England, UK), with higher radiation of wavelength between 300 and 400 nm along with the light spectrum having an intensity of 800 μW · cm−2 for UV-A, 115 μW · cm−2 for UV-B, and 25 μW · cm−2 for UV-C were used as light sources. An ultrasonic bath model EURONDA SPA 4D, 40 kHz (Vicenza, Italy) was used to mix the components.
SEM image and EDS spectra were obtained using a Philips XL30 scanning electron microscope (Amsterdam, Netherlands). The crystalline structure of the TiO2/SrTiO3 nanocomposite was characterized by XRD (Bruker D8 Discover X-ray diffractometer, Karlsruhe, Germany).
TiO2/SrTiO3 nanocomposite preparation
Six milligrams of nano-TiO2 and 4 mg of nano-SrTiO3 were added into 100 mL of distilled water. After 1 h of sonication and irradiation (UV-A light) of the suspension, the mixture was centrifuged and then dried at 30°C. Finally, the obtained product was calcined at 300°C for 2 h.
Procedure
The concentration of the dye in the solution was calculated by a computer program using a calibration curve. The program determines the absorbance of the dye solution at a maximum wavelength of dyes: dark green 6, 623 nm; reactive orange 72, 433 nm. The first step was the preparation of dye solution by distilled water. Then, nanomaterials were added. Firstly, the solution mixture was stirred for 15 min without irradiation in order to get equilibrium of dye adsorption. Then, the solution was irradiated with two lamps (20 and 400 W) for 1 h, and during irradiation, it was continuously stirred with a rate of 200 rpm and a temperature of 25°C, and the real dye solution pH for the direct and reactive dyes were 6.6 and 6.4, respectively. After irradiation, the samples were purified with the Millipore filter. The decolorization and photocatalytic degradation efficiency have been calculated as follows:
where C0 and Ce correspond to the initial and final concentrations of the dyes before and after photo irradiation. In this equation, Efficiency % shows the dye photocatalyst degradation percent [25]. For the investigation of the TiO2/SrTiO3 nanocomposite photocatalytic efficiency, its degradation percent was compared with nano-TiO2 and nano-SrTiO3. Basic parameters were photocatalyst concentration of 0.1% (on weight of bath), 20 mg/L dye, 25°C temperature, 1 h irradiation and real pH. Figure 5 shows the schematic of dye photocatalytic degradation.
References
Song S, Xu L, He Z, Ying H, Chen J, Xiao X, Yan B: Photocatalytic degradation of C.I. Direct Red 23 in aqueous solutions under UV irradiation using SrTiO 3 /CeO 2 composite as the catalyst. J. Hazard. Mater. 2008, 152: 1301–1308. 10.1016/j.jhazmat.2007.08.004
Lee J, Choi SP, Thiruvenkatachari R, Shim WG, Moon H: Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes Pigm 2006, 69: 196–203. 10.1016/j.dyepig.2005.03.008
Madhavan J, Grieser F, Ashokkumar M: Degradation of orange-G by advanced oxidation processes. Ultras. Sonochem. 2010, 17: 338–343. 10.1016/j.ultsonch.2009.10.008
Isaev AB, Aliev ZM, Adamadzieva NK, Alieva NA, Magomedova GA: The photocatalytic oxidation of azo dyes on Fe 2 O 3 nanoparticles under oxygen pressure. Nanotech. Rus. 2009, 4: 475–479. 10.1134/S1995078009070088
Mahvi AH, Ghanbarian M, Nasseri S, Khairi A: Mineralization and discoloration of textile wastewater by TiO 2 nanoparticles. Desalination 2009, 239: 309–316. 10.1016/j.desal.2008.04.002
Xua J, Aoa Y, Fua D, Yuana C: Low-temperature preparation of anatase titania-coated magnetite. J. Phys. Chem. Sol. 2008, 69: 1980–1984. 10.1016/j.jpcs.2008.02.015
Hegde M, Nagaveni K, Roy S: Synthesis, structure and photocatalytic activity of nano TiO 2 and nano Ti 1 - x M x O 2 -δ (M = Cu, Fe, Pt, Pd, V, W, Ce, Zr). PRAMANA J. Phys. 2005, 65: 641–645. 10.1007/BF03010452
Linsebigler A, Lu G, Yates JT: Photocatalysis on TiO 2 surfaces: principles, mechanisms, and selected results. Chem. Rev. 1995, 95: 735–758. 10.1021/cr00035a013
Wang J, Wen FY, Zhang ZH, Zhang XD, Pan ZJ, Zhang P, Kang PL, Tong J, Wang L, Xu L: Investigation on degradation of dyestuff wastewater using visible light in the presence of a novel nano TiO 2 catalyst doped with upconversion luminescence agent. J. Photochem. Photobiol. A 2006, 180: 189–195. 10.1016/j.jphotochem.2005.10.016
Zyoud AH, Zaatar N, Saadeddin I, Ali C, Park D, Campet G, Hilal HS: CdS-sensitized TiO 2 in phenazopyridine photo-degradation: Catalyst efficiency, stability and feasibility assessment. J. Hazard. Mater. 2010, 173: 318–325. 10.1016/j.jhazmat.2009.08.093
Ueda M, Matsuo SOY: Preparation of tabular TiO 2 –SrTiO 3 -δ composite for photocatalytic electrode. Sci. Tech. Adv. Mater. 2004, 5: 187–193. 10.1016/j.stam.2003.09.012
Montazer M, Behzadnia A, Moghadam MB: Superior self-Cleaning features on wool fabric using TiO 2 /Ag nanocomposite optimized by response surface methodology. J. Appl. Polym. Sci. 2012, 125: E356-E363. 10.1002/app.36768
Montazer M, Behzadnia A, Pakdel E, Rahimi MK, Moghadam MB: Photo induced silver on nano titanium dioxide as an enhanced antimicrobial agent for wool. J. Photochem. Photobiol. B 2011, 103: 207–214. 10.1016/j.jphotobiol.2011.03.009
Sung-Suh HM, Choi JR, Hah HJ, Koo SM, Bae YC: Comparison of Ag deposition effects on the photocatalytic activity of nanoparticulate TiO 2 under visible and UV light irradiation. J. Photochem. Photobiol. A 2004, 163: 37–44. 10.1016/S1010-6030(03)00428-3
Subramanian V, Wolf E, Kamat P: Semiconductor–metal composite nanostructures. To what extent do metal nanoparticles improve the photocatalytic activity of TiO 2 films? J. Phys. Chem. B 2001, 105: 11439–11446. 10.1021/jp011118k
Boudali A, Khodja MD, Amrani B, Bourbie D, Amara K, Abada A: First-principles study of structural, elastic, electronic, and thermal properties of SrTiO 3 perovskite cubic. Phys. Lett. A 2009, 373: 879–884. 10.1016/j.physleta.2008.12.017
Subramanian V, Roeder RK, Wolf EE: Synthesis and UV-Visible-light photoactivity of noble-metal-SrTiO 3 composites. Ind. Eng. Chem. Res. 2006, 45: 2187–2193. 10.1021/ie050693y
Wang J, Yin S, Zhang Q, Saito F, Sato T: Mechanochemical synthesis of SrTiO 3−x F x with high visible light photocatalytic activities for nitrogen monoxide destruction. J. Mater. Chem. 2003, 13: 2348–2352. 10.1039/b303420h
Niishiro R, Kato H, Kudo A: Nickel and either tantalum or niobium-codoped TiO 2 and SrTiO 3 photocatalysts with visible-light response for H 2 or O 2 evolution from aqueous solutions. Phys. Chem. Chem. Phys. 2005, 7: 2241–2245. 10.1039/b502147b
Chang C, Ray B, Paul DK, Demydov D, Klabunde KJ: Photocatalytic reaction of acetaldehyde over SrTiO 3 nanoparticles. J. Molec. Catal. A: Chem. 2008, 281: 99–106. 10.1016/j.molcata.2007.08.014
Wei X, Xu G, Ren Z, Xu C, Shen G, Han G: PVA-assisted hydrothermal synthesis of SrTiO 3 nanoparticles with enhanced photocatalytic activity for degradation of RhB. J. Am. Ceram. Soc. 2008, 91: 3795–3799. 10.1111/j.1551-2916.2008.02716.x
Zhang J, Bang JH, Tang C, Kamat PV: Tailored TiO 2 − SrTiO 3 heterostructure nanotube arrays for improved photoelectrochemical performance. ACS Nano 2010, 4: 387–395. 10.1021/nn901087c
Karimi L, Zohoori S, Yazdanshenas ME: Photocatalytic degradation of azo dyes in aqueous solutions under UV irradiation using nano-strontium titanate as the nanophotocatalyst. J. Saudi Chem. Soc 2011. in press in press
Karimi L, Mirjalili M, Yazdanshenas ME, Nazari A: Effect of nano TiO 2 on self-cleaning property of cross-linking cotton fabric with succinic acid under UV irradiation. Photochem. Photobiol. 2010, 86: 1030–1037. 10.1111/j.1751-1097.2010.00756.x
Chen CY: Photocatalytic degradation of azo dye reactive orange 16 by TiO 2 . Water Air Soil Pollut. 2009, 202: 335–342. 10.1007/s11270-009-9980-4
Acknowledgments
This work was supported by the Young Researchers and Elites Club, Yazd Branch, Islamic Azad University, Yazd, Iran under Grant no. 88363.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LK participated in the idea of the study, the design of the study, interpretation of the results, and writing the manuscript for publication. SZ carried out the experiment and participated in its design, coordination, and sequence of alignments. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Karimi, L., Zohoori, S. Superior photocatalytic degradation of azo dyes in aqueous solutions using TiO2/SrTiO3 nanocomposite. J Nanostruct Chem 3, 32 (2013). https://doi.org/10.1186/2193-8865-3-32
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-32