Abstract
This finding focuses on the optimization of synthesis time for the transformation of Fe-filled spherical-like graphene shell (GS) to almost catalyst-free carbon nanotube (CNT) structure using two-stage catalytic chemical vapor deposition apparatus. The camphor oil and ferrocene were used as carbon precursor and catalyst respectively, following the variety growth of graphene-family nanomaterials for 2, 4, 6, 8, 10, 30, and 60 min at 800°C synthesis temperature. The graphene-family nanomaterial properties were characterized using field emission scanning electron microscope, high resolution transmission electron microscope, micro-Raman spectrometer, thermogravimetric, and carbon-hydrogen-nitrogen-sulfur/oxygen (CHNS/O) analyzer. The result of field emission scanning electron microscopy analysis reveals that the CNTs were observed with high aspect ratio at 60-min synthesis time. The dependence of integrated intensity ratio of D-band and G-band (ID/IG) presented that ID/IG ratio sharply decreases with longer synthesis time. At higher synthesis time, thermogravimetric and CHNS/O analysis of CNT can obviously improve with decreases of non-carbonaceous material and transition metal catalyst. The nucleation-growth model of Fe-filled spherical-like GS to almost catalyst-free CNT has been highlighted to explain the change in growth mode.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Carbon nanotubes (CNTs) play a large part in a new dimension into the knowledge of carbon science. Almost every day, new conceptual and experimental framework for the goals of CNT is identified [1–3]. Compared with more conventional materials, this carbon allotrope has extraordinary physical and chemical properties. In 1990s, single-walled CNT (SWCNT) was discovered by two different groups: (1) Bethune et al. from IBM, California, (2) and Iijima and Ichihashi who were affiliated with NEC Corporation, Japan, and made their own route of CNT synthesis [4, 5]. However, the problem arises when Monthioux and Kuznetsov [6] raised this question few years ago, ‘why did the scientific community seem to discover CNT in 1991 when they had actually been known for forty years?’. We believe this major issue is quite interesting, and one that we will never know. Oberlin et al. [7] unknowingly discovered SWCNT of a diameter less than 100 Å in 1976; however, they did not fully recognize the discovery and described their SWCNT as a carbon filament. At that time, journalists, industrialists, and the scientific community were not concerned with nano level structure. Anticipating a growing demand, large volumes of CNT seem to be in progress and ready for commercialization. For this reason, we could see that valuable outputs from CNT-based products will be available in the markets [8–15].
In recent years, there are many fundamental issues concerning CNT growth patterns which are still not clear. From a scientific point of view, there is an immediate challenge to produce CNT of the desired properties to suit individual applications. In this experiment, the effect of synthesis time (as short as 2 min) on the formation of graphene-family nanomaterials grown on transition metal catalyst without the use of a substrate was investigated. One possible explanation is the important role of synthesis time in the formation of CNT. Our modified two-stage catalytic chemical vapor deposition (CVD) apparatus is simple and economic equipment for high-quality formation of CNT. This synthesis method facilitates an improvement of the nucleation-growth mechanism of carbon-based material without using substrate. Detailed information will be discussed in the following sections.
Results and discussion
Surface morphology
Figure 1 illustrates the FESEM micrographs of the carbon black in powder form and shows the structural variation of low-dimensional graphene-family nanomaterial influenced by the effect of synthesis time of 2, 4, 6, 8, 10, 30, and 60 min. In 2, 4, and 6 min (Figure 1a,b,c), it is shown that the appearance of the microsphere structure of the samples might be consisting of either partially amorphous carbon (a-C) or graphene shell [16, 17]. The size of the microsphere structure is clearly seen decreasing with synthesis time. The diameter of the microsphere structure is approximately 1.26 μm, 654 nm, and 591 nm as shown in Figure 1a,b,c, respectively. However, after the synthesis time was further increased (Figure 1d,e,f,g), the microsphere structure vanished from the surface morphology of the sample. As synthesis time is increased from 8 to 60 min (as shown in Figure 1d,e,f,g), a tubular structure on the top surface of the carbon microsphere was formed. It is clearly observed that the diameter of the tubular structure decreases with the increase in synthesis time, resulting in narrower tubular structure. This result is in excellent agreement with earlier findings [18] and could be an additional information on the growth mechanism of carbon-based materials with respect to synthesis time. In a separate report, we have similar findings with those in the study by Kumar and Ando [19], where carbon microspheres consist of carbon and Fe elements as evident in Figures 1a and 2a. Based on the EDX spectrum, it is confirmed that the carbon was the dominant element, and small amounts of Fe were found in the sample. At longer synthesis times, the carbon microsphere diameter decreases which may be attributed to the catalytic chemical reaction. This suggests that up to the synthesis time of 8 min (as in Figure 1d), the nucleation energy is strong enough to break the outer surface of the carbon microsphere; thus, as shown diagrammatically in Figure 3, (1) the activated Fe catalyst leaves the core area of the carbon microsphere and (2) synthesizes a tubular structure on top of the carbon microsphere surface. Broadly speaking, substrate-catalyst interaction plays an important role in the formation of CNT. It can be either ‘base- or tip-growth model’ [20]. However, Kumar and Ando [19] failed to prove the nucleation-growth mechanism of the free-substrate CNT. It has been observed that the free-substrate CNT in this work demonstrates a new growth mechanism of CNT, supplementary to the previously studied tip- or base-growth model. In the next discussion, we try to promote extended nucleation-growth mechanism outline, which leads to the formation of free-substrate CNT, known as ‘root-growth’ mechanism for CNT. An optimal synthesis time for CNT formation in our experimental is 60 min due to (1) narrower diameter with higher aspect ratio of nanotubes and (2) less a-C produced. As presented in Figure 2b, the EDX spectrum of the as-grown CNT at 60-min synthesis time clearly indicates that 99.9 at.% of carbon element is present. From Figures 2b and 4, less a-C and Fe catalyst left after 60 min, producing 99.9 at.% of pure CNT. No characteristic peaks of impurities can be detected.
Possible growth mechanism for the formation of Fe-filled spherical GS to almost catalyst-free CNT structure. A two-stage catalytic CVD was used. (a) Formation of Fe-filled sphere-like GS, (b) a Fe-filled sphere-like GS consists of graphitic layers and reduced micro-size Fe catalyst, (c) activation of catalytic chemical reaction, (d) possibilities for re-ordering of carbon structure, (e) formation of nanotube structure and (f) formation of Fe nanoparticle catalyst.
TG analysis
Normally, TG analysis is used to estimate the purity of the sample in terms of carbonaceous material and metal catalyst present. A thermogram of the weight loss of the as-grown free-standing carbon black powder on the ferrocene synthesized at 800°C with different synthesis times is presented in Figure 4. In this experimental work, the TG analysis was performed in ambient oxygen, with a temperature increase of 20°C/min. For every case, TG analysis was done up to 1,000°C to evaluate the thermal stability and purity of the carbon black powder. We found that almost the same sharp decline in weight loss was consistent in the range of 500°C to 600°C (single-step decomposition of carbon black powder); this can be attributed to the oxidation behavior of carbonaceous material, including GS, SWCNT, and multi-walled CNT (MWCNT). It is surprising that the oxidation temperature and thermal stability of our best sample are much higher and lower, respectively, compared to those of previous research works by Scheibe et al. [21] and Antunes et al. [22]. As in Figure 4, the purity and the residual weight of the crystalline carbon black powder and Fe catalyst respectively can be estimated by comparing the base line and the residual line. For 2, 4, 6, 8, 10, and 30-min synthesis time, the residual weights of the Fe catalyst were 7.81%, 5.65%, 5.10%, 4.73%, 4.64%, and 4.25% which imply that 92.19%, 94.35%, 94.9%, 95.27%, 95.36%, and 95.75% of the mass product, respectively, came from carbon. Under this condition, the highest CNT purity achieved is about 99.99% for 60-min synthesis time. To our knowledge, this is the first time that 99.9% purity CNT was treated using low temperature (approximately 800°C). Previous work by Huang et al. [23] reported 99.99% purity MWCNT with propylene on Fe-Mo-Al2O3 catalyst treated at higher temperature between 1,500°C and 2,150°C. It is observed that the oxidation temperature, purity, and thermal stability of CNT are dependent on the synthesis time of CNT sample preparation. In order to maintain the optimal performance of the CNT, high purity CNT is needed. This conclusion is consistent with the observations from the EDX analysis. The presence of impurities may significantly influence the behaviors of the CNT in existing and wide area of new application [24, 25].
Micro-Raman analysis
The micro-Raman spectra obtained at different synthesis time are shown in Figure 5a,b, with radial breath mode (RBM) at low wave number regions between 100 and 500 cm−1[26] and tangential mode at higher wave number regions between 1,000 and 2,000 cm−1[27] observed at room temperature. Here, the authors concern is on the quality of the graphene-family nanomaterials. From the micro-Raman analysis (results are shown in Figure 5a), obvious RBM peaks corresponding to the existence of SWCNT are present. According to Bandon’s equation, the diameter (d, in nanometers) of the SWCNT can be estimated from the sharp RBM peak position, ωRBM where d = 223.75 (cm−1)/ωRBM (cm−1). We notice that in the micro-Raman spectra (presented in Figure 5a), there were two ωRBM peaks originating from 210.52 and 273.95 cm−1 (60-min synthesis time). This corresponds to the tube diameters of 1.063 and 0.897 nm for 60-min synthesis time. There is a narrow diameter distribution in SWCNT produced here. No growth of SWCNT was obtained at 2, 4, 6, 8, 10, and 30 min as there is insufficient nucleation energy of Fe to efficiently catalyze the hydrocarbon decomposition. According to the micro-Raman result as shown in Figure 5a, longer synthesis time was found to produce CNTs with narrower diameter which is associated with large specific surface area availability. This characteristic is very important to any application because their performance depends on the surface reaction process [28].are two prevalent peaks in the micro-Raman spectrum of the graphitic mode (G-peak) and the disorder mode (D-peak) which are normally at 1,580 and 1,350 cm−1 in CNT, respectively. The G-peak is attributed to the ordered graphite structure, while the D-peak corresponds to non-crystal structure consisting of defects, dislocations, and lattice distortions in the carbon structure. As shown in Figure 5b, the G- and D-peak positions for 2, 4, 6, 8, 10, 30 and 60 min are always consistent at the same wave numbers (1,580 and 1,360 cm−1, respectively). The graphitization of graphene-family nanomaterial can be obtained by comparing the relative integrated intensity of D-peak to G-peak (ID/IG). The lower the ID/IG value, the higher is the degree of graphitization. The ID/IG is used also as a purity index to assess the quality of CNT with minor defect and with very low a-C content [29]. The lowest ID/IG ratio was found to be 0.791 for 60-min synthesis time, while the ID/IG values of the samples synthesized for 2, 4, 6, 8, 10, and 30 min (Figure 5b) were almost 1 (Table 1).
HRTEM analysis
The theoretical- and experimental-based understanding of the growth mechanisms of CNT would not have been possible without the use of TEM, officially invented by Siemens in 1939. TEM has enabled the confirmation of the crystallographic and morphologic structures of a variety of carbon-based materials. It is important to point out that in 1958, Radushkevich and Lukyanovich [30] highlighted the wealth of structural information that was possible from HRTEM, making this technique very interesting in the characterization of nanotubular-sized carbon growth, as it can be done in situ, allowing for direct observation in the characterized sample. In our experimental analysis, we hypothesize that the carbon microspheres and the nanotubes tested were of solid carbon structures. It was difficult to identify whether the cores of the carbon microsphere and CNT were hollow or solid. Based on a previous discussion on FESEM analysis, there are inherent limitations in FESEM analysis; this is only suitable for the analysis of the outer surface of the material, as its magnification is too low for the crystal structure to be observed in atomic level. Moreover, the analyzed sample could be either in a crystalline or amorphous phase. However, further investigation is needed to confirm the crystallographic structure of carbon microspheres and nanotubular structures.
-
The important consideration is how to ensure (1) whether the nanofibers are nanotubes or not, and (2) whether the solid microspheres are core-shell microspheres or not, i.e., how to make them hollow-like structures.
-
The other issue is how to ensure whether the crystal growth of CNT is in good agreement or not with the ‘anisotropy of carbon deposition theory’.
Figure 6a displays a typical low magnification HRTEM image of a dispersed carbon microsphere and small tubular structure. The result indicates a clear spherical GS and CNT. As shown in Figure 6a,b, two types of materials can be clearly distinguished. The black spot represents the Fe nanoparticles (pointed with a black arrow). The electron beam cannot penetrate through the Fe element; therefore, it appears as a darker black color. The areas filled by the carbon element are semi-transparent; therefore, they appear gray. However, some studies suggest that those areas are Fe nanoparticles encapsulated by graphitic layers. This is supported by Figure 7b (inset of Figure 7a); this figure clearly shows that the nanotube is a multi-walled centrally hollow tube, not a solid fiber. The lack of fringes (represent as individual cylindrical graphitic layers) inside the nanotube, as well as the lighter contrast as compared to the nanotube walls, is an evidence that the core of structure is hollow. The closing-cap CNT has been observed in the sample as shown in Figure 7c.
HS-SPME/GC-MS analysis
Previous studies [31] have used camphor oil as hydrocarbon precursor in CNT growth; however, such studies have not described the flavor volatile compounds. Therefore, HS-SPME/GC-MS analysis was undertaken as part of this study, as shown in Table 2. A total of 28 components were identified. This investigation has revealed further evidence of chromatogram structure for camphor oil. This consideration emphasizes the potential value of the nanostructured carbon material before it is submitted for the synthesis process.
Fe-filled sphere-like GS to almost catalyst-free CNT nucleation-growth mechanism: the possibilities are endless
Theoretically, the mystery of carbon growth at nanoscale is not completely solved [32]. A simple nucleation-growth mechanism of Fe-filled sphere-like GS to almost catalyst-free CNT was carried out to overcome some major experimental stumbling blocks. Our aim is to determine how the nanotube-like structure was grown from GS surfaces. The nature of camphor oil (C10H16O) itself is a monoterpene, a molecule that is built up biosynthetically from two isoprene units which obey the isoprene rule, (C5H8) n , where n is the number of linked isoprene units. Interestingly, a previous study on camphor reported by Kareem [33] showed that the decomposition end-product sublimated from solid to volatile material at ordinary room temperature, and ambient air was calculated by fractional life method. Kumar and Ando [34, 35] reported the mass production of CNT which was synthesized from botanical camphor oil rather than conventional hydrocarbon precursors. Unlike any other precursors, camphor oil is a non-toxic, reproducible precursor and is abundantly grown in Asian countries. Hence, the hydrocarbon precursor decomposition study is strongly dependent on environmental conditions [36] as evaluated using suitable analytical tools attached with a mass spectrometer [37] to extract derivative compounds from hydrocarbon precursor. This could explain why it is important to observe the preliminary study of kinetics of hydrocarbon precursor decomposition in selected gas ambient before proceeding to the selection of synthesis time. Bahrami et al. [38] reported and proposed the synthesis and nucleation-growth mechanism of CNT on Fe/MgO catalyst at 900°C to 1,000°C for 1 to 60 min. They found that Fe x O y was coated on MgO surface and reduced in situ in hydrocarbon precursor to Fe nanoparticles. Indirectly, tube-like structure was grown on Fe nanoparticles. This phenomenon can be either tip- or base-growth mechanism of CNT as supported by Charlier et al. [39].
Based on our understanding of the FESEM, EDX, micro-Raman, TG, and HRTEM analyses, the possibility of a nucleation-growth model of Fe-filled sphere-like GS to almost catalyst-free CNT is proposed as in Figure 3 as follows: A selected area in a large group of sphere-like GS structure was observed using HRTEM during exposure to heat under nitrogen ambient at 800°C under varying synthesis times (Figure 3a). The shape and diameter size of the Fe-graphene shell-CNT influenced by the synthesis time were investigated. Since the nature of the ferrocene compound consists of one iron atom, ten carbon atoms, and ten hydrogen atoms, in the first few minutes, cyclopentadienyl rings bound to the opposite sides of a central Fe atom break and decompose into free micro-sized Fe atomic cluster. At the same time, micro-sized Fe atomic cluster acts as a catalyst, promoting further growth on micro-sized Fe structure. Encapsulation of few-layer graphene consisting of free-carbon network due to the decomposition of camphor oil on micro-sized Fe structure surface was observed, as shown in Figure 3b. However, recently, Schaper and co-workers reported that it was a formation of intermediate iron carbide (Fe3C) determined by the dissolution of carbon element in the quasi-liquid catalyst particle and its segregation in the form of graphitic layers in the case of super-saturation [40]. Recently, it was supported by Fedoseeva et al. [41] who also reported on the synthesis of defect-like carbon (also known as iron-containing rods covered by graphite-like shells and embedded into carbon matrix) by air oxidation and carbonization methods. As in Figures 6b and 3c, we could observe a portion of the nano-size Fe catalyst seeds from micro-size Fe catalyst domain. Thereby, the axial movements of nano-size Fe catalyst inside the sphere-like GS is due to the buildup competition between the interfacial force and the expulsive force between the graphitized tube walls and core material (micro-size Fe catalyst), respectively. On the other hand, during these structural transformations, the outer CNT diameter size remained almost constant due to compressive stress during the thickness growth of the graphitic tube walls. Ren et al. [42] proposed the thinner catalyst layer, producing thinner diameter size nanotubes. The length of a nanotube can be easily tailored by simply changing the synthesis time. Previous findings, such as those of Xiang et al. [43, 44], have theoretically and experimentally confirmed that higher synthesis time is beneficial with regard to growing longer CNT. Reit et al. [45] reported the length of nanotube was slightly increased as synthesis time was increased. As a result, the electrochemical performance of the CNT super-capacitor is dependent on synthesis time. One possible explanation why the synthesis time could affect the length of nanotube is presented here. The CNT growth increases rapidly once a carbon atom becomes sufficiently saturated onto the catalyst surface as illustrated in Figure 1g. At current stage, it is found that our samples are free of camphor-feedstock diffusion resistance and Fe catalyst deactivation (i.e., causes the growth to decelerate), allowing CNT to grow on sphere-like GS surface. However, further investigation is needed to predict the critical length of CNT in different synthesis parameters, subjecting the CNT array to strong diffusion resistance. Moreover, further investigation is needed to demonstrate the possibility of cap-opening and cap-closing mechanisms as referred to in Figure 3e,f. Finally, this mechanism demonstrates how nanotube growth is being made and supports the validity of the concept of carbon-through-metal diffusion and of the dissolution of carbon element in metal catalyst.
Conclusion
In summary, we have presented a few explanations of camphor-grown sphere-like GS and CNT using a practical method (two-stage catalytic CVD) for various synthesis times. It is shown that the 60-min synthesis time is the ideal time in controlling the phase of structure graphitization, gives highest aspect ratio, renders the lowest integrated intensity (ID/IG) ratio and diameter size distribution, and provides excellent purity and thermal stability of CNT. It is reasonable to believe that growing high-quality CNT using two-stage catalytic CVD has great promises, which is more economical, have simple operating conditions, and reproducibility. However, to understand the actual growth mechanism inside the system, a future investigation optimizing the growth parameters is needed. Through this, it is hoped that these desirable properties of CNT will be recommended for existing and wide range of new applications.
Methods
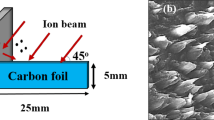
The synthesis of CNT using two-stage catalytic CVD is very attractive because of its simplicity. The experimental details of the synthesis of CNT were described. Briefly, in this work, camphor oil has been explored (obtained from SAFC (Steinheim, Germany) with 99.8% purity) as a hydrocarbon source [46] to investigate the changes of structural and thermal properties due to synthesis time in two-stage catalytic CVD. The ferrocene (obtained from Sigma-Aldrich (MO, USA) with 98% purity) was used as a catalyst without pre-treatment. The optimized condition 10% w/v (weight-volume percentage) of ferrocene relative to camphor oil was placed in different alumina boats [47]. The alumina boats were positioned side by side along a quartz tube in the first furnace of the two-stage catalytic CVD apparatus. In this approach, we appraise the effect of short synthesis time (2, 4, 6, 8, 10, 30, and 60 min) which was systematically studied with fixed growth temperature around 800°C [48], followed by thermal annealing process for 30 min through a nitrogen gas ambient [49] in the second furnace. The experimental setup in Figure 8a is similar to that reported earlier from our group [50]. The process involved as-synthesized titanium dioxide (TiO2) which was used as a catalyst prepared through a sol–gel method. However, in this process, the organometallic compound (ferrocene) was used as a catalyst. Under these conditions, sublimation and vaporization occurred on ferrocene and camphor oil, respectively, around 180°C in the first furnace. It has been observed that a ‘mat-like’ structure of carbon black powder grew inside the quartz tube of the second furnace; this was cooled down to room temperature. The as-grown free-standing carbon black powder was collected from the quartz tube as shown in Figure 8b and ready for characterization.
The characterization of the as-grown free-standing carbon black powder was carried out by field emission scanning electron microscopy (FESEM; JSM-7600-F, JEOL Ltd., Tokyo, Japan) operating at 10-kV electron high tension coupled to an energy dispersive X-ray (EDX). The protocol used for the sample preparation prior to FESEM characterization of each sample is as follows: The lint-free glove was used when touching the sample, sample holder, or stage to avoid contaminant and moisture. Small amounts of as-grown free-standing carbon black powder were mounted using carbon tape (carbon tape sticks on the top surface of the sample holder). Since our sample has good electrical properties, it was not coated with gold or platinum. An EDX analysis was based on dot mapping, which depends on the sample structure, to investigate the elemental analysis of the sample.
The diameter distribution, crystallinity, and impurities of the CNT were characterized by micro-Raman spectrometer (HR 800, Horiba Jobin Yvon, Edison, NJ, USA) at room temperature with Ar+ ion laser line (λ = approximately 514.532 nm) wavelength source and 20-mW power. In our facility, micro-Raman spectroscopy requires minimal sample handling and preparation. We have examined small amounts of carbon black powder mounted on to glass slide and secured with slide holder. The focal point with ×100 magnification is the area where the specific part of the sample will be analyzed. Three measurements per sample were taken across it to check the homogeneity of the sample. The data have been computed with the help of the LabSpec® software (version 5.45.09; Hamilton, New Zealand).
Further information on the as-grown free-standing carbon black powder structure have been obtained using a thermogravimetric analyzer (TGA; PE Pyris 1 TGA Thermo Balance, Perkin Elmer Inc., Waltham, MA, USA) with a heating rate of 20°C/min under ambient oxygen and a CHNS/O analyzer (PE 2400 CHNS/O Elemental Analyzer) heated at 975°C with constant flow of helium stream and enriched with oxygen of 99.995% purity to evaluate significant synthesis time-dependent thermal change.
Micrographs were obtained using high-resolution transmission electron microscope (HRTEM; FEI, Technai G2 20S Twin) operating at an accelerating voltage of 200 kV. The sample was suspended in an ethanol-methanol mixture sonicated for 15 min. Using a pipette, a droplet of the suspension was taken, placed onto a holey carbon-coated 400 mesh copper grid, and dried overnight.
Finally, the qualitative and quantitative analyses of the camphor oil were examined by gas chromatography–mass spectrometry (GC-MS). Prior to this study, headspace solid-phase micro extraction (HS-SPME) technique was used in order to collect the flavor volatiles of the camphor oil. GC separation and identification were performed on a GC system coupled with a mass detector (6890 N Network GC System, Agilent Technologies Inc., Santa Clara, CA, USA), and the mass spectra of the extracted volatile compounds were computed using the normalization method through the ChemStation® Data Analysis software.
Authors’ information
MSS is currently a graduate research assistant at the University of Southampton Malaysia Campus (USMC), where his studies focus on the growth of graphene. He is also one of the members in the Centre of Nanoscience and Nanotechnology, Universiti Teknologi MARA, where his research interest involves the synthesis and characterization of carbon nanotubes. He received a BSc degree in Physics from the Universiti Teknologi MARA, Malaysia in 2010. He has authored and co-authored over 20 peer-reviewed papers in journals and conference proceedings in the field of nanostructured carbon materials including Advances in Condensed Matter Physics, Journal of Spectroscopy, Journal of Nanomaterials, and Nano Hybrids. He also serves as a reviewer for several national and international journals. SAMZ is currently a postgraduate student at the Universiti Teknologi MARA, where his research expertise is on the chemistry of carbon-carbon hybrids and oxide materials. He has published outstanding articles in several high reputation journals such as Carbon, Materials Letters, Journal of Nanomaterials, and Materials Chemistry and Physics related to the research area. MM joined the Centre of Nanoscience and Nanotechnology, Universiti Teknologi MARA in 2009 and currently is a postgraduate student under the supervision of Professor Mohamad Rusop. She holds a BSc in Physics from the Universiti Teknologi MARA in 2010. Her primary research interest focuses on the mechanistic study of carbon nanotubes from palm olein as a precursor. NAA is a postgraduate student in nanocarbon group at the Centre of Nanoscience and Nanotechnology, Universiti Teknologi MARA. Her research includes nanotube-porous silicon interface problems associated with the fabrication of field electron emission device. NAA has a BSc degree in Materials Technology from the same institution (2009). She is the author and co-author of more than 13 papers in refereed journals and conference proceedings including Ionics and Microelectronic Engineering. SAB is the head in the Nanotechnology Research Centre, Universiti Pendidikan Sultan Idris. Since 2012, she is also one of associate members in the Centre of Nanoscience and Nanotechnology, Universiti Teknologi MARA. She earned a PhD degree in nanomaterials in 2011, MSc degree in Materials Physics in 2005, and BSc degree in Industrial Physics from the same institution in 2002. Her main research interest is in the field of low dimensional carbon materials such as amorphous carbon, carbon nanotubes, and graphene from promising renewable hydrocarbon feedstock. She is also active in research publication, and until now, she has authored and co-authored more than 40 articles including conference proceedings, journals, patents, and book chapters such as Materials Letters, Japanese Journal of Applied Physics, Journal of the Ceramic Society of Japan, and Carbon and Oxide Nanostructures (Advanced Structured Materials). In 2011, she was awarded with a silver medal at the 39th Exhibition of Inventions Geneva organized by WIPO, Geneva. SA is a professor of Physics at the Faculty of Applied Sciences, Universiti Teknologi MARA since 2009. In 2004, he holds a PhD degree in nanostructured materials at the University of Malaya, where his study focuses on the fabrication of porous silicon nanostructure. Currently, he extends his research interest in the fabrication of silicon quantum dot nanoparticles and one-dimensional materials (e.g., TiO2, ZnO, and carbon nanotubes). He is also active in research publication such as Surface Review and Letters, Optical Materials, and Microelectronic Engineering. SYSY is an associate professor of Physics at the Faculty of Applied Sciences, Universiti Teknologi MARA. In 2005, he obtained a PhD degree at the School of Applied Physics, National University of Malaysia. He is also one of council member in American Society of Non Destructive Testing, Malaysian Association of Solid State Science, and Malaysian Institute of Physics. He has published several papers in conference proceedings such as Advanced Materials Research and American Institute of Physics Proceedings related to the low-dimensional carbon materials and superconductor physics. MRM is the head of the Centre of Nanoscience and Nanotechnology and a research fellow in the Centre of Nanoelectronic. He received a PhD degree in opto-electronic devices and nanotechnology in 2003 from the Nagoya Institute of Technology, Japan. He is a professor in exceptional class at Universiti Teknologi MARA. His research focuses on the opto-electronic devices and nanotechnology. He is the editor or editorial advisory board member of five international journals. He has published over 9 patents and 400 research papers in peer-reviewed journals in the field of nanomaterials with an H-index of 15 and a total citation over than 100.
References
Zobir SAM, Zainal Z, Keng CS, Sarijo SH, Yusop M: Synthesis of carbon nanohorn–carbon nanotube hybrids using palm olein as a precursor. Carbon 2013, 54: 492–494.
Azmina MZ, Suriani AB, Salina M, Azira AA, Dalila AR, Rosly J, Roslan MN, Rusop M: Variety of bio-hydrocarbon precursors for the synthesis of carbon nanotubes. Nano Hybrids 2012, 2: 43–63.
Yusop MZM, Ghosh P, Yaakob Y, Kalita G, Sasase M, Hayashi Y, Tanemura M: In situ TEM observation of Fe-included carbon nanofiber: evolution of structural and electrical properties in field emission process. ACS Nano 2012, 11: 9567–9573.
Bethune DS, Kiang CH, de Vries MS, Gorman G, Savoy R, Vazquez J, Beyers R: Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363: 605–607. 10.1038/363605a0
Iijima S, Ichihashi T: Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363: 603–605. 10.1038/363603a0
Monthioux M, Kuznetsov VL: Who should be given the credit for the discovery of carbon nanotubes? Carbon 2006,44(9):1621–1623. 10.1016/j.carbon.2006.03.019
Oberlin A, Endo M, Koyama T: Filamentous growth of carbon through benzene decomposition. J. Cryst. Growth 1976,32(3):335–349. 10.1016/0022-0248(76)90115-9
Oriňáková R, Oriňák A: Recent applications of carbon nanotubes in hydrogen production and storage. Fuel 2011,90(11):3123–3140. 10.1016/j.fuel.2011.06.051
Ren X, Chen C, Nagatsu M, Wang X: Carbon nanotubes as adsorbents in environmental pollution management: a review. Chem. Eng. J. 2011,170(2–3):395–410.
Upadhyayula VKK, Deng S, Mitchell MC, Smith GB: Application of carbon nanotube technology for removal of contaminants in drinking water: a review. Sci. Total. Environ 2009,408(1):1–13. 10.1016/j.scitotenv.2009.09.027
Ferguson AJ, Blackburn JL, Kopidakis N: Fullerenes and carbon nanotubes as acceptor materials in organic photovoltaics. Mater. Lett. 2013, 90: 115–125.
Khodakovskaya MV, Kim B-S, Kim JN, Alimohammadi M, Dervishi E, Mustafa T, Cernigla CE: Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community. Small 2013,9(1):115–123. 10.1002/smll.201201225
Grobert N: Carbon nanotubes–becoming clean. Mater Today 2007,10(1–2):28–35.
Gould P: Carbon nanotubes help bones grow: biomaterials. Mater Today 2006,9(5):19.
Sealy C: Water on the nanoscale: carbon nanotubes. Mater Today 2006,7(12):12.
Zobir SAM, Abdullah S, Zainal Z, Sarijo SH, Rusop M: Synthesis of carbon nano- and microspheres using palm olein as the carbon source. Mater. Lett. 2012, 78: 205–208.
Wu H-C, Hong C-T, Chiu H-T, Li Y-Y: Continuous synthesis of carbon spheres by a non-catalyst vertical chemical vapor deposition. Diamond Relat. Mater 2009,18(4):601–605. 10.1016/j.diamond.2008.10.047
Suriani AB, Azira AA, Nik SF, Roslan MN, Rusop M: Synthesis of vertically aligned carbon nanotubes using natural palm oil as carbon precursor. Mater. Lett 2009,63(30):2704–2706. 10.1016/j.matlet.2009.09.048
Kumar M, Ando Y: Controlling the diameter distribution of carbon nanotubes grown from camphor on a zeolite support. Carbon 2005,43(3):533–540. 10.1016/j.carbon.2004.10.014
Dijon J, Szkutnik PD, Fourier A, de Monsabert TG, Okuno H, Quesnel E, Muffato V, de Vito E, Bendiab N, Bogner A, Bernier N: How to switch from a tip to base growth mechanism in carbon nanotube growth by catalytic chemical vapour deposition. Carbon 2010,48(13):3953–3963. 10.1016/j.carbon.2010.06.064
Scheibe B, Borowiak-Palen E, Kalenczuk RJ: Oxidation and reduction of multiwalled carbon nanotubes—preparation and characterization. Mater. Charact. 2010,61(2):185–191. 10.1016/j.matchar.2009.11.008
Antunes EF, de Resende VG, Mengui UA, Cunha JBM, Corat EJ, Massi M: Analyses of residual iron in carbon nanotubes produced by camphor/ferrocene pyrolysis and purified by high temperature annealing. Appl. Surf. Sci. 2011,257(18):8038–8043. 10.1016/j.apsusc.2011.04.090
Huang W, Wang Y, Luo G, Wei F: 99.9% purity multi-walled carbon nanotubes by vacuum high-temperature annealing. Carbon 2003,41(13):2585–2590. 10.1016/S0008-6223(03)00330-0
Köhler AR, Som C, Helland A, Gottschalk F: Studying the potential release of carbon nanotubes throughout the application life cycle. J. Cleaner. Prod. 2008,16(8–9):927–937.
Huang B-R, Lin T-C: A novel technique to fabricate horizontally aligned CNT nanostructure film for hydrogen gas sensing. Int. J. Hydrogen. Energy. 2011, 36: 15919–15926. 10.1016/j.ijhydene.2011.09.048
Liu P, Liu L, Zhang Y: Alignment characterization of single-wall carbon nanotubes by Raman scattering. Phys. Lett. A 2003,313(4):302–306. 10.1016/S0375-9601(03)00764-3
Ritter U, Scharff P, Dmytrenko OP, Kulish NP, Prylutskyy YI, Belyi NM, Gubanov VA, Komarova LA, Lizunova SV, Shlapatskaya VV, Bernas H: Radiation damage and Raman vibrational modes of single-walled carbon nanotubes. Chem. Phys. Lett. 2007,447(4–6):252–256.
Nijkamp MG, Raaymakers JEMJ, van Dillen AJ, de Jong KP: Hydrogen storage using physisorption—materials demands. Appl. Phys. A. 2001,72(5):619–623. 10.1007/s003390100847
Azira AA, Suriani AB, Rusop M: Carbon nanotubes formation from Fe/Ni/Mg by camphor oil decomposition. J. Ceram. Soc. Jpn. 2011,119(1386):125–128. 10.2109/jcersj2.119.125
Radushkevich LV, Lukyanovich VM: About the structure of carbon formed by thermal decomposition of carbon monoxide on iron substrate. Soviet J. Phys. Chem. 1952, 26: 88–95.
Kumar M, Ando Y: Carbon nanotubes from camphor: an environment-friendly nanotechnology. J. Phys. Conf. Ser. 2007, 61: 643–646.
Yuan Q, Xu Z, Yakobson BI, Ding F: Efficient defect healing in catalytic carbon nanotube growth. Phys. Rev. Lett. 2012,108(24):245505.
Kareem SA: Kinetics of camphor decomposition in ambient condition. Res. Commun. Chem. 2000,1(1):8–11.
Kumar M, Ando Y: Gigas growth of carbon nanotubes. Defense Sci. J. 2008,58(4):496–503.
Kumar M, Ando Y: Camphor—a botanical precursor producing garden of carbon nanotubes. Diamond Relat. Mater. 2003,12(3–7):998–1002.
Sharma S, Kalitaa G, Hirano R, Hayashi Y, Tanemura M: Influence of gas composition on the formation of graphene domain synthesized from camphor. Mater. Lett. 2013, 93: 258–262.
Suriani AB, Roslan MN, Rusop M: Vertically aligned carbon nanotubes synthesized from waste cooking palm oil. J. Ceram. Soc. Jpn. 2010,118(10):963–968.
Bahrami B, Khodadadi A, Mortazavi Y, Esmaieli M: Short time synthesis of high quality carbon nanotubes with high rates by CVD of methane on continuously emerged iron nanoparticles. Appl. Surf. Sci. 2011,257(23):9710–9716. 10.1016/j.apsusc.2011.05.086
Charlier J-C, De Vita A, Blasé X, Car R: Microscopic growth mechanisms for carbon nanotubes. Sci. 1997,275(5300):647–649. 10.1126/science.275.5300.647
Schaper AK, Hou H, Greiner A, Phillipp F: The role of iron carbide in multiwalled carbon nanotube growth. J. Catal. 2004,222(1):250–254. 10.1016/j.jcat.2003.11.011
Fedoseeva YV, Bulusheva LG, Okotrub AV, Vyalikh DV, Huo J, Song H, Zhou J, Chen X: Effect of oxidation and heat treatment on the morphology and electronic structure of carbon-encapsulated iron carbide nanoparticles. Mater. Chem. Phys 2012,135(1):235–240. 10.1016/j.matchemphys.2012.04.063
Ren ZF, Huang ZP, Xu JW, Wang JH, Bush P, Siegal MP, Provencio PN: Synthesis of large arrays of well-aligned carbon nanotubes on glass. Sci. 1998, 282: 1105–1107.
Xiang R, Yang Z, Zhang Q, Luo G, Qian W, Wei F, Kadowaki M, Einarsson E, Maruyama S: Growth deceleration of vertically aligned carbon nanotube arrays: catalyst deactivation or feedstock diffusion controlled? J. Phys. Chem. C. 2008,112(13):4892–4896. 10.1021/jp710730x
Xiang R, Luo G, Yang Z, Zhang Q, Qian W, Wei F: Temperature effect on the substrate selectivity of carbon nanotube growth in floating chemical vapor deposition. Nanotechnol. 2007,18(41):415703–415706. 10.1088/0957-4484/18/41/415703
Reit R, Nguyen J, Ready WJ: Growth time performance dependence of vertically aligned carbon nanotube supercapacitors grown on aluminum substrates. Electrochim. Acta. 2013, 91: 96–100.
Asli NA, Suriani AB, Shamsudin MS, Yusop SFM, Rusop M, Abdullah S: Novel method: coral like structure of align carbon nanotubes (A-CNTs) on porous silicon template (PSiT) without catalyst; green approach. Adv. Mater. Res. 2012, 364: 222–227.
Shamsudin MS, Abdullah S, Rusop M: Structural and thermal behaviors of iron-filled align carbon nanotubes formulated by two-stage catalytic chemical vapor deposition. Adv. Mater. Res. 2012, 364: 191–195.
Shamsudin MS, Asli NA, Abdullah S, Yahya SYS, Rusop M: Effect of synthesis temperature on the growth iron-filled carbon nanotubes as evidenced by structural, micro-raman, and thermogravimetric analyses. Adv. Condens. Mat. Phys. 2012, 2012: 420619.
Shamsudin MS, Suriani AB, Abdullah S, Yahya SYS, Rusop M: Impact of thermal annealing under nitrogen ambient on structural, micro-Raman, and thermogravimetric analyses of camphoric-CNT. J. Spectro. 2013, 2013: 167357.
Shamsudin MS, Achoi MF, Asiah MN, Ismail LN, Suriani AB, Abdullah S, Yahya SYS, Rusop M: An investigation on the formation of carbon nanotubes by two-stage chemical vapor deposition. J. Nanomater. 2012, 2012: 972126.
Acknowledgments
The authors are so grateful to Prof. Dr. Norlida Kamarulzaman and Mrs. Norashikin Kamarudin (Centre of Nanomaterials, Institute of Science) for their valuable technical assistance and useful discussions on HRTEM analysis, and to Mr. M.Z. Borhan (School of Chemistry and Environmental Studies, Faculty of Applied Sciences) for his continuous support and helpful discussions on HS-SPME/GC-MS analysis. This project was supported by the Malaysian Ministry of Higher Education through the Fundamental Research Grant Scheme and MyMaster Scholarship program, the Research Management Institute through Excellence Fund under contact no. 600-RMI/ST/DANA 5/3/Dst 435/2011, and the Institute of Graduate Studies through UiTM Postgraduate Teaching Assistance Scheme. MSS thanks the University of Southampton for the provision of facility. The authors gratefully acknowledge the anonymous reviewers for making recondite information available.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MSS, MM, SAMZ, NAA, SAB, SA, SYSY, and MRM carried out the nanocarbon studies and participated in the experimental work on the growth and characterization of carbon nanotubes. All authors provided the same contributions in this article. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Shamsudin, M., Mohammad, M., Zobir, S. et al. Synthesis and nucleation-growth mechanism of almost catalyst-free carbon nanotubes grown from Fe-filled sphere-like graphene-shell surface. J Nanostruct Chem 3, 13 (2013). https://doi.org/10.1186/2193-8865-3-13
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-13