Abstract
A novel, simple and economic reverse phase high performance liquid chromatography (RP-HPLC) method has been developed for the quantification of Meloxicam in bulk and tablet dosage form with greater precision and accuracy. Separation was achieved on Develosil ODS HG-5 RP C18, (15 cm × 4.6 mm i.d. 5 μm) column in isocratic mode with mobile phase consisting of acetonitrile: phosphate buffer(pH 3.4) (60:40) with a flow rate of 1 mL/min. The detection was carried out at 268 nm. The retention time of Meloxicam was found to be 2.09 min. The method was validated as per ICH guidelines. Linearity was established for Meloxicam in the range 20 – 120 μg/ml with R2 value 0.996. The percentage recovery of Meloxicam was found to be in the range 99.99-100.46%. The high recovery and low relative standard deviation confirm the suitability of the proposed method for the estimation of the drug in bulk and tablet dosage forms. Validation studies demonstrated that the proposed RP-HPLC method is simple, specific, rapid, reliable and reproducible for the determination of Meloxicam for Quality Control level.
Similar content being viewed by others
Introduction
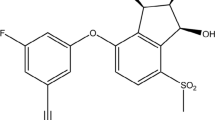
Meloxicam (MEL), shown in Figure 1, is chemically, 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H,1,2-benzothaizine-3-carboxamide-1, 1-dioxide. It is used for the treatment of osteoarthritis, rheumatoid arthritis, and pauciarticular and polyarticular course juvenile rheumatoid arthritis. Its analgesic, antipyretic and anti-inflammatory activity is due to the inhibition of COX-2 enzyme (Noble & Balfour 1996; Davies & Skjodt 1999). Meloxicam pharmacological activity is greatly affected by the substituents on parent oxicam moiety e.g. change in methyl to ethyl at 2 positions causes complete suppression of activity (Lemke & Williams 2008). Several analytical methods for the determination of meloxicam by flourimetry (Hassan 2002), capillary electrophoresis (Nemutlu & Kir 2003), spectrophotometry (Hassan 2002), HPLC (Arayne et al. 2005; Farzana & Pradeep 2009; Damle et al. 2009), LC/MS (Wiesner et al. 2003) and polarographic (Altıokka et al. 2000; Radi et al. 2001a; Altınöz et al. 2002; Radi et al. 2001b; Rao et al. 2005) have been reported. The aim of the present work was to develop and validate a sensitive RP-HPLC method that can be implemented for the quantification of meloxicam in bulk as well as in its tablet dosage forms.
Experimental
Materials
Pure Meloxicam (MEL) used as working standards, was purchased from Yarrow chem. Products, Mumbai, India. Tablets containing 15 mg and 7.5 mg of meloxicam (MUVERA15 and MOVAC) were obtained from Apollo Pharmaceuticals Pvt. Ltd, Visakhapatnam, India and used within their shelf life period. Acetonitrile and water (HPLC-grade) were purchased from Merck, India. All other chemicals and reagents employed were of analytical grade, and purchased from Merck, India.
Instrumentation
The chromatographic system used comprised of Analytical technologies ltd UV 2230 UV–vis detector. Data integration was carried out using A-4000 version software. Samples were injected into Develosil ODS HG-5 RP C18 (5 μm, 15 cm × 4.6 mm i.d) column. An Analytical technologies ltd.sonicator was used for enhancing the dissolution of the compounds. A Wenster digital pH meter was used for pH adjustment.
Chromatographic conditions
The high performance liquid chromatographic (HPLC) system used was operated isocratically with the column temperature maintained at 30°C, using a mobile phase composition of acetonitrile and phosphate buffer (pH adjusted to 3.4 with O-Phosphoric acid) in the ratio of 60:40 v/v at a flow rate of 1.0 mL/min within a run time of 7 min. Prior to use, the mobile phase was degassed by an ultrasonic bath and filtered by a Millipore vacuum filter system equipped with a 0.45 μm high vacuum filter. The drug was detected and quantified at 268 nm.
Preparation of standard solutions
The stock solution was prepared by transferring 100 mg of Meloxicam into 100 mL volumetric flask. Then it was added with small amount of diluent [acetonitrile: water (50:50)], and the mixture was sonicated to dissolve and made up to volume with mobile phase. From this stock solution different concentrations were prepared to give final concentrations of 20 – 120 μg/mL for standard calibration curve.
Assay of meloxicam from marketed tablets
Twenty tablets were accurately weighed and crushed to a fine powder in a mortar in each of the marketed formulation separately. An amount of the powder equivalent to 100 mg was transferred into a 100 mL volumetric flask and 10 mL of diluent was added to it followed by 10 ml of 0.1 N NaOH. The mixture was sonicated to dissolve the exipients and then made up to volume with mobile phase. Following 15 min of mechanical shaking, it was kept in an ultrasonic bath for 15 mins, and the solution was filtered through a 0.45 μm filter paper. Suitable aliquots (1 ml each) of the filtered solution were transferred to 50 ml volumetric flasks and made up to volume with mobile phase to yield six concentrations of Meloxicam (20 μg/mL). A 20 μL volume of the sample solution was injected into the chromatographic system, six times, under optimized chromatographic conditions. The peak areas were measured at 268 nm and concentrations in the samples were determined by interpolation from standard calibration curve of each drug previously obtained.
Method validation
The method was validated in accordance with ICH guidelines (International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use 2010). The parameters assessed were linearity, accuracy, and limit of detection (LOD), limit of quantification (LOQ), precision, reproducibility, robustness and system suitability.
Accuracy
Accuracy was best determined by the standard addition method. Previously analyzed samples of Meloxicam API were added with standard drug solutions and are analyzed by the proposed method. Recovery (%), RSD (%) and bias (%) were calculated for each concentration.
Accuracy is reported as percentage bias, which is calculated from the expression
Precision
Precision was determined as both repeatability and intermediate precision, in accordance with ICH guidelines. Repeatability of sample injection was determined as intraday variation and intermediate variation. For these determinations, single concentration (20 μg/ml) at different time intervals and different days, of the solution of Meloxicam API was used.
Robustness
The concept of robustness of an analytical procedure has been defined by the ICH as “a measure of its capacity to remain unaffected by small but deliberate variations in method parameters”. To determine the robustness of the method experimental conditions are purposely altered and chromatographic characters are evaluated. Influence of small changes in chromatographic conditions such as change in flow rate (± 0.1 ml/min), wavelength of detection (±2 nm) and acetonitrile content in mobile phase (±2%) were studied to determine the robustness of the method.
Limit of detection (LOD)
The limit of detection (LOD) of an analytical method may be defined as the concentration, which gives rise to an instrument signal that is significantly different from the blank. For spectroscopic techniques or other methods that rely upon a calibration curve for quantitative measurements, the IUPAC approach employs the standard deviation of the intercept (Sa), which may be related to LOD and the slope of the calibration curve, b, by
Limit of quantitation (LOQ)
The LOQ is the concentration that can be quantitated reliably with a specified level of accuracy and precision. The LOQ represent the concentration of analyte that would yield a signal-to-noise ratio of 10.
Where, Sa is the standard deviation of the peak area ratio of analyte to IS (6 injections) of the drugs and b is slope of the corresponding calibration curve.
Results and discussion
Optimization of chromatographic conditions
The chromatographic conditions were optimized by different means i.e. using different column, different mobile phase, different flow rate, different detection wavelength and different diluents for standard drug and marketed tablets are summarized in Table 1 and different chromatograms (Figure 2) are shown.
Linearity & range
The standard calibration curve(Figure 3) showed good linearity in the range of 20 – 120 μg/ml (Table 2), for Meloxicam API with correlation coefficient (r2) of 0.996. A typical calibration curve has the regression equation of y = 37461x + 158481 for Meloxicam.
Accuracy: recovery study
The recovery of the method, determined by adding a previously analyzed test solution with additional drug standard solution at three levels of concentration, was 99.99 - 100.46%. The values of recovery (%), RSD (%) and % Bias listed in Table 3 indicate the method is accurate.
Precision: Intra-assay & inter-assay
The intra & inter day variation of the method was carried out and the high values of mean assay and low values of standard deviation and % RSD (% RSD < 2%) within a day and day to day variations for Meloxicam revealed that the proposed method is precise (Table 4).
Robustness
Influence of small changes in chromatographic conditions such as change in flow rate (± 0.1 ml/min), Wavelength of detection (±2 nm) & acetonitrile content in mobile phase (±2%) studied to determine the robustness of the method are also in favor of (Table 5, % RSD < 2%) the developed RP-HPLC method for the analysis of Meloxicam API.
LOD & LOQ
The Minimum concentration level at which the analyte can be reliable detected (LOD) & quantified (LOQ) were found to be 0.42 & 1.29 μg/ml respectively.
Assay of meloxicam in tablet dosage forms
Assay was performed by using the regression equation (y = 37461x + 158481, R2 = 0.996) obtained from the standard calibration curve of Meloxicam API. Results obtained are given in Table 6. The assay of Muvera 15 and Movac tablet containing Meloxicam was found to be 99.99 and 100.01% respectively as per the method. The chromatograms are represented in Figures 4 and 5.
The method is having linearity in the range 20 to 120 μg/ml with regression coefficient value 0.99. When compared to previous methods for recovery study, the % recovery is nearly 100%. Precision results are having low% RSD value i.e. below 0.1 in comparison to previous methods, which is an added advantage. Similarly in previous methods assay in marketed formulation is carried out by considering single formulation, but the present method uses two different formulations. Also at very low concentration it can be detected and quantified i.e. at 0.42 & 1.29 μg/ml, which is not observed in previous methods. For these reasons the method is proved to be novel in comparision to existed methods.
Conclusion
A New RP-HPLC method indicating assay of MEL in bulk and in pharmaceutical dosage forms is established. This method is simple, reliable, linear, accurate, sensitive and reproducible as well as cost effective for the effective quantitative analysis of MEL in bulk and tablet formulations. The method was completely validated showing satisfactory data for all the method validation parameters tested and method is free from interference of the other active ingredients and additives used in the formulations. Therefore the method is suitable for use of the routine quality control analysis of Meloxicam in API or in pharmaceutical dosage forms.
References
Altınöz S, Nemutlu E, Kır S: Polarographic behaviour of meloxicam and its determination in tablet preparations and spiked plasma. Farmaco 2002, 57: 463-468. 10.1016/S0014-827X(02)01239-9
Altıokka G, Atkosar Z, Tuncel M: Pulse polarographic determination of meloxicam. Pharmazie 2000, 56: 184-185.
Arayne MS, Sultana N, Siddiqui FA: A new RP-HPLC method for analysis of meloxicam in tablets. Pak J Pharm Sci 2005, 18: 58-62.
Damle MC, Sinha PK, Jeswani RM, Topagi KS: A validated RP-HPLC method for determination of Meloxicam in the Presence of its Impurities. Int J Pharm Tech Res 2009, 1: 1051-1060.
Davies NM, Skjodt NM: Clinical pharmacokinetics of meloxicam. Clin Pharmacokinet 1999, 36: 115-122. 10.2165/00003088-199936020-00003
Farzana SB, Pradeep RV: A stability indicating HPLC method for the determination of meloxicam in bulk and commercial formulations. Trop J Pharm Res 2009, 8: 257-264.
Hassan EM: Spectrophotometric and fluorimetric methods for the determination of meloxicam in dosage forms. J Pharm Biomed Anal 2002, 27: 771-793. 10.1016/S0731-7085(01)00530-1
International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use: Harmonized Triplicate Guideline on Validation of Analytical Procedures: Methodology, Recommended for Adoption at Step 4 of the ICH Process on November 1996 by the ICH Steering Committee. Switzerland: IFPMA; 2010.
Lemke LT, Williams AD: Foye’s Principles of Medicinal Chemistry. New York: W. Lippincot and wilkin; 2008.
Nemutlu E, Kir S: Method development and validation for the analysis of meloxicam in tablets by CZE. J Pharm Biomed Anal 2003, 31: 393-405. 10.1016/S0731-7085(02)00641-6
Noble S, Balfour JA: Meloxicam. Drugs 1996, 51: 424-431. 10.2165/00003495-199651030-00007
Radi A, El-Ries MA, El-Anwar F, El-Sherif Z: Electrochemical oxidation of meloxicam and its determination in tablet dosage form. Anal Lett 2001a, 34: 739-748. 10.1081/AL-100103216
Radi AE, Ghoneim M, Beltagi A: Cathodic adsorptive stripping square-wave voltammetry of the anti-inflammatory drug meloxicam. Chem Pharm Bull 2001b, 49: 1257-1260. 10.1248/cpb.49.1257
Rao RN, Meena S, Rao AR: An overview of the recent developments in analytical methodologies for determination of COX-2 inhibitors in bulk drugs, pharmaceuticals and biological matrices. J Pharm Biomed Anal 2005, 39: 349-367. 10.1016/j.jpba.2005.03.040
Wiesner JL, De Jager AD, Sutherland FCW, Hundt HKL, Swart KJ, Hundt AF, Els J: Sensitive and rapid liquid chromatography-tandem mass spectrometry method for the determination of meloxicam in human plasma. J Chromatogr B 2003, 785: 115-121. 10.1016/S1570-0232(02)00862-0
Acknowledgements
The authors would like to acknowledge the contributions of Yalamarty Pharmacy College, Tarluwada, Anandapuram, Visakhapatnam, Andhra Pradesh, India for providing necessary facilities to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NK has designed the entire procedure and done the entire work. M has done method optimisation. PS and G has done some validation parameters. JN and N have done rest of validations. All the authors have read the article and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sahoo, N.K., Sahu, M., Rao, P.S. et al. Validation of assay indicating method development of meloxicam in bulk and some of its tablet dosage forms by RP-HPLC. SpringerPlus 3, 95 (2014). https://doi.org/10.1186/2193-1801-3-95
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-1801-3-95