Abstract
Introduction
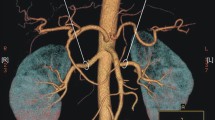
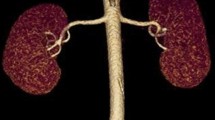
The commonest variation to the classic anatomic description of renal arterial supply is the presence of accessory renal arteries. The incidence varies widely according to ethnicity. There is no data on the prevalence of these anomalies in persons of Caribbean ethnicity.
Methods
All CT scans done over two years from July 1, 2010 to June 30, 2012 were retrospectively evaluated. The anatomy of the renal arterial supply was reported from these studies and the anatomy of accessory renal arteries was documented.
Results
There were 302 CT scans evaluated and accessory renal arteries were present in 109/302 (36.1%) CT scans, 95% confidence interval 30.6%, 41.4%. There were 71/309 (23.5%) patients with accessory arteries on the left and 54/309 (17.9%) had them on the right (p 0.087). Of these, 16 (14.7%) patients had bilateral accessory renal arteries present. The most common origin for the accessory arteries was the abdominal aorta in 108 (99.1%) cases and in 1 case the accessory artery arose from the coeliac trunk. There were 80 left sided accessory renal arteries: 17 (21.3%) upper polar and 27 (33.8%) lower polar arteries. Of 62 right sided accessory arteries, 14 (22.6%) were upper polar and 26 (42%) were lower polar arteries.
Conclusion
This is the first population-based report of anatomic anomalies in renal arterial supply in a Caribbean population. These are important findings that may affect vascular and urologic procedures on persons of Caribbean ethnicity.
Similar content being viewed by others
Background
The classic description of renal arterial anatomy describes a single renal artery arising from the abdominal aorta and entering the renal hilum. It branches at or near the hilum into anterior and posterior branches and then progressively into lobar, inter-lobar and arcuate arteries, eventually supplying the parenchyma as end arteries(Anatomy 2005; Merklin & Michels 1958; Michels 1955). However, many patients have variations to this classic description (Harrison et al. 1978; Khamanarong et al. 2004; Sampaio & Passos 1992; Urban et al. 2001; Beregi et al. 1999; Ozkan et al. 2006). The most common variation is the presence of accessory renal arteries (Harrison et al. 1978; Khamanarong et al. 2004; Sampaio & Passos 1992; Urban et al. 2001; Beregi et al. 1999; Ozkan et al. 2006). The reported incidence ranges from 11.3% (Sungura 2012) to 59.5% (Budhiraja et al. 2013) of persons and varies widely with ethnicity (Ozkan et al. 2006;Sungura 2012; Budhiraja et al. 2013; Saldarriaga et al. 2008; Papaloucas et al. 2007; Santos-Soares et al. 2013).
Accessory arteries are clinically significant because they affect several operative procedures including graft deployment during endovascular aneurysm repair, open aneurysmorrhaphy, nephrectomy and renal transplantation. Therefore, it is important to have knowledge of anatomic variants in each population.
A literature review in Medscape, Medline, Pubmed, Embase and Scilo was performed on April 30, 2013 using the keywords: Caribbean, renal, artery, vascular, perfusion, anomaly and variants. This returned two case reports describing the presence of accessory renal arteries encountered during cadaveric dissections for anatomic teaching (Adekunle & Uche-Nwachi 2007; Rao et al. 2011). There were no reports of large population-based studies from the Caribbean. We carried out this retrospective study to evaluate the incidence and distribution of accessory renal arteries in unselected patients undergoing contrast enhanced computer tomography (CT) scanning of the abdomen at a regional referral centre in the northern Caribbean.
Methods
This study was done at the University Hospital of the West Indies (UHWI) in Jamaica. This is a 500 bed hospital that serves as a major tertiary referral centre for Jamaica and a regional referral centre for northern Caribbean countries (Johnson et al. 2013). Therefore, the findings in this study should be representative of anatomic variations in the general population across the Anglophone Caribbean.
At the UHWI, multiphase CT and angiography (CTA) were introduced routinely in the radiology department in 2007 with the introduction of a Phillips Brilliance® 64 slice multi-detector CT scanner. Currently over 5000 CT scans are performed yearly at the UHWI. The institutional review board (University of the West Indies Faculty of Medical Science Ethics Committee) granted ethical approval to access these studies to evaluate the distribution of anatomic patterns. Written informed consent was obtained from the patient for the publication of this report and any accompanying images.
Two practicing radiologists independently evaluated images from all CT scan series of the abdomen and pelvis performed on a 64-slice CT scanner over a period of two years from July 1, 2010 to June 30, 2012. The anatomy of the renal arterial supply on each scan was reported from these studies. The images evaluated in this study included: all CTAs of the abdominal aorta and iliac arteries; all abdomino-pelvic CT scans with an arterial phase covering the abdominal aorta and iliac arteries; and all CT scans of the chest that adequately covered the entire abdomen in the arterial phase. We excluded the following studies: any CT scans done at referring facilities; any CT with incomplete demographic data; duplicated CT scans; CT scans without adequate arterial phases; and CT scans in patients who had prior nephrectomy as these patient would be expected to have disturbed anatomy. This study was performed to investigate renal arterial anatomy in patients with scans for a variety of indications. Therefore we could not reliably comment on the relationship of the ureters to any accessory arteries encountered.
Merklin and Michels 1958 proposed a classification for accessory arteries based on their origin and entry into the kidney. We utilized this classification to describe the variants encountered in our study. The main renal artery was considered to be a single dominant artery arising from the abdominal aorta that entered the renal hilum and branched into segmental arteries. Accessory renal arteries did not branch prior to entry into the renal parenchyma. They were classified based on the Merklin and Michels’ classification based on their origin from: (1) abdominal aorta; (2) main renal artery and (3) origin from other sources. We also classified the variations according to their entry into the kidney into: (1) upper polar; (2) hilar and (3) lower polar accessory arteries.
Data were extracted from the CT scans and recorded in a Microsoft Excel chart formatted with the above information. Descriptive statistics were generated using the SPSS statistical software and a statistical test of differences in proportions was used to compare the frequency or accessory arteries on the right and left sides.
Results
There were 302 CT scans done over the study period that met the inclusion criteria. Accessory renal arteries were present in 109 (36.1%) patients, (95% confidence interval (CI): 30.6%, to 41.4%). There were accessory renal arteries present in 71/302 (23.5%) patients on the left and 54/302 (17.9%) patients on the right side. Sixteen (5.3%) patients had bilateral accessory renal arteries present. Although accessory renal arteries were commoner on the left, the difference did not attain statistical significance (23.5% vs 17.9%, p = 0.087).
According to the Merklin and Michels’ classification (Merklin & Michels 1958), the most common origin for accessory renal arteries was directly from the abdominal aorta in all cases. There was a single case in which the origin of the accessory renal artery came directly off the aorta antero-laterally at the same level as the coeliac trunk, coursed infero-laterally to the right to enter the upper pole of the right kidney. There were no cases in which the accessory artery took an origin from the main renal artery or any other vessel.
The anatomy was also described according to the entry point on the kidneys. There were 80 accessory renal arteries on the left side in 71 patients. Table 1 classifies these arteries according to their renal entry point. There were 62 accessory renal arteries on the right side in 54 patients and this is tabulated in Table 2.
Discussion
In the developing human embryo the mesonephros, metanephros, adrenals and gonads are supplied by paired mesonephric arteries arising from the dorsal aorta (Hlaing et al. 2010; Felix 1911). The 3rd, 4th and 5th pairs of lateral mesonephric arteries supply the metanephros (Felix 1911). The caudal branches usually disappear, leaving a single persistent renal artery (Felix 1911). When more than one of these lateral mesonephric arteries persist, multiple accessory renal arteries result (Budhiraja et al. 2013; Hlaing et al. 2010; Felix 1911).
The presence of accessory renal arteries is one of the most common urogenital variants (Harrison et al. 1978; Khamanarong et al. 2004; Sampaio & Passos 1992; Urban et al. 2001; Beregi et al. 1999; Ozkan et al. 2006). It is well documented that the incidence of accessory renal arteries varies widely with ethnicity, ranging from 11.4% in Kenyans (Sungura 2012) to 59.5% in Indians (Budhiraja et al. 2013). Table 3 documents the variation in incidence according to ethnicity in published medical literature.
Renal arterial anatomy was traditionally evaluated with catheter angiography but it has now been superseded by CT angiography (Urban et al. 2001; Sebastia et al. 2010; Das 2008; Smith et al. 1998) that reportedly can identify renal arterial anatomy with 66-100% sensitivity and 75-100% specificity (Smith et al. 1998; Sahani et al. 2005; Dachman et al. 1998; Pozniak et al. 1998). There is also the advantage of providing other information that may impact treatment such as demonstrating venous and collecting system anatomy that conventional angiography cannot offer (Sebastia et al. 2010).
Using CT angiography we have shown that the prevalence of accessory renal arteries is 36.1% in the Caribbean population. This incidence is greater than that seen in most other ethnicities (Table 2), exceeded only by the reported incidence in Northern India, where Budhiraja et al. (Budhiraja et al. 2013) reported an incidence of 59.5%. However, Budhiraja’s study only evaluated a small convenience sample of 37 cadavers used in anatomic teaching (Budhiraja et al. 2013). Most of the published reports in medical literature with more than 200 patients or cadavers (Ozkan et al. 2006; Sungura 2012; Budhiraja et al. 2013; Saldarriaga et al. 2008; Papaloucas et al. 2007; Santos-Soares et al. 2013) reported much lower incidence ranging from 11.2% in Kenya (Sungura 2012) to 27.4% in Greece(Papaloucas et al. 2007). When compared to these larger population-based studies (Ozkan et al. 2006; Sungura 2012; Budhiraja et al. 2013; Saldarriaga et al. 2008; Papaloucas et al. 2007; Santos-Soares et al. 2013), the incidence in persons of Caribbean ethnicity is higher than we expected. Similar to international data (Khamanarong et al. 2004; Sampaio & Passos 1992; Urban et al. 2001; Beregi et al. 1999; Ozkan et al. 2006; Sungura 2012; Budhiraja et al. 2013; Saldarriaga et al. 2008; Papaloucas et al. 2007; Santos-Soares et al. 2013), the accessory renal arteries were encountered more commonly on the left side (23.5% vs 17.9%) in our population.
Knowledge of the presence and distribution of accessory arteries is of paramount importance in renal transplantation. The presence of accessory arteries in a donor kidney is usually considered a contraindication to its use in transplant surgery (Sebastia et al. 2010). Since these are end arteries, the accessory arteries must be re-implanted and this would require several anastomoses and a prolonged ischemic time, leading to a theoretically higher incidence of renal failure, graft rejection and reduced graft function (Budhiraja et al. 2013; Sebastia et al. 2010; Das 2008).
Using Merklin and Michels’ classification (Merklin & Michels 1958), we evaluated the accessory origins. There were no cases of an accessory artery originating from the main renal artery. This was similar to the original findings published by Merklin and Michels (Merklin & Michels 1958), although other investigators have reported renal artery origins being present in 8% (Budhiraja et al. 2013) to 14% of cases (Talovic et al. 2007). In our series all of the accessories originated from the aorta. This was greater than the incidence reported in the literature where aortic takeoff is reported to occur in only 30.8% (Talovic et al. 2007) to 47.3% (Budhiraja et al. 2013).
The accessory origin may have significant impact on endovascular aneurysm repair because graft deployment and seating may need to be adjusted in the face of these anomalies. Even in open aneurysmorrhaphy, there must be careful planning of aortic cross clamp placement to prevent inadvertent occlusion of these end arteries and renal ischaemia (Schepens et al. 1994; Safi et al. 1996). In these cases, operative techniques would need to be modified and the informed consent process adjusted to reflect an increase in operative risk. For this reason, CT is well established as the primary modality to evaluate abdominal aortic aneurysms in terms of diagnosis and treatment planning (Errington et al. 1997; Simoni et al. 1996).
The entry point on the kidney is also important, especially with polar accessory arteries that enter the renal parenchyma directly outside of the hilum (Sebastia et al. 2010). In our series, 42% of the accessory renal arteries were lower polar arteries on the right side and 33.8% were lower polar arteries on the left side. Lower polar accessories tend to give important arterial supply to the upper ureter so any occlusion or inadvertent ligation could result in graft necrosis at the uretero-pelvic junction resulting in a leak or stenosis (Sebastia et al. 2010). With an aortic origin, they may also cause obstruction at the uretero-pelvic junction leading to impaired renal function (Shoja et al. 2008).
Upper polar arteries were less common, accounting for 22.6% (14/62) of the accessory arteries on present the right and 21.3% (17/80) of those on the left. They are still important because they are at risk for injury during dissection, especially those with an aortic origin that have a horizontal course (Sampaio & Passos 1992; Budhiraja et al. 2013).
Conclusion
Approximately 36% of persons in a Caribbean population can be expected to have accessory renal arteries present. This is greater than the incidence reported in most large population-based studies in medical literature. The finding is commoner on the left side and in most cases they arise directly from the aorta. These are important findings that may affect vascular and urologic procedures on persons of Caribbean ethnicity.
References
Adekunle A, Uche-Nwachi EO: Abnormal testicular vascular patterns amongst Trinidadians: a case report. Online J of Biol Sci 2007, 1(1):18-20.
Anatomy G’s: The anatomic basis of clinical practice. Edinburgh: Elsevier Churchil Livingstone; 2005.
Beregi JP, Mauroy B, Willoteaux S, Mounier-Vehier C, Remy-Jardin M, Francke JP: Anatomic variation in the origin of the main renal arteries: spiral CTA evaluation. Eur J Radiol 1999, 9(7):1330-1334. 10.1007/s003300050843
Budhiraja V, Rastogi R, Anjankar V, Babu CSR, Goel P: Supernumerary renal arteries and their embryological and clinical correlation: a cadaveric study from north India. ISRN Anatomy 2013. [http://dx.doi.org/10.5402/2013/405712] []
Dachman AH, Newmark GM, Mitchell MT, Woodle ES: Helical CT examination of potential kidney donors. AJR Am J Roentgenol 1998, 171: 193-200. 10.2214/ajr.171.1.9648788
Das S: Anomalous renal arteries and its clinical implications. Bratisl Lek Listy 2008, 109(4):182-184.
Errington ML, Ferguson JM, Gillespie IN, Connell HM, Ruckley CV, Wright AR: Complete pre-operative imaging assessment of abdominal aortic aneurysm with spiral CT angiography. Clin Radiol 1997, 52: 369-377. 10.1016/S0009-9260(97)80132-8
Felix W: Mesonephric arteries (aa. mesonephrica). In Manual of Human Embryology. 22nd edition. Edited by: Keibel F, Mall FP. Philadelphia, USA: Lippincott; 1911:820-825.
Harrison LHJ, Flye MW, Seigler HF: Incidence of anatomical variants in renal vasculature in the presence of normal renal function. Ann Surg 1978, 188: 83-89. 10.1097/00000658-197807000-00014
Hlaing KP, Sulaiman IM, Latiff AA, Gahar NA, Suhaimi FH, Othman F: Accessory renal vessels at the upper and lower pole of the kidney: a cadaveric study with clinical implications. Bratisl Lek Listy 2010, 111(5):308-310.
Johnson PB, Cawich SO, Roberts P, Shah S, Gardner MT, Gordon-Strachan G, Pearce NW: Variants of hepatic arterial supply in a Caribbean population: a computed tomography based study. Clin Radiol 2013, 68: 823-827. doi:10.1016/j 10.1016/j.crad.2013.03.020
Khamanarong K, Prachaney P, Utraravichien A, Tong-Un T, Sripaoraya K: Anatomy of renal arterial supply. Clin Anat 2004, 17: 334-336. 10.1002/ca.10236
Merklin RJ, Michels NA: The variant renal and suprarenal blood supply with data on the inferior phrenic, ureteral and gonadal arteries: a statistical analysis based on 185 dissections and review of the literature. J Int Coll Surg 1958, 29(1):41-76.
Michels NA: Blood supply and anatomy of the upper abdominal organs with a descriptive atlas. Philadelphia: Lippincott; 1955.
Ozkan U, Oˇguzkurt L, Tercan F, Kizilkilic O, Koc Z, Koca N: Renal artery origins and variations: angiographic evaluation of 855 consecutive patients. Diagn Interv Radiol 2006, 12(4):183-186.
Papaloucas C, Fiska A, Pistevou-Gombaki K, Kouloulias VE, Brountzos EN, Argyriou P, Demetriou T: Angiographic evaluation of renal artery variation amongst Greeks. Aristotle Univ Med J 2007, 34(2):43-47.
Pozniak MA, Balison DJ, Lee FTJ, Tambeaux RH, Uehling DT, Moon TD: CT angiography of potential renal transplant donors. Radiographics 1998, 18: 565-587.
Rao TR, Shetty P, Rao S: Unusual course of accessory renal artery and its clinical significance: a case report. Int J Anatom Varia 2011, 4: 197-199.
Safi HJ, Harlin SA, Miller CC, et al.: Predictive factors for acute renal failure in thoracic and thoracoabdominal aortic aneurysm surgery. J Vasc Surg 1996, 24: 338-344. discussion 344–5 10.1016/S0741-5214(96)70189-1
Sahani DV, Rastogi N, Greenfield AC, et al.: Multi-detector row CT in evaluation of 94 living renal donors by readers with varied experience. Radiology 2005, 235: 905-910. 10.1148/radiol.2353040496
Saldarriaga B, Pinto SA, Ballesteros LE: Morphological expression of the renal artery. A direct anatomical study in a Colombian half-caste population. Int J Morphol 2008, 26(1):31-38.
Sampaio FJ, Passos MA: Renal arteries: anatomic study for surgical and radiological practice. Surg Radiol Anat 1992, 14: 113-117. 10.1007/BF01794885
Santos-Soares TR, Ferraz JS, Dartibale CB, Oliveira IRM: Variations in human renal arteries. Acta Scientiarum Biol Scie 2013, 35(2):277-282. 10.4025/actascibiolsci.v35i2.11178
Schepens MA, Defauw JJ, Hamerlijnck RP, Vermeulen FE: Risk assessment of acute renal failure after thoracoabdominal aortic aneurysm surgery. Ann Surg 1994, 219: 400-407. 10.1097/00000658-199404000-00011
Sebastia C, Peri L, Salvador R, et al.: Multidetector CT of living renal donors: lessons learned from surgeons. Radiographics 2010, 30: 1875-1890. 10.1148/rg.307105032
Shoja MM, Tubbs RS, Shakeri A, Ardalan MR, Rahimi-Ardabili B, Ghabili K: Asymptomatic bilateral ureteropelvic junction obstruction due to supernumerary renal arteries. Saudi J of Kid Dis and Transplantation 2008, 19(5):806-808.
Simoni G, Perrone R, Cittadini GJ, De Caro G, Baiardi A, Civalleri D: Helical CT for the study of abdominal aortic aneurysms in patients undergoing conventional surgical repair. Eur J Vasc Endovasc Surg 1996, 12: 354-358. 10.1016/S1078-5884(96)80256-5
Smith PA, Ratner LE, Lynch FC, Corl FM, Fishman EK: Role of CT angiography in the preoperative evaluation for laparoscopic nephrectomy. Radiographics 1998, 18: 589-601.
Sungura RE: CT angiography pattern of renal arterial anatomy among Africans and its implication on renal transplantation: a cross sectional descriptive study at Kenyatta national hospital. Dig Repository 2012, 56: 307. available online: http://erepository.uonbi.ac.ke:8080/xmlui/handle/123456789/43790
Talovic E, Kulenovic A, Voljevica A, Kapur E: Review of supernumerary renal arteries by dissection method. Acta Medica Academica 2007, 36(5):56-69.
Urban BA, Ratner LE, Fishman EK: Three-dimensional volume-rendered CT angiography of the renal arteries and veins: normal anatomy, variants, and clinical applications. Radiographics 2001, 21: 373-386.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PBJ designed the research protocol and collected data. SOC designed the research protocols, and wrote the manuscript. SDS assisted with data collection and analysis. WA carried out data collection and statistical analysis. HB assisted with design of research protocols. RM collected data and assisted with research protocols. MTG assisted with data collection and statistical analysis. All authors have read and approved the final version of this manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Johnson, P.B., Cawich, S.O., Shah, S.D. et al. Accessory renal arteries in a Caribbean population: a computed tomography based study. SpringerPlus 2, 443 (2013). https://doi.org/10.1186/2193-1801-2-443
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-1801-2-443