Abstract
Logging and forest loss continues to be a major problem within Southeast Asia and as a result, many species are becoming threatened or extinct. The present study provides the first detailed and comprehensive ecological data on the Siberut macaque (Macaca siberu), a primate species living exclusively on the island of Siberut off the west coast of Sumatra. Our results show that M. siberu is ecologically similar to its closest relative M. nemestrina occurring on the mainland, both species being semi-terrestrial, mainly frugivorous (75-76%), exhibit a large daily travel distance for their group size and spend more time on traveling than any other macaque species. The habitat of Siberut macaques was floristically very diverse (Simpson’s index D=0.97), although somewhat impoverished in tree species richness, and had a lower tree basal area and a lower rattan density compared to other forests in Malesia (both rattan and palm tree fruit being an important food resource for Macaca siberu due to their long fruiting periods). These factors may lead to a lower diversity and abundance of fruit resources, and coupled with a high degree of frugivory of Siberut macaques, may explain the large amount of traveling observed in this species. The large home range requirements and strong dependence on fruit are important factors that need to be considered when developing conservation measures for this IUCN-listed (Category Vulnerable) species.
Similar content being viewed by others

Background
Tropical rainforests occupy only 7% of our Earth’s land surface but are home to over half of all the species on the planet (Thomas and Baltzer 2002). They are of great importance for the world’s economy and ecology by providing timber, food products and pharmaceuticals, and because they play a major role in the global carbon cycle (also as carbon sinks) and shape local climate patterns (Laurance 1999;Thomas and Baltzer 2002;FAO 2010). While the area of planted forest and conservation efforts are steadily increasing, forest loss and conversion still continue globally at high rates (1990-2000: 16 million ha, 2000-2010: 13 million ha; FAO 2010). Especially in Southeast Asia, which has experienced fast development in the last decades, deforestation rates increased drastically (1880-1980: 0.3%, 1990-1997: 0.91%, 2000-2010: 1%; Flint 1994;Achard et al. 2002;Miettinen et al. 2011), with Indonesia being one of the most critical countries (FAO 2010), and Sumatra among the most critical regions (1985-2007: 48% forest cover loss; Achard et al. 2002;Hansen et al. 2009;Laumonier et al. 2010;Miettinen et al. 2011). While forest loss continues, many of the species existing in these tropical habitats still remain to be discovered or described. The role of these species within the ecosystem is still unknown, and reduction in species diversity may lead to the loss of important services for the ecological community (Díaz et al. 2006).
In this environmentally critical region lies the Mentawai Archipelago, consisting of four small islands 85 to 135 km off the west coast of Sumatra, Indonesia (Whitten 1982c; Fuentes1997/1997;Whittaker 2006). It is part of the biodiversity hotspot Sundaland, which covers Malaysia, Brunei, Singapore and the western half of Indonesia (Mittermeier et al. 1999;Myers et al. 2000;Myers 2001;Mittermeier et al. 2004). Among all 34 biodiversity hotspots worldwide, Sundaland has the highest number of endemic plant species (15,000 - same as Tropical Andes) and the highest number of endemic mammal species (173), of which 81% are already listed as threatened by IUCN (Mittermeier et al. 2004: p. 32-33, 64). This high species richness and endemism in Sundaland cannot be attributed to the amount of habitat alone, as Sundaland only ranks twelfth among all hotspots, with 100,571 km2 of moist broadleaf forest (Mittermeier et al. 2004: p. 32). Rather, it is most likely the result of a dynamic geological past of Quaternary glaciations and episodic sea-level changes, during which Sundaland was repeatedly connected to the Asian mainland, enabling species migrations from the mainland to the islands of Sundaland (Gathorne-Hardy et al. 2002;Meijaard 2003;Sodhi et al. 2004;Woodruff 2010;Gower et al. 2012). Additionally, the rise in sea-level and increased isolation of islands which occurred during interglacial periods facilitated the speciation process (Sodhi et al. 2004). Furthermore, during the Pleistocene, some parts of Borneo and the northern and western part of Sumatra, including the Mentawai Islands, acted as rainforest refugia, enabling the survival of these rainforest biota (Gathorne-Hardy et al. 2002;Meijaard 2003).
The Mentawai Islands, of which Siberut is the largest and northernmost island, have been separated from the mainland for over 0.5 m years (Mitchell and Tilson 1986;Voris 2000;Bird et al. 2005). Even at times when sea level was so low that the rest of Sundaland was connected, the 1,700 m deep Mentawai Basin maintained the separation of the islands from the mainland (Brune et al. 2010). In fact, the Mentawai Islands were never connected to Sundaland directly, but were linked to Sumatra via a land bridge from Siberut through the Batu Islands (Whittaker 2006). As a result of this long period of geographic isolation, the Mentawai Archipelago has evolved a distinct flora and fauna with a high level of endemism, and allowed the survival of a number of “primitive” forms of considerable evolutionary interest (WWF 1980).
The flora of Siberut is estimated to comprise about 15% endemic plant forms, but the figure is out of date and new research is needed (WWF 1980). Of those species also known from other areas in Southeast Asia, some developed distinct traits on Siberut (WWF 1980: p. 13). The Mentawaian fauna includes 43 mammal species, of which 42% are endemic to Mentawai, and without bats, the endemism level increases to 71% (Thorington Jr. et al. 2012;Wilting et al. 2012). That the fauna of Siberut still remains understudied was recently shown by Kemp (2000), who recorded 28 new bird species for the island.
The ecosystems on small isolated islands such as Siberut are usually shaped by a range of different factors: Firstly, small islands often have an impoverished flora and fauna compared to the mainland, since species richness has been shown to decrease with land area (MacArthur and Wilson 1967;Simberloff 1974;Heaney 1984;Burkey 2002;Kreft et al. 2008;Nijman and Meijaard 2008). Usually the poorly dispersing species or large animals with large home range requirements are absent on small islands (Simberloff 1974;Heaney 1984). Secondly, a lack of certain species or whole taxa is usually associated with increased density of a few other species (density compensation) and a broader niche of island species compared to their relatives on the mainland (niche expansion), where competition for the same resources is higher (MacArthur et al. 1972;Buckley and Jetz 2007;Yoder et al. 2010). Niche expansion or niche shifts between islands and the mainland can concern habitat, vertical foraging strata, altitudes, foraging techniques and diet (MacArthur et al. 1972;Yoder et al. 2010). On Siberut, such niche expansion has been demonstrated for the spangled drongo (Dicrurus hottentotus) and three squirrel species (: WhittenCallosciurus melanogaster, Sundasciurus (lowii) fraterculus, Lariscus obscurus 1981;Whitten 1982e). On islands, fewer species compete for the same niche, so that evolutionary pressure becomes lower and populations or species evolve less rapidly. Thus, more “primitive” (archaic) forms can be maintained than on the mainland (WWF 1980;Patou et al. 2010). Despite these general trends geographical isolation can also lead to the evolution of new forms (Yoder et al. 2010).
Primate species richness on Mentawai is unusually high. On a land surface area of only 6,549 km2, Mentawai harbors five endemic primate species (Fuentes1997/1997), the Kloss gibbon (Hylobates klossii), the Mentawai langur (Presbytis potenziani), the pig-tailed langur or pig-tailed snub-nosed monkey (Simias concolor), the Pagai island macaque (Macaca pagensis) and the Siberut macaque (Macaca siberu). Whereas the first three species occur on all four islands, M. pagensis only occurs on the three southern islands, and M. siberu exclusively on Siberut. All Mentawaian primates are included in the IUCN Red List of Threatened Species (IUCN 2012). They are threatened by habitat loss due to legal and illegal logging, conversion of the forest into oil palm plantations, forest clearing, extraction of forest products (such as rattan), hunting and pet trade (Whittaker 2006). In Siberut, forest cover has decreased to 60% (by 2005; Whittaker 2006), but part of it is protected by the national park, which includes 465 km2 of protected “no-use” sanctuary zone where no hunting and logging is allowed (WWF 1980; Fuentes1997/1997;Whittaker 2006).
Of the four primate species occurring on Siberut, the Kloss gibbons, the Mentawai langurs and the pig-tailed langurs have been reasonably well studied (e.g. Tenaza 1975;Tilson 1977;Watanabe 1981;Tilson and Tenaza 1982;Whitten 1982a;Whitten 1982b;Whitten 1982c;Tenaza and Tilson 1985;Fuentes 1997;Hadi et al. 2009a;Erb et al. 2012b;Hadi et al. 2012). In contrast, studies on the Siberut macaque are mainly limited to investigations on population size, acoustic traits, phylogenetic relationships and some preliminary observations on ecology (Whitten and Whitten 1982;Abegg and Thierry 2002b;Roos et al. 2003;Ziegler et al. 2007;Schneider et al. 2008;Waltert et al. 2008;Quinten et al. 2009). Initially, Siberut macaques were thought to be a subspecies of southern pigtail macaques ( FoodenMacaca nemestrina pagensis: 1975) or of the Mentawai/ Pagai macaque of the southern Mentawai Islands (Macaca pagensis siberu: Fuentes and Olson1995;Groves 1997). Recently, however, genetic and morphological studies have allowed their classification as a distinct species called M. siberu (Kitchener and Groves 2002;Roos et al. 2003), being more closely related to M. nemestrina on Sumatra and Malaysia than to M. pagensis on the neighboring Mentawai Islands (Ziegler et al. 2007). So far, no detailed and comprehensive systematic behavioral or ecological studies have been conducted on Siberut macaques.
The present study aimed to gather basic ecological knowledge about the endemic Siberut macaques, including home range requirements, habitat and forest structure use and feeding habits. The data will add substantially to what is known about the ecological range of the genus Macaca, which is among the most successful non-human primate genera with more than 20 species distributed from North Africa throughout Asia up to Japan (Abegg and Thierry 2002a). Siberut macaques, as part of the silenus-sylvanus lineage, are thought to represent a relict species from the earliest wave of macaque dispersal and thus may also help to understand the ancestral traits of this genus (Fooden 1980;Abegg and Thierry 2002a;Roos et al. 2003;Ziegler et al. 2007). We compared the new data on Siberut macaques with available data from other macaque species, in order to understand the range of ecological variations, and with data on Siberut’s other primate species with the aim of investigating possible niche differentiations. In addition, we present data on the habitat of Siberut macaques to investigate whether the forest is impoverished as island biogeography theory would predict, which could have important impacts on the behavior of Siberut macaques. The data should also be useful in the development of much needed conservation guidelines for the species.
Results
Home range size and daily travel distance
The total area used by the group during one year was 134.9 ha, based on 100% MCP. When excluding outliers, the area was 80.6 ha (95% MCP), with a core home range of 26.1 ha (50% MCP). Using the fixed kernel method with reference bandwidth, the 95% contour included 84.1 ha, and the 50% contour 26.2 ha (Figure 1). Excluding days with short contact times (<6 h per day) did not change the shape or the size (average change of 0.1 ha) of the home ranges. Home range size plateaued at ~900 fixes, i.e. 5 full months of observation.
Home range areas of the study group. Left: 100%, 95% and 50% minimum convex polygons (MCP’s). Right: 95% and 50% contour of the fixed kernel home range using reference bandwidth. The star is indicating the research station and the cloud of small dots represents the locations of group scan observations. Grid shows UTM coordinates (UTM Zone 47 South). One grid square equals 400x400m.
The average travel speed of the group (from group scan data) was 169.6 m/h (SD ± 35.6 m/h, range: 87.8 m – 280.0 m, N=78 days), the average travel speed of single adult individuals (from focal observations) was 206.1 m/h (SD ±143.9 m/h, N=120 focal follows). The monthly average daily travel distance of the group equaled 2,048.4 m (SD ±205.5 m, N= 10 months with 6-9 “full day follows” per months), with travel distances of single days ranging between 1,054 m and 3,360 m. Monthly average daily travel distances tended to increase with the percentage of fruit in the diet (rs= 0.64, N=10, p=0.055; see Figure 2). The monthly average daily travel distance was 303 m more than needed to cross the 100% MCP home range at its widest point. The largest observed distance moved between two consecutive group scan observations was 657 m within 30 min, and the largest distance moved of a single individual during a focal observation was 542 m in 67 min (by an adult male). Compared to other macaque species, Siberut macaques traveled more per day than other macaque species of similar group size, but they were similar to Macaca nemestrina, to whom they are genetically most closely related (Figure 3). Overall, group size was significantly correlated with the distance traveled per day in this comparative data set (Spearman rank: rs = 0.46, N=32, p<0.01).
Diet and average daily travel distance per month. Diet is calculated as percent feeding time on fruit, arthropods, mushrooms, leaves (mainly young leaves and young leave petioles), pith (the soft core of the palm stem) and other food items, which includes flowers, sap and shoots. Data on travel distance for March 2010 and November 2010 were omitted because they were too scarce.
Mean daily travel distances (in km) of free-ranging macaque groups and species in undisturbed habitats unless otherwise stated. Sources and study sites: M. assamensis: Schülke et al (2011), Phu Kieo Wildlife Sanctuary, Thailand; M. fascicularis: 3 groups from C. Girard-Buttoz (pers. comm.), Ketambe, Sumatra, tropical lowland evergreen forest, Mar. ’10 – Apr. ’11, data from 2 adult males per group; 7 groups (A, G, K77, H77, K, H, T, A) from van Schaik et al (1983), Ketambe, Sumatra, 1 group (A) from Aldrich-Blake (1980), Kuala Lompat, W-Malaysia; 1 group from MacKinnon & MacKinnon (1980), Kuala Lompat, W-Malaysia; M. f. fuscata: data from the studies of Ikeda (1982) at Kawaradake, Japan, Wada (1979), Shiga heights, Japan, and Izumiyama (1999), Kamikochi, Japan, as in Tsuji (2010); M. mulatta: 3 groups (B, C, E) from Neville (1968), Uttar Pradesh, N-India, 1 group (Asarori II) from Lindburg (1971) and Lindburg (1977: Sugiyama (), Uttar Pradesh, India; M. radiata 1971: Caldecott (), Dharwar, S-India; M. n. nemestrina 1986a), Lima Belas, W-Malaysia, forest surrounded by oil palm plantations; MacKinnon & MacKinnon (1980), Kuala Lompat, W-Malaysia; M. (n.) leonina: HQ troop from Albert (2012), Khao Yai National Park, Thailand, close to human settlement, only day range data used from when the group was not using human food (high fruit abundance time); forest group (Ch troop) from J. M. José Domínguez (pers. comm.), Khao Yai National Park, Thailand, May – Jun. ’11 and May – Aug. ‘12; M. nigra: O’Brian & Kinnaird (1997), Tangkoko, Sulawesi; M. siberu: this study; M. sylvanus: 2 groups from Ménard & Vallet (1997) at Djurdjura and Akfadou, Algeria; 1 group (group 6) from Deag (1974), Ain Kahla, Marocco; M. tonkeana: 2 groups from Pombo et al (2004), Lore Lindu National Park, Sulawesi, 1 group in disturbed forest.
Habitat and forest structure use
Siberut macaques spent most of their time in the dense, continuous forest (average over all months: 95.7%), and used windthrow areas only 2.6%, canopy gaps 1.4%, and swamp areas 0.3% of their time. However, as can be seen in Figure 4, variation across months was high. The two windthrow areas were created by storms in May 2009 (before observations commenced) and June 2010. The use of windthrow areas and swamp were both correlated to the amount of fruit in the diet but in opposite ways. While windthrow areas were used more when less fruit was consumed (rs= -0.67, N=12, p=0.02), time spent in swamp areas was positively related to the proportion of fruit in the diet (rs = 0.69, N=12, p=0.02). The former effect may result from generally reduced fruit availability after the storm that created the second windthrow area while the latter effect suggests that swamps are only visited after sufficient amounts of fruit have been ingested.
In terms of the absolute forest height, Siberut macaques mainly used the lower strata of the forest (64.9% of their day-time activities in 0-10 m height, with 53.0% being between 0-5 m), and only to a much lesser extent the upper forest strata (16.5% in 11-20 m, 11.5% in 21-30 m, 5.8% in 31-40 m, and 1.3% in >41 m; see also Table 1 and Figure 5). Regarding the relative height of the forest, we find that the group spent 28.9% of their time on the ground, 36.2% in the lower-story, 23.5% in the mid-story, and only 11.4% in the canopy (Figure 6). Adult males spent more time on the ground than adult females and juveniles. The predominant activities on the ground were traveling (80.2%) and to some extent foraging (10.1%), with feeding, resting and social behavior only accounting for small proportions. Compared to other forest-living macaques in the tropics and subtropics, Siberut macaques fall on the side of more terrestrial macaque species (Table 2) and are more terrestrial than M. nemestrina in W-Malaysia (longterm study data), but similarly terrestrial than M. nemestrina in Sumatra (only survey data).
Activity budget
The activity budget revealed that the group spent most of its time traveling, with a monthly average (±SD) of 57.3% (±6.6), 14.6% (±5.6) resting, 12.1% (±9.0) foraging, 10.1% (±4.0) feeding, and only 5.9% (±2.2) on social activities (Figure 7). There was a significant sex-difference in resting (Mann-Whitney-U: W=18, p=0.02), with adult males resting 6.4% more than their female counterparts. There was also a significant sex-difference in time spent foraging (Mann-Whitney-U: W=0, p=0.02), with females spending nearly two and a half times more on searching for food then males (Figure 7, Fisher’s Omnibus Test to control for multiple testing: X2=19.6, df=10, p=0.03). For feeding, traveling and social activities, males and females did not differ significantly. The most striking differences between juveniles and adults were the much higher amount of time spent traveling in juveniles (juveniles: 60.7%, females: 48.4%, males: 51.1%), a lower amount of time spent feeding (juveniles: 9.5%, females: 12.9%, males: 14.5%), and only very little time spent on social behavior (juveniles: 4.8%, females: 7.6%, males: 7.1%), which is surprising as playing is part of this category. However, these differences cannot be tested for significance as the majority of the juveniles were not identified. Most of the activities showed large variations throughout the months, and only social behavior was relatively stable (~5%, Figure 8). High variations in feeding time are probably driven by fruit availability in the forest, and as feeding time decreases, the time spent foraging (searching for food) increases, along with an increase of time spent traveling, both at the cost of time spent resting (Figure 8).
Compared to other macaque species, Siberut macaques spent more time traveling (Table 3), which is surprising, given their small group size, but is in accordance with the long daily travel distance observed (Figure 3). Among the 15 species examined in Table 3, only M. nemestrina travels more than Siberut macaques. Due to the large amount of time they have to devote to traveling, they only have very little time left for social activities (Table 3).
Diet
Across the study period, the diet of Siberut macaques was on average composed of 75.7% fruit (72.8% ripe fruit, 20.2% half-ripe fruit, 4.3% unripe fruit, 2.7% fruit of unknown ripeness), 11.9% arthropods (mainly ants, termites, spiders), 4.5% mushrooms, 4.4% leaves (59.7% young leaves, 13.4% young leave petioles, 25.4% leaves of unknown age, 1.5% mature leaves), 2.6% pith, 0.6% sap, 0.2% shoots and 0.2% flowers. Compared to other macaques, the degree of frugivory in Siberut macaques was high (third highest among the 14 macaque species examined) and the percentage of leaves eaten low (fifth lowest of the 14 macaque species, Table 4). According to local people, Siberut macaques also occasionally catch and consume crabs and shrimp from the rivers, but this was only observed once. In contrast to other macaque species, Siberut macaques were never observed to prey on bird eggs, birds, squirrels or other small mammals. Although the majority of the diet was comprised of fruit, the proportion of fruit varied largely from 43.2% (Dec. 2010) to 96.1% (Apr. 2010, Figure 2). With decreasing proportion of fruit eaten per month, the time spent feeding on arthropods, pith and leaves increased significantly (Spearman rank correlations: fruit vs. arthropods: rs = -0.97, p<0.01, fruit vs. pith: rs = -0.86, p<0.01, fruit vs. leaves: rs = -0.59, p<0.05, for all N=12 months). The correlation between fruit and mushrooms was not significant. The increase in pith eating was mainly due to adult males, since all males fed on pith whereas only 2 of the 6 adult females did. Pith was the only food item which was significantly different in the diet of males and females (Mann-Whitney-U: W=18, p=0.02). Observations suggested that only males were strong enough to break the palm trunks open to get access to the pith, whereas females were only observed feeding pith after they found a trunk already opened. In sum, when the abundance of fruit decreased, they used arthropods and (young) leaves as fallback foods (for annual availability of leaves and fruit see Erb et al. 2012a).
Dietary diversity, measured by the Shannon-Wiener index H’, was low in months when the proportion of fruit in the diet exceeded 80% (average H’: 1.1) and high in months when fruit only made up a smaller part of the diet (average H’: 2.1). Overall average dietary diversity was 1.6. The monthly dietary diversity index was negatively correlated with the monthly feeding time (Spearman rank correlation: rs = -0.82, N= 12, p=0.02), indicating that the monkeys need to invest more time in feeding when the diversity of food items is low.
Comparison of Siberut’s primates
Of all four sympatric primate species on Siberut, Siberut macaques have the largest group size and by far the largest home range size (see Table 1 for data and references). The size of home range increases with the percentage of fruit in the diet across species, with Siberut macaques being the most frugivorous species. At similar proportions of fruit and leaves in the diet, Kloss gibbons spend twice as much time feeding on arthropods, and Siberut macaques included more other food items instead, thus having a broader diet (Table 1). The amount of frugivory also seems to be related to the daily travel distance, with both folivorous colobine species (Presbytis potenziani and Simias concolor) traveling the shortest distances, Kloss gibbons being intermediate and Siberut macaques having 3-4 times the travel distances of the two colobine species; the same pattern emerges for travel time (Table 1). For the forest strata use we find a niche differentiation. Whereas Siberut macaques mainly used the lower strata (0-10 m) of the forest, the sympatric colobine species mainly stayed within heights of 11 to 20 m (Table 1). For Kloss gibbons, no data are available for the same study site but data collected in Central Siberut indicate that the gibbons spent 94% of their time in the middle and upper canopy (Whitten 1982c).
Habitat analysis
Within the 3 ha of forest sampled within the home range, a total of 1,807 individuals of trees, palm trees, rattan and lianas, belonging to 167 species, 107 genera and 46 families were recorded. 107 individuals (5.9%; 5 lianas and 102 trees) could not be identified at the family level. From the remaining individuals, 83% could be determined to species level, the rest to genus level. Trees were the dominant growth form, with 40 families and 133 species. The liana flora comprised 12 families and 19 species. This natural and undisturbed forest had 3 species of palm trees (Arenga obtusifolia, Oncosperma horridum, Pinanga sp.) and 10 species of rattan (Calamus: 6, Korthalsia: 3, Plectocomia: 1). We found two strangler species (Ficus annulata, Ficus sp., Moraceae). The estimated potential species richness for all categories (trees, palm trees, lianas, rattan and strangler) ranged between 186 and 225, with a mean of 200 species for the different estimators (ACE: 188, ICE: 203, Chao 1: 193, Chao 2: 200, Jack 1: 207, Jack 2: 225, Bootstrap: 186; MMMeans (1st run): 200). Estimated tree species richness ranged between 150 and 189, with a mean of 166 species for the different estimators (ACE: 154, ICE: 169, Chao 1: 164, Chao 2: 177, Jack 1: 170, Jack 2: 189, Bootstrap: 150: MMMeans (1st run): 160). For lianas, a mean of 22 species was estimated, with a range of 20 to 24 (ACE: 21, ICE: 22, Chao 1 & Chao 2: 20, Jack 1 & Jack 2: 23, Bootstrap: 21: MMMeans (1st run): 24). For palm trees, rattan and strangler, the estimated species richness was the same as the observed one. In sum, total species richness observed was 167 and expected was 200, which is mainly due to the tree community which was undersampled. These estimates were in line with field observations on species occurrence outside the plots.
Looking at species diversity, which combines the information of species richness and relative abundance, we found a Simpson’s diversity index of 0.97 for all categories together (trees, palm trees, liana, rattan, strangler), or 0.98 when only considering trees. As this index ranges between 0 and 1, with 1 being the highest diversity, this indicates a very high species diversity of the studied forest habitat. Similarly, the Shannon-Wiener diversity index was also high, with 4.30 for all categories, and 4.32 for trees alone. The 12 botanical plots studied showed a mean similarity in terms of species composition ranging from 0.32 to 0.63, depending on the index (incidence based indices: Jaccard: 0.32, Sørensen: 0.49, but both are usually biased downward when species richness is large; abundance based indices: Morisita-Horn: 0.60, Bray-Curtis: 0.43, Chao-Jaccard: 0.47, Chao-Sørensen: 0.63), resulting in a mean of 0.49, which is a medium similarity within the indices possible range between 0 and 1.
The average total density of all recorded individuals per area was 602.3 per ha (±118.1 SD) (trees: 402.3 individuals/ha (±86.8), palm trees: 73.0 individuals/ha (±24.8), lianas: 41.0 individuals/ha (±19.9), rattan: 83.3 individuals/ha (±52.7), strangler: 2.7 individuals/ha (±3.1)).
The height distribution of the forest showed tree heights ranging from 2 m (Chionanthus glomerata, Oleaceae) up to maximum 52 m (Sloanea javanica, Elaeocarpaceae). Other high trees taller than 40 m were Nauclea sp. (Rubiaceae), Scorodocarpus borneensis (Olacaceae), Syzy-gium palembanicum (Myrtaceae) and Palaquium obova-tum (Sapotaceae). Most of the trees (76%) fall into the three height categories from 6 to 20 m (Figure 5). When considering trees and palm trees together (as Siberut macaques often used palm trees as food resource), these three categories account for 78% (Figure 5). The category with the highest proportion of trees, 11-15 m, accounted for one third of all trees (32.6% for trees, 33.1% for trees & palm trees). Only a small proportion of trees was higher than 30 m (trees: 4.1%, trees & palm trees: 3.6%). The distribution of time the macaques spent in different height classes during their normal, daylight activities was different from the abundance of different height classes in the forest (Χ2 = 1841.5, df = 8, p<0.001, Figure 5) The macaques spent 51% of their time at less than 5 m height, and only 30% of their time between 6 and 20 m, the three categories which comprise the majority of the forest trees.
The diameter distribution of trees showed an average of 99 cm dbh in all plots together, which is mainly driven by the large trees characteristic for Plot A, the only plot on the top of a hill. After excluding this plot, the average dbh of trees decreased to 38 cm. The largest trees >100 cm dbh belonged to the family Dipterocar-paceae (Dipterocarpus elongates, Shorea ovalis, Shorea pauciflora) in Plot A, and to the family Euphorbiaceae (Endospermum malaccense) and Moraceae (Artocarpus maingayi, Ficus sp.) in 2 plots on the dry level ground. The distribution of trees in the botanical plots per dbh class followed a negative exponential distribution, with trees of smaller diameters being the most abundant, with a gradual decrease with increasing diameter (Figure 9). The sleeping trees used by the study group showed a very different distribution from the actual forest tree distribution (Figure 9; Χ2 = 647.8, df = 9, p<0.001). The majority of sleeping trees (85%) had a dbh between 40 and 90 cm. Nearly 7% of sleeping trees were larger than 100 cm dbh, indicating that monkeys favored large trees as sleeping trees. As large trees occur at low abundance they are an important requirement for a suitable habitat of Siberut macaques.
The distribution of feeding tree sizes was more even (Figure 9). A large proportion of feeding trees were small trees between 10 and 20 cm dbh (24.6%), but there was also a high percentage of feeding trees larger than 50 cm dbh (40.6%). The distribution of feeding trees used by the group was significantly different from the distribution of the same tree species in the botanical plots (Χ2 = 74.3, df = 9, p<0.001), thus indicating that large feeding trees are a critical key component for the survival of Siberut macaques.
The tree flora was clearly dominated by the family Euphorbiaceae, both in terms of number of individuals (156) and species richness (21 species). For Siberut macaques, this family was important because it included the most favorite species of sleeping trees (Endospermum malaccense). As fruit resource, Euphorbiaceae appeared to be less important, which may be due to the irregularity of fruit production. Five of the seven species used by the macaques were fruiting in one year (habituation period), but not in the other (data collection period). The second most important tree family in terms of number of individuals (119) was Myristicaceae. It had a high importance as fruit resource for Siberut macaques (Knema sp.), but not as much as sleeping trees. Other important families in the forest based on number of individuals were Dipterocarpaceae (78), Sapotaceae (68) and Myrtaceae (63). In terms of species richness however, Euphorbiaceae, the family with the highest species richness, was followed by Lauraceae (9), Myristicaceae (8), Annonanceae (7) and Rubiaceae (7).
Considering all studied plant categories in the botanical plots and not only trees, the species with the highest abundance was a rattan species, Korthalsia echinometra (Palmae), reaching an abundance of 158 individuals in the 3 ha of forest examined, followed by the palm tree Oncosperma horridum (157 individuals). The most abundant tree species Vatica pallida (Dipterocarpaceae) was about three times rarer in number (61 individuals). Rattan and palm trees were very important for Siberut macaques because they provided fruit for a longer time period. This can be seen from the number of months each species was recorded as fruit resource during scan observations over a period of 1 year. In average, palm trees provided fruit during 6.0 months (N= 4 species), rattan during 3.3 months (N= 8), whereas lianas and trees only during 2.1 or 2.0 months respectively (N= 7; N= 17).
The importance of palms for the forest and their dominance can also be seen from the basal area per family: Palmae (or Arecaceae) was the top-ranking family in terms of basal area (4.86 m2/ha), followed by Dipterocarpaceae (2.98 m2/ha), Euphorbiaceae (2.68 m2/ha), Myristicaceae (2.51 m2/ha), Moraceae (2.46 m2/ha), Myrtaceae (2.27 m2/ha), and Sapotaceae (2.09 m2/ha). All other families had a basal area less than 2 m2/ha. Total basal area of trees, palm trees, lianas, rattan and strangler combined was 33.68 m2/ha, that of trees alone 28.46 m2/ha.
Discussion and conclusions
Interspecies comparisons: the genus Macaca
The present study provides first comprehensive data on the ecology of a new representative within the ecologically very diverse macaque genus. The study group comprised 29 individuals, which is twice the size suggested by the preliminary observations of Whitten & Whitten (1982). Our second semi-habituated group consisted of at least 13 individuals, which is probably an underestimate as the number of adult males (3) which were frequently seen and vocalized was the same as in our much larger study group. All these observations suggest that group size for Siberut macaques falls within the range reported for M. silenus (9-31 individuals; 9 groups: Kumara & Singh (2004)), M. sinica (5-47 individuals; 20 groups Dittus (1988)) and M. radiata (16-44 individuals; 12 groups: Sugiyama (1971)).
(CaldecottOur comparative data sets revealed that Siberut macaques are ecologically most similar to pigtail macaques, M. nemestrina 1986a;Caldecott et al. 1997), their sister taxa according to genetic analyses (Ziegler et al. 2007). Siberut macaques are semi-terrestrial, traveling large distances per day relative to their group size, spending a very high percentage of their daily activities on traveling and using a mainly frugivorous diet. In the following, we discuss these traits in more detail.
The Siberut macaques studied here spent a very large amount of their daily activities on traveling, ranking second among all 15 macaque species examined. These differences in traveling time appear to be true differences, as they cannot be attributed to different sampling methods (69% scan sampling, 21% other, 10% unknown observation method; Table 3) or different definitions, because even when taking both traveling and foraging time together, Siberut macaques are still different from other macaque species. Siberut macaques also traveled longer distances per day (highest value for their group size, but similar to M. nemestrina). The high amount and distance traveled may be linked to the degree of terrestriality. For pigtail macaques, ground foraging and traveling is an adaptation to the habitat of Sundaic dipterocarp forest with scarce food resources which are patchy and slow to renew (Caldecott 1986a). Ground traveling allows fast traveling between widely dispersed fruit resources, and additionally allows the exploitation of food resources on the ground (Caldecott 1986a;Caldecott et al. 1997). Similarly, Siberut macaques inhabit dipterocarp and mixed forest where food seemed to be dispersed and often in small patches, for example the various different rattan fruit or Aren (Arenga obtusifolia) fruit which were common fruit resources throughout the year (CR, unpublished data). Similar to pigtail macaques, Siberut macaques mainly used the ground for traveling and to some extent for foraging. They were usually searching for insects or spiders and mushrooms under old foliage or on fall-down trees, and were occasionally picking young leaves or leaf petioles of herbs from the ground vegetation (e.g. from Curculigo latifolia, Hypoxidaceae), while searching for fruiting trees within their home range. As adaptation to those scattered and often cryptic resources, they would often forage alone or together with only a few other individuals in close vicinity, with sometimes large group spreads of ~200 m, or in rare cases of over 400 m. A similar pattern was reported for pigtail macaques (Caldecott 1986a;Caldecott et al. 1997). Another explanation for the high amount of traveling in Siberut macaques could be the lower percentage of trees bearing fruit (max. 5% in Central Siberut (median 3.5%) compared with max. 16% (median 4%) in Malay Peninsula (Whitten 1980)).
Comparison of the diets of macaques revealed that Siberut macaques were mainly frugivorous, which is similar to M. nemestrina, M. nigrescens and M. tonkeana (Table 4). Ménard (2004) suggested that the most frugivorous macaque species also spend the most time moving. We tested this using the comparative data sets from Tables 3 and 4 and by only including those groups and species for which both the time spent feeding on fruit and the time spent moving/ traveling were available for the same study site or group, we avoided potential confounding effects of habitat differences. There was a positive significant correlation (Spearman rank: rs = 0.53, N=20, p<0.02), i.e. the more frugivorous a species (or group) is, the more time of its daily activities it has to spend traveling, which could partly explain the large amount of time spent traveling in Siberut macaques.
Figs (Ficus spp.) are a common food source for many primates (Shanahan et al. 2001) and can make up a large percentage of the fruit diet of macaques ( (Ungar20-40% for M. fascicularis 1995;Kinnaird and O’Brien 2005), 44% for M. nigra (Kinnaird and O’Brien 2005(Kohlhaas), 47% for M. nigrescens 1993); see also Riley (2007) for the importance for M. tonkeana). For Siberut macaques however, figs only accounted on average for 6.9% of the total amount of known fruit eaten per month. The abundance of figs (≥ 10 cm dbh) on Siberut was relatively low, with a density of 2.7 figs/ha, similar to the figure for South Sumatra (1.4 figs/ha, Kinnaird & O’Brian (Kinnaird and O'Brien 2005)), but very different to Sulawesi (11.8 figs/ha in the habitat of M. nigra in North Sulawesi (Kinnaird and O'Brien 2005), 33.2 figs/ha in the habitat of M. tonkeana in Central Sulawesi (Riley 2007)). As figs are not so abundant in the habitat of Siberut macaques, they are of lesser importance than, for example, rattan or palm fruit (rattan fruit: 8.5%, palm tree fruit: 22.3% of the monthly fruit diet). A high degree of frugivory and thus dependence on fruiting trees within the forest has important conservation consequences for Macaca siberu, which will be discussed below.
In addition, as island biogeography theory would predict, we may expect a possible niche shift or niche expansion in Siberut macaques, since longtail macaques which are the main competitors of pigtail macaques on the mainland Sumatra (Crockett and Wilson 1980), are completely absent in Mentawai. However, as the activity budget and diet of Siberut macaques and pigtail macaques are very similar, there is no indication for a behavioral or dietary niche differentiation. For forest strata use, no detailed data are available for pigtail macaques, but judging from the degree of terrestriality, there is also no indication for a broadened niche.
Interspecies comparisons: Siberut’s primates
Of all primate species on Siberut, Siberut macaques have the largest group size and the largest home range requirements (see also Whitten and Whitten 1982). Although Kloss gibbons and Siberut macaques are both mainly frugivorous, Siberut macaques have a broader diet (i.e. also feed on herbs, mushrooms, sap and resin, pith etc) and dietary overlap between the two species seems low (only 25.6%, i.e. 10 out of 39 fruit species recorded for Kloss gibbons (Whitten 1982a) were also used by Siberut macaques, but different species may fruit in different years). Our present findings showed that Siberut macaques consumed a remarkably small amount of leaves for a rainforest macaque species (Table 4) and in this respect they resemble Kloss gibbons which include a much lower proportion of leaves in their diet compared to Malaysian gibbon species (Whitten 1982a). One possible explanation may be that due to the high annual rainfall and a very nutrient poor soil on Siberut, competition between trees is especially high resulting in a high concentration of secondary compounds in (tree) leaves and thus in a decreased digestability for mainly frugivorous primates, leading to a decreased choice of leaves as food resource compared to other primate species (Whitten 1980;Whitten 1982a). Quantitative data to test this, however, are missing. Siberut macaques still fed on more leaves than Kloss gibbons, probably because they also used young herbaceous leaves from the understory vegetation, as they frequently foraged and travelled on the ground.
Forest comparison
The behavior of every animal is closely linked and determined by its habitat (Krebs and Davies 1997). Differences in the behavior of Siberut macaques compared to other macaque species could be attributed to differences in the forest habitat. To investigate this possibility, we compared species, family richness and other important forest characteristics of the habitat of Siberut macaques with other forests in the same phytogeographical region of Malesia.
In our study, we recorded a tree species richness of 133, which is lower than in North-Sumatra (184 species, Kartawinata et al. 2004), and thus in line with island biogeography theory (MacArthur and Wilson 1967;Simberloff 1974), as islands usually have an impoverished flora. This impoverishment, however, was only seen at the species level, not at the family level, as we recorded a similar number of tree families as in North-Sumatra (40 families in this study; 41 in North Sumatra: Kartawinata et al. 2004). The basal area of trees in our study site was very similar to that recorded in Central Siberut (28.5 m2/ha of trees ≥10 cm dbh in this study; 27.7 m2/ha of trees ≥15 cm dbh in Central Siberut, Whitten (1982d)), indicating that our botanical plots are representative for the forest of Siberut Island. In North-Sumatra, however, basal area is much higher (40.6 m2/ha of trees ≥10 cm dbh, Kartawinata et al (2004). The three most species rich families were Euphorbiaceae, Lauraceae and Myristicaceae, with Euphorbiaceae and Lauraceae also being among the three most species rich families in another study at the same site in Siberut (Hadi et al. 2009b), as well as in West and East Malaysia (Kochummen et al. 1990;Lee et al. 2002). The tree families with the highest richness of individuals were Euphorbiaceae, Myristicaceae and Dipterocarpaceae, which is the same as in Central Siberut but in a different order (Dipterocarpaceae, Myristicaceae, Euphorbiaceae: Whitten 1982d), and Euphorbiaceae and Dipterocarpaceae were also the two top-ranking families in terms of tree richness in mainland Sumatra, West and East Malaysia (Kochummen et al. 1990;Laumonier 1997;Lee et al. 2002).
Rattan, which are centered in their distribution on the Sunda Shelf (Whitmore 1984), were an important feature of the studied forest, reaching densities of 83.3 individuals (apparent genets) per hectare. Although this density is much lower than in West Malaysia (115 clumps/ ha, Abdul Hamid and Suratman 2010) or Sulawesi (314 mature genets/ha, Siebert 2005), rattan diversity (10 species in this study) was higher than in East Malaysia (6 species, Putz and Chai 1987) and similarly high in West Malaysia (11 species, Abdul Hamid and Suratman 2010). Apart from rattan, palm trees were a common element in the forest, as already noticed for Central Siberut before (Whitten 1982d), reaching relatively high densities (Oncosperma horridum: 52.3 palms ≥10 cm dbh/ha, Arenga obtusifolia: 17.3 palms ≥5 cm dbh/ha or 9.7 palms ≥10 cm dbh/ha). They are no indicator of disturbance, as they are common in primary forests (Laumonier 1997). Densities of other species which are more light demanding and thus common in secondary forests (Kochummen et al. 1990) were all low or average, thus there was no floristic indication for disturbances in the plots (Endospermum malaccensis, Euphorbiaceae: 2.3 trees/ha, Dillenia obovata, Dilleniaceae: 3.0 trees/ha, Macaranga sp, Euphorbiaceae: 0.7 trees/ha, Campnosperma auriculatum, Anacardiaceae: 1 tree/ha; compared to a mean of all tree species of 2.7 trees/ha, or the maximum density of 20.3 trees/ha of Vatica pallida, Dipterocarcpaceae).
Our comparison has shown differences between the forest in Siberut and other forests in Malesia in terms of an impoverishment of tree species richness, lower tree basal area and lower rattan density. Collectively, this may lead to a lower diversity and abundance of fruit resources, and this could possibly explain the large travel distances and the high amount of time devoted to traveling in Siberut macaques.
Conservation and future of Siberut macaques
Based on the present results, we can give the following advice for future conservation action plans for Siberut macaques. First, it has to be taken into account that Siberut macaques need a much larger home range than any of the other primate species on Siberut. An appropriate conservation area would be an area large enough to sustain several groups to facilitate emigration processes and gene flow, and large enough to include sufficient fruit resources during seasons of low fruit availability. The area should consist of an intact, rather than fragmented, forest area as the dense, continuous forest was the most frequently used habitat type and was the basis of their food supply. Large forest trees in particular are important both as fruit resources and sleeping trees, as was already assumed by Whitten and Whitten (1982). Thus, selective logging of the large trees would immensely disturb their livelihood.
The future of Siberut macaques will unavoidably be closely connected to habitat degradation and loss. As macaques are generally omnivorous, they have the advantage of being able to adapt more easily to habitat changes than more specialized primates. However, as fruit constitutes a large percentage of their diet, they are likely to raid crops on farms if their original forest habitat does not supply enough fruit anymore, and this would increase conflicts with local people. In Siberut, local people traditionally hunt and trap primates, and Siberut macaques are caught in ground traps baited with sago, with which they can trap a whole group at once (pers. comm. by C. Abegg). Thus, Siberut macaques will become more vulnerable with increasing habitat loss and degradation.
The habitat of Siberut macaques is decreasing continuously. From the total land area of Siberut probably all covered with rainforest in the past, 87% was left in 1980 and only 60% in 2005 (2,400 km2,Table one in Whittaker 2006). From the remaining forest cover, 1,926 km2 are assigned to the Siberut National Park, but only 465 km2 are a protected no-use sanctuary zone where no hunting and logging is allowed (Fuentes1997/1997). A recent investigation has shown that the density of Siberut macaques in the national park is about three times lower than in the SCP area where this study was conducted (data from M. Quinten, unpublished), which indicates that the national park alone might not be enough to conserve Siberut macaques in the long-term. Although the status of Siberut macaques is not yet listed as critical, their population decreased from ~39,000 individuals in 1980 to 17,000-30,000 individuals in 2005 (Whittaker 2006) and if this decline continues, their future may soon be at risk.
Methods
Study area
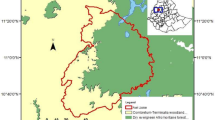
The study was conducted on Siberut Island, which comprises 4,030 km2 and a human population of ~25,000 people (Fuentes1997/1997;Whittaker 2006). Siberut has a strongly dissected, rugged landscape of numerous steep slopes and ravines (highest elevation: 384 m a.s.l.), and many rivers and streams (WWF 1980;Watanabe 1981). The island is covered by tropical lowland evergreen broadleaf rainforest (UNEP-WCMC classification) or tropical moist broadleaf forest (WWF classification). Different vegetation types can be distinguished: primary dipterocarp forest on high ridges (dominated by Dipterocarpaceae), primary mixed forest on slopes and lower hills (mixed composition of tree families with none being dominant), freshwater swamp forest, mangrove forest and Barringtonia forest on the West coast of Siberut (WWF 1980). The soil on Siberut is less fertile than on the Malay Peninsular (Whitten 1980;Whitten 1982a). The study was conducted at the field site of the Siberut Conservation Programme (SCP; www.siberut-island.org), situated within the Peleonan Forest in North Siberut (Figure 10). The Peleonan Forest comprises 4,500 ha rented by SCP for conservation purposes, surrounded by logging concessions and the Indian Ocean. It consists of undisturbed primary rainforest (i.e. with no signs of human impact) and some secondary forest at late successional stage. The climate is equatorial, with no seasonal changes in temperature. Temperature recorded during February 2010 and March 2011 ranged between 20.7°C and 35.2°C, with a monthly average of 25-27°C. There are only small seasonal changes in rainfall, and during our study, March was the driest month, and October to January the wettest period (max. rainfall per day: 150 mm/m3). Long-term climate data over 50 years show a mean annual rainfall of 3,601 mm at our study site, and every month of the year is perhumid, receiving at least 200 mm of rain (see Figure 1 in Erb et al.2012a).
Location of Siberut, the Peleonan Forest (rented by SCP for conservation purpose) and the study site (SCP research station). The right map shows the 95% MCP home ranges of the studied group A (bottom), the semi-habituated group B (top) and locations of encounters with other neighboring groups of Siberut macaques. Small squares within the study group’s home range indicate the 12 botanical plots. Data source: 90 m digital elevation data come from Shuttle Radar Topography Mission (SRTM) by USGS/ NASA (http://srtm.csi.cgiar.org).
Study group
The study group (group A) was habituated from December 2008 until March 2010. Habituation was done by searching the group silently from sunrise to sunset with two to four teams simultaneously on six days per week. Once macaques were encountered, geographical position, subgroup size and activity was recorded. Beginning of 2010, at the end of the habituation period and when data collection started, group size was 29. There were 3 permanent adult males and 8 adult females in the group. Adult individuals were the main focus of this study and were all identified, except two of the adult females which were first seen at the end of the study. We assume that they were already part of the group throughout the data collection period, but were never reliably identified before as they might have stayed very peripheral and shy. Adults were defined as those individuals who were sexually fully mature, i.e. had large testes in males and visible nipples in females. By the end of the study period, during the mating season in January and February 2011, 3 juvenile females developed their first sexual swelling. From the juveniles, only a few individuals were identified, the rest was recorded as age-sex category during data collection.
As this group of Siberut macaques is the first one ever studied in detail, we report some information on the life history and demography. Infants are born with white fur and reddish facial skin, hands and feet. After few weeks, their fur coloration changes to juvenile/adult like dark brown coat with only small white fur patches left around the temples and a slightly lighter breast fur. At an age of about 2.5 months, the coloration change is complete. During the course of this study several births occurred (Mar.-Apr. 2009 3 birth, Sep.-Oct. 2009 4 birth (1 died), Jan. 2010 1 birth, Jul. 2010 1 birth (died at 2 month age)).
The studied group was surrounded by several other groups of conspecifics (Figure 10), including one group (group B) which was habituated from May to November 2009 and occasionally came to the research station when trees were fruiting there.
Data collection
Group scan observations (scan sampling: Martin and Bateson 1993) were conducted from March 2010 until March 2011, from 6 am until latest 7:30 pm, by 1 to 3 observers simultaneously (69.3% by 1 observer, 27.0% by 2 observers, 3.7% by 3 observers). Scans were taken every full hour (sampling duration: 10 min) in the first month, and were changed to every 30 min at half and full hours for the rest of the time period (sampling duration: 5 min per scan). Data in March 2011 were too scarce and were omitted for some analyses. During group scans, the following data were recorded: time, identity of the monkeys, type of habitat (forest, canopy gap, windthrow area, swamp), relative and absolute height of the monkey in the forest, activity and in case they were feeding, the food item and species, as well as the GPS coordinates.
To calculate the monthly percentage of habitat use, we scored for each scan the habitat type used by the majority (>50%) of individuals. Canopy gap was defined as a small open area within the forest caused by a treefall. Windthrow area was a forest area hit by a heavy storm, which destroyed nearly all trees in that specific area.
Forest strata use was measured as the absolute forest height used by the monkeys (in 5 m steps) and the relative height used. Values for the whole group are based on the average of the values of each age-sex class (adult males, adult females, juveniles). The relative forest height describes the height of the monkey relative to the forest at a certain place in the forest, and was divided into 4 categories: ground (soil and leaf litter), lower-story (substrates on the ground, including fall-down trees, up to the mid-story), mid-story (either a tree that ends below the canopy at this place in the forest or the lower branches of a tree that make up the canopy) and canopy (the crown of the tree that makes up the canopy at this place in the forest).
The general activity of the monkeys at a scan time was classified as either traveling, resting, feeding (inserting food into the mouth, handling/ manipulating food; but not processing food which was already stored in the cheek pouch), foraging (searching for food), and social activities (allogrooming, mating, playing). Activity budget is given as the monthly average of 12 months, from March 2010 until February 2011.
Diet was based on the percentage of feeding time on different food items based on scan data. Food item categories were fruit, flower, arthropods, mushrooms, leaves, pith (soft core of palm stems), sap and shoot (young stems). The overall diet was calculated as the average over 12 months (Mar. 2010 – Feb. 2011). The dietary diversity index was calculated using the Shannon-Wiener index H’ (Pielou 1966;Krebs 1999). The index combines information on species richness as well as relative abundance. For calculation of H’, only food items of known species were used, which included fruit, flower, pith, sap and some of the leaves. Mushrooms and arthropods were not identified to species level and were thus not included in the index.
GPS points were recorded for 2,267 scans in a geographic coordinate system in a Lat/Lon format (Datum: WGS 84) and later converted into the projected coordinate system WGS 1984 UTM Zone 47 South. We only used GPS coordinates of permanent group members for home range analysis. Home range was calculated as Minimum Convex Polygon (MCP, Worton 1987;Harris et al. 1990;White and Garrott 1990;Börger et al. 2006) to allow comparison with older studies, and with Kernel methods using reference bandwidth h ref , which equaled 63.85 m (Silverman 1986;Worton 1989;Wand and Jones 1995;Worton 1995;Seaman and Powell 1997;Kenward 2001). We also applied the “ad hoc” bandwidth h ad hoc (Berger and Gese 2007;Jaques et al. 2009;Kie et al. 2010), but results were similar to h ref and are not reported. For MCP calculation (with “fixed means”), we used 4,839 unique point locations (duplicate fixes removed) and calculated 100% (the maximum area in which the group was ranging), 95% (full home range, reducing the outlier effect), and 50% MCPs (core home range). For kernel polygons, average GPS coordinates were calculated for each scan as the center of the group, as GPS points of single individuals per scan are not independent, resulting in 2,410 point locations. Points were jittered by ±0.5 m to avoid point duplicates, and kernels were constructed using fixed kernel approach, Gaussian (bivariate normal) kernels, a raster cell size of 20 m and a buffer of 25 m (the median distances to the group center). Although group center coordinates per scan were autocorrelated, we used all for kernel analysis because removing autocorrelation would also remove biologically significant information (Lair 1987;Reynolds and Laundre 1990;de Solla et al. 1999;Blundell et al. 2001;Cushman et al. 2005). We calculated the 95% contour as the full home range and the 50% contour as the core home range. Home range (and travel distance) calculations were done in ArcGIS® 9 (ArcEditor™ 9.3.1), using the Home Range Tools (HRT) extension (Version 1.1), except for the asymptote analysis of home range area, which was done with the extension HoRAE (Nov. 2011) in OpenJUMP 1.4.3.
The monthly average daily travel distance was calculated from group center coordinates per scan (see above). We only used days with ≥9 h observation time per day (called “full day follows”). Travel speed (m/h) is calculated as the travel distance per observation day divided by the observation time on that day. As the active period of the group was usually about 12 hours, from 6:30 am to 6:30 pm, this calculated travel speed was multiplied by 12 to obtain the daily travel distance. Straight line distances of groups in 30 min intervals can be different from actual travel distances of individuals (Isbell et al. 1999), so that we also report the average travel speed of single individuals. This is based on focal animal observations of all adult males and females, from August 2010 until February 2011, which aimed to be at least 30 min long (mean 33 min, max. 127 min). For analysis, we only used observations of at least 20 min.
For habitat analysis, we established 12 permanent botanical plots of 50 m x 50 m size (0.25 ha each) within the group’s home range (Figure 10), in which 25 subplots of 10 m x 10 m were nested. A total of 3 ha was sampled, covering 3.6% of the 95% kernel home range. Plots were distributed semi-randomly, while taking habitat variation, altitude and distance to rivers into account (top of hill: 1, slope: 2, slope/ riverine: 1, riverine/ level ground: 1, dry level ground: 6, edge of swamp: 1). Plots were mainly covering mixed forest, and to a low extent dipterocarp forest. We recorded all trees ≥10 cm dbh (diameter at breast height), all palm trees ≥10 cm dbh (Oncosperma horridum) or ≥5 cm dbh respectively (Arenga obtusifolia, Pinanga sp.), all lianas (woody vines) and stranglers (strangler figs) ≥5 cm dbh and all rattan (i.e. climbing palms) longer than 5 m. They were marked, measured for dbh, and height (or length for lianas and rattan) was estimated by eye after training with a measuring tape. For trees with buttress roots or prop roots, dbh was measured ~20 cm above the rooting point. For clustering (rhizomatous) rattan and palm trees which produce multiple stems, we counted “apparent” genets, following Gerwing et al (2006). For the rhizomatous and very spiny palm tree Oncosperma horridum, we recorded the number of stems ≥10 cm dbh (important for basal area calculation), and for height and dbh, a mean was estimated. For rhizomatous rattan, dbh and length was measured for each stem separately. We collected two specimen per species with the help of a local plant specialist (31% of species with fruit and/or flower), and described, photographed and dried them in a self-made herbarium oven at 60-75°C in the field. They were identified (using Kooders 1913;Sinclair 1955;Kostermans 1969;Kostermans 1970;van Steenis 1972;Polunin 1988;van Balgooy 1997;van Balgooy 1998;de Wilde 2000;Symington et al. 2004;Yoneda 2004;Berg et al. 2005;Min et al. 2006;Soepadmo et al. 2007) and stored at the Herbarium ANDA of the Andalas University Padang, W-Sumatra. For analysis, we classified species into trees, palm trees, lianas, stranglers and rattan to enhance comparability between plots and other studies (Hadi et al. 2009b). The dbh distribution of trees from botanical plots is compared to 235 sleeping trees (Dec. 2008 – Mar. 2011) and to 73 feeding trees (Mar. 2010 – Mar. 2011; palm trees excluded). Basal area (m2) was calculated as 3.142 * (dbh in cm/ 200)2. We measured species diversity with the Simpson’s diversity index D, calculated as D = 1 - (Σ n * (n - 1)/N * (N - 1)), with n being the total number of individuals of a particular species, and N being the total number of individuals of all species. D ranges between zero (no diversity) and one (max. diversity). In addition, we also report the Shannon-Wiener diversity index.
Statistical analysis: Spearman rank correlations were conducted in Excel®, and p-values were based on 10,000 permutations. Mann-Whitney-U tests and Chi-Square tests were carried out in R 2.14.0© 2011. Species richness estimators, similarity indices and the Shannon-Wiener diversity index were calculated using EstimateS 8.2.0© 2009 (R.K. Colwell; http://purl.oclc.org/estimates). The species richness estimators ACE, ICE, Chao 1, Chao 2, Jack 1, Jack 2 and Bootstrap are reported as the mean of 10,000 randomizations, without replacement. For all analyses, we adopted an α-level of 0.05.
References
Abdul Hamid NH, Suratman MN: Assessment of rattan abundance and species composition at Kuala Keniam, Taman Negara Pahang. In 2010 International Conference on Science and Social Research (CSSR 2010). Kuala Lumpur, Malaysia: ; 2010:1041-1046.
Abegg C, Thierry B: Macaque evolution and dispersal in insular south-east Asia. Biol J Linn Soc 2002, 75: 555-576. 10.1046/j.1095-8312.2002.00045.x
Abegg C, Thierry B: The phylogenetic status of Siberut macaques: Hints from the bared-teeth display. Primate Rep 2002, 63: 73-78.
Achard F, Eva HD, Stibig H-J, Mayeux P, Gallego J, Richards T, Malingreau J-P: Determination of deforestation rates of the world's humid tropical forests. Science 2002, 297: 999-1002. 10.1126/science.1070656
Agetsuma N: Dietary selection by Yakushima macaques ( Macaca fuscata yakui ): The influence of food availability and temperature. Int J Primatol 1995, 16: 611-627.
Agetsuma N: Foraging strategies of Yakushima macaques ( Macaca fuscata yakui ). Int J Primatol 1995, 16: 595-609.
Agetsuma N, Nakagawa N: Effects of habitat differences on feeding behaviors of Japanese monkeys: Comparison between Yakushima and Kinkazan. Primates 1998, 39: 275-289. 10.1007/BF02573077
Aggimarangsee N: Survey for semi-tame colonies of macaques in Thailand. Nat Hist Bull Siam Soc 1992, 40: 103-166.
Ahsan MF: Feeding ecology of the primates of Bangladesh. Ecology and Evolution. In Current Primatology. Volume I. Edited by: Thierry B, Anderson JR, Roeder JJ, Herrenschmidt N. Strasbourg: Université Louis Pasteur; 1994:79-86.
Albert A: Feeding and ranging behavior of northern pigtailed macaques ( Macaca leonina ): Impact on their seed dispersal effectiveness and ecological contribution in a tropical rainforest at Khao Yai National park, Thailand, Unpublished PhD thesis. In Département de Biologie, Ecologie et Environment. Université de Liege: ; 2012:220.
Albert A, Savini T, Huynen M-C: Sleeping site selection and presleep behavior in wild pigtailed macaques. Am J Primatol 2011, 73: 1222-1230. 10.1002/ajp.20993
Aldrich-Blake FPG: Long-tailed macaques. In Malayan Forest Primates. Edited by: Chivers DJ. New York, London: Plenum Press; 1980:147-165.
Berg CC, Corner EJH, Nooteboom HP: Flora Malesiana, Series 1: Volume 17, Part 2: Moraceae (Ficus). Leiden: National Herbarium of the Netherlands; 2005.
Berger KM, Gese EM: Does interference competition with wolves limit the distribution and abundance of coyotes? J Anim Ecol 2007, 76: 1075-1085. 10.1111/j.1365-2656.2007.01287.x
Bernstein IS: A field study of the pigtail monkey ( Macaca nemestrina ). Primates 1967, 8: 217-228. 10.1007/BF01731038
Bird MI, Taylor D, Hunt C: Palaeoenvironments of insular Southeast Asia during the Last Glacial Period: A savanna corridor in Sundaland? Quat Sci Rev 2005, 24: 2228-2242. 10.1016/j.quascirev.2005.04.004
Blundell GM, Maier JAK, Debevec EM: Linear home ranges: Effects of smoothing, sample size, and autocorrelation on kernel estimates. Ecol Monogr 2001, 71: 469-489. 10.1890/0012-9615(2001)071[0469:LHREOS]2.0.CO;2
Börger L, Franconi N, de Michele G, Gantz A, Meschi F, Manica A, Lovari S, Coulson T: Effects of sampling regime on the mean and variance of home range size estimates. J Anim Ecol 2006, 75: 1393-1405. 10.1111/j.1365-2656.2006.01164.x
Brune S, Babeyko AY, Gaedicke C, Ladage S: Hazard assessment of underwater landslide-generated tsunamis: A case study in the Padang region, Indonesia. Nat Hazards 2010, 53: 205-218. 10.1007/s11069-009-9424-x
Buckley LB, Jetz W: Insularity and the determinants of lizard population density. Ecol Lett 2007, 10: 481-489. 10.1111/j.1461-0248.2007.01042.x
Burkey TV: Extinction rates in archipelagoes: Implications for populations in fragmented habitats. Conserv Biol 2002, 9: 527-541.
Caldecott JO: An ecological and behavioural study of the pig-tailed macaque. Volume 21. Basel, New York: Karger; 1986.
Caldecott JO: A summary of the ranging and activity patterns of the pig-tailed macaque ( Macaca n. nemestrina ) in relation to those of sympatric primates in Peninsular Malaysia. In Current perspectives in primate social dynamics. Edited by: Taub DM, King FA. New York: van Nostrand Reinhold Company; 1986b:152-158.
Caldecott JO, Feistner ATC, Gadsby EL: A comparison of ecological strategies of pig-tailed macaques, mandrills and drills. In Evolution and ecology of macaque societies. Edited by: Fa JE, Lindburg DG. Cambridge: Cambridge University Press; 1997:73-94.
Chalise MK: Some behavioral and ecological aspects of Assamese monkeys ( Macaca assamensis ) in Makulu-Barun Area, Nepal. Nepal J Sci Technol 1999, 1: 85-90.
Chalise MK: Assamese macaques ( Macaca assamensis ) in Nepal. Primate Conserv 2003, 19: 99-107.
Chatani K: Positional behavior of free-ranging Japanese macaques ( Macaca fuscata ). Primates 2003, 44: 13-23.
Chopra PK, Seth S, Seth PK: Behavioural profile of free-ranging rhesus monkeys. Primate Rep 1992, 32: 75-105.
Choudhury A: Ecology and behaviour of the pig-tailed macaque Macaca nemestrina leonina in some forests of Assam in North-East India. J Bombay Nat Hist Soc 2008, 105: 279-291.
Crockett CM, Wilson WL: The ecological separation of Macaca nemestrina and Macaca fascicularis in Sumatra. In The macaques: Studies in ecology, behavior and evolution. Edited by: Lindburg D. New York: van Nostrand Reinhold Company; 1980:148-181.
Cushman SA, Chase M, Griffin C: Elephants in space and time. Oikos 2005, 109: 331-341. 10.1111/j.0030-1299.2005.13538.x
de Solla SR, Bonduriansky R, Brooks RJ: Eliminating autocorrelation reduces biological relevance of home range estimates. J Anim Ecol 1999, 68: 221-234. 10.1046/j.1365-2656.1999.00279.x
de Wilde WJJO: Flora Malesiana, Series 1: Volume 14: Myristicaceae (Seed Plants). Leiden: National Herbarium of the Netherlands; 2000.
Deag JM: Social behaviour and ecology of wild Barbary macaques (M. sylvanus), Part I, Unpublished PhD thesis. Bristol: ; 1974.
Deag JM: The diurnal patterns of behaviour of the wild Barbary macaque Macaca sylvanus . Journal of Zoology, London 1985, 206: 403-413.
Díaz S, Fargione J, Stuart Chapin F III, Tilman D: Biodiversity loss threatens human well-being. PLoS Biol 2006, 4: 1300-1305.
Dittus WPJ: The socioecological basis for the conservation of the Toque monkey ( Macaca sinica ) of Sri lanka (Ceylon). In Primate Conservation. Edited by: Monaco HSHPRI, Bourne GH. New York, San Francisco, London: Academic Press; 1977:237-265.
Dittus WPJ: Group fission among wild toque macaques as a consequence of female resource competition and environmental stress. Anim Behav 1988, 36: 1626-1645. 10.1016/S0003-3472(88)80104-0
Erb WM: Male-male competition and loud calls in one-male groups of simakobu (Simias concolor), Unpublished PhD thesis. Stony Brook University: Department of Anthropology; 2012:202.
Erb WM, Borries C, Lestari NS, Hodges JK: Annual variation in ecology and reproduction of wild simakobu ( Simias concolor ). Int J Primatol 2012, 33: 1406-1419. 10.1007/s10764-012-9630-3
Erb WM, Borries C, Lestari NS, Ziegler T: Demography of Simakobu ( Simias concolor ) and the impact of human disturbance. Am J Primatol 2012, 74: 580-590. 10.1002/ajp.22010
FAO (2010) Global forest resources assessment: In: FAO Forestry Paper 160. Food and Agriculture Organization of the United. Rome: Nations; 2010.
Flint EP: Changes in land use in South and Southeast Asia from 1880 to 1980: A data base prepared as part of a coordinated research program on carbon fluxes in the tropics. Chemosphere 1994, 29: 1015-1062. 10.1016/0045-6535(94)90166-X
Fooden J: Taxonomy and evolution of liontail and pigtail macaques (Primates: Cercopithecidae). Fieldiana Zool 1975, 67: 1-169.
Fooden J: Classification and distribution of living macaques ( Macaca Lecepede 1799). In The macaques: Studies in ecology, behavior and evolution. Edited by: Lindburg DG. New York: van Nostrand Reinold; 1980:1-9.
Fuentes A: Feeding and ranging in the Mentawai island langur ( Presbytis potenziani ). Int J Primatol 1997, 17: 525-548.
Fuentes A: Current status and future viability for the Mentawai Primates. Primate Conserv 1997, 17: 111-116.
Fuentes A, Olson M: Preliminary observations and status of the Pagai macaque. Asian Primates 1995, 4: 1-4.
Gathorne-Hardy FJ, Syaukani DRG, Eggleton P, Jones DT: Quaternary rainforest refugia in South-East Asia: Using termites (Isoptera) as indicators. Biol J Linn Soc 2002, 75: 453-466. 10.1046/j.1095-8312.2002.00031.x
Gerwing JJ, Schnitzer SA, Burnham RJ, Bongers F, Chave J, DeWalt SJ, Ewango CEN, Foster R, Kenfack D, Martínez-Ramos M, Parren M, Parthasarathy N, Pérez-Salicrup DR, Putz FE, Thomas DW: A standard protocol for liana censuses. Biotropica 2006, 38: 256-261. 10.1111/j.1744-7429.2006.00134.x
Giyarto: Aktivitas harian dan penggunaan home range oleh monyet hitam Sulawesi ( Macaca nigra ) di Cagar Alam Tangkoko-Batuangus, Sulawesi Utara, Unpublished BSc thesis. In Biology Faculty. Yogyakarta: Gadjah Mada University; 2010:42.
Goldstein SJ: Feeding ecology of rhesus monkeys (Macaca mulatta) in Northwestern Pakistan, PhD thesis, Yale University. Michigan: UMI Dissertation Services, A Bell & Howell Company; 1984.
Goldstein SJ, Richard AF: Ecology of rhesus macaques ( Macaca mulatta ) in Northwest Pakistan. Int J Primatol 1989, 10: 531-567. 10.1007/BF02739364
Gower D, Johnson K, Richardson J, Rosen B, Rüber L, Williams S: Biotic evolution and environmental change in Southeast Asia. Cambridge: Cambridge University Press; 2012.
Groves CP: The nomenclature of the Tanzanian mangabey and the Siberut macaque. Aust Primatol 1996, 10: 2-5.
Hadi S: Niche differentiation of two sympatric colobines Simias concolor and Presbytis potenziani on the Mentawaian Island of Siberut, Indonesia, Unpublished PhD thesis. In Faculty of Biology. Göttingen: Georg-August University Göttingen; 2012:104.
Hadi S, Ziegler T, Hodges JK: Group structure and physical characteristics of Simakobu monkeys ( Simias concolor ) on the Mentawai Island of Siberut, Indonesia. Folia Primatol 2009, 80: 74-82. 10.1159/000214226
Hadi S, Ziegler T, Waltert M, Hodges JK: Tree diversity and forest structure in northern Siberut, Mentawai Islands, Indonesia. Trop Ecol 2009, 50: 315-327.
Hadi S, Ziegler T, Waltert M, Syamsuri F, Mühlenberg M, Hodges JK: Habitat use and trophic niche overlap of two sympatric colobines, Presbytis potenziani and Simias concolor , on Siberut Island, Indonesia. Int J Primatol 2012, 33: 218-232. 10.1007/s10764-011-9567-y
Hansen MC, Stehman SV, Potapov PV, Arunarwati B, Stolle F, Pittman K: Quantifying changes in the rates of forest clearing in Indonesia from 1990 to 2005 using remotely sensed data sets. Environ Res Lett 2009, 4: 1-12.
Hanya G: Diet of a Japanese macaque troop in the coniferous forest of Yakushima. Int J Primatol 2004, 25: 55-71.
Hanya G: Seasonal variations in the activity budget of Japanese macaques in the coniferous forest of Yakushima: Effects of food and temperature. Am J Primatol 2004, 63: 165-177. 10.1002/ajp.20049
Harris S, Cresswell WJ, Forde PG, Trewhella WJ, Wooland T, Wray S: Home-range analysis using radio-tracking data - A review of problems and techniques as applied to the study of mammals. Mammal Rev 1990, 20: 97-123. 10.1111/j.1365-2907.1990.tb00106.x
Hashimoto C: Differences in feeding behavior between adult and juvenile Japanese macaques in Kinkazan Island, Japan. In Primatology Today. Edited by: Ehara A, Kimura T, Takenaka O, Iwamoto M. Amsterdam, New York, Oxford: Elsevier Science Publishers; 1991:111-114.
Heaney LR: Mammalian species richness on islands on the Sunda Shelf, Southeast Asia. Oecologia 1984, 61: 11-17. 10.1007/BF00379083
Hill DA: Seasonal variation in the feeding behavior and diet of Japanese macaques ( Macaca fuscata yakui ) in lowland forest of Yakushima. Am J Primatol 1997, 43: 305-322. 10.1002/(SICI)1098-2345(1997)43:4<305::AID-AJP2>3.0.CO;2-0
Ikeda H: Population changes and ranging behavior of wild Japanese monkeys at Mt. Kawaradake in Kyushu, Japan. Primates 1982, 23: 338-347. 10.1007/BF02381318
Isbell LA, Pruetz JD, Musyoka Nzuma B, Young TP: Comparing measures of travel distances in primates: Methodological considerations and socioecological implications. Am J Primatol 1999, 48: 87-98. 10.1002/(SICI)1098-2345(1999)48:2<87::AID-AJP1>3.0.CO;2-G
IUCN: IUCN red list of threatened species, Version 2012.2. 2012. http://www.iucnredlist.org
Izumiyama S: Seasonal changes in travelling distance of Japanese macaques in Kamikochi: Effects of meteorological conditions in snowy period. Primate Res 1999, 15: 343-352. 10.2354/psj.15.343
Jaques CN, Jenks JA, Klaver RW: Seasonal movements and home-range use by female pronghorns in sagebrush-steppe communities of western South Dakota. J Mammal 2009, 90: 433-441. 10.1644/07-MAMM-A-395.1
Kartawinata K, Samsoedin I, Heriyanto M, Afriatini JJ: A tree species inventory in a one-hectar plot at the Batang Gadis National Park, North Sumatra, Indonesia. Reinwardtia 2004, 12: 145-157.
Kemp N: The birds of Siberut, Mentawai Islands, West Sumatra. Kukila 2000, 11: 73-96.
Kenward RE: A manual for wildlife radiotracking. London: Academic Press; 2001.
Khan MAR, Wahab MA: Eco-ethology of the crab-eating macaque of Bangladesh. J Asiat Soc Bangladesh 1983, 9: 101-109.
Kie JG, Matthiopoulos J, Fieberg J, Powell RA, Cagnacci F, Mitchell MS, Gaillard J-M, Moorcroft PR: The home-range concept: Are traditional estimators still relevant with modern telemetry technology? Philos Trans R Soc B 2010, 365: 2221-2231. 10.1098/rstb.2010.0093
Kinnaird MF, O'Brien TG: Fast foods of the forest: The influence of figs on primates and hornbills across Wallace's line. In Tropical fruits and frugivores: The search for strong interactions. Edited by: Dew JL, Boubli JP. Dordrecht: Springer; 2005:155-184.
Kitchener AC, Groves C: New insights into the taxonomy of Macaca pagensis of the Mentawai Islands, Sumatra. Mammalia 2002, 66: 533-542.
Kochummen KM, LaFrankie JV Jr, Manokaran N: Floristic composition of Pasoh Forest Reserve, a lowland rain forest in Peninsular Malaysia. J Trop For Sci 1990, 3: 1-13.
Kohlhaas AK: Behavior and ecology of Macaca nigrescens : Behavioral and social responses to the environment and fruit availability, Unpublished PhD thesis. In Department of Environmental, Population, and Organismic Biology. Colorado: University of Colorado; 1993:241.
Kooders SH: Exkursionsflora von Java. Jena: Gustav Fischer; 1913.
Kostermans AJGH: Materials for a revision of Lauraceae II. Reinwardtia 1969, 7(5):451-536.
Kostermans AJGH: Materials for a revision of Lauraceae III. Reinwardtia 1970, 8(1):21-196.
Krebs CJ: Ecological Methodology. 2nd edition. Inc: Addison-Welsey Educational Publishers; 1999.
Krebs JR, Davies NB: Behavioural Ecology. An Evolutionary Approach. 4th edition. Oxford: Blackwell; 1997.
Kreft H, Jetz W, Mutke J, Kier G, Barthlott W: Global diversity of island floras from a macroecological perspective. Ecol Lett 2008, 11: 116-127.
Kumar RS, Mishra C, Sinha A: Foraging ecology and social behaviour of the Arunachal macaque - A preliminary study. In Ecology and conservation of the Arunachal macaque Macaca munzala. NCF Technical Report No. 15, Nature Conservation Foundation, National Institute of Advanced Studies, International Snow Leopard Trust Edited by: Sinha A, Kumar RS, Mishra C. 2006, 45-60.
Kumara HN, Singh M: Distribution and abundance of primates in rain forests of the Western Ghats, Karnataka, India and the conservation of Macaca silenus . Int J Primatol 2004, 25: 1001-1018.
Kurup GU, Kumar A: Time budget and activity patterns of the lion-tailed macaque ( Macaca silenus ). Int J Primatol 1993, 14: 27-39. 10.1007/BF02196501
Lair H: Estimating the location of the focal center in red squirrel home ranges. Ecology 1987, 68: 1092-1101. 10.2307/1938381
Laumonier Y: The vegetation and physiography of Sumatra. Dordrecht, Boston, London: Kluwer Academic Publishers; 1997.
Laumonier Y, Uryu Y, Stüwe M, Budiman A, Setiabudi B, Hadian O: Eco-floristic sectors and deforestation threats in Sumatra: Identifying new conservation area network priorities for ecosystem-based land use planning. Biodivers Conserv 2010, 19: 1153-1174. 10.1007/s10531-010-9784-2
Laurance WF: Reflections on the tropical deforestation crisis. Biol Conserv 1999, 91: 109-117. 10.1016/S0006-3207(99)00088-9
Lee HS, Davies SJ, LaFrankie JV, Tan S, Yamakura T, Itoh A, Ohkubo T, Ashton PS: Floristic and structural diversity of mixed dipterocarp forest in Lambir Hills National Park, Sarawak, Malaysia. J Trop For Sci 2002, 14: 379-400.
Lindburg DG: The rhesus monkey in North India: An ecological and behavioral study. Academic Press. In Primate behavior: Developments in field and laboratory research. Volume 2. Edited by: Rosenblum LA. London: New York; 1971:1-106.
Lindburg DG: Feeding behaviour and diet of rhesus monkeys ( Macaca mulatta ) in a Siwalik forest in North India. In Primate ecology: Studies of feeding and ranging behaviour in lemurs, monkeys and apes. Edited by: Clutton-Brock TH. London, New York, San Francisco: Academic Press; 1977:223-249.
Lu JF, Lin Y-S, Lee L-L: Troop composition, activity pattern and habitat utilization of Formosan macaque ( Macaca cyclopis ) at Nanshi logging road in Yushan National Park. In Primatology today: Proceedings of the XIIIth congress of the International Primatological Society Nagoya and Kyoto, 18-24 July 1990. Edited by: Ehara A, Kimura T, Takenaka O, Iwamoto M. Amsterdam, New York, Oxford: Elsevier Science Publishers; 1991:93-96.
MacArthur RH, Diamond JM, Karr JR: Density compensation in island faunas. Ecology 1972, 53: 330-342. 10.2307/1934090
MacArthur RH, Wilson EO: The theory of island biogeography. Princeton, New Jersey: Princeton University Press; 1967.
MacKinnon JR, MacKinnon KS: Niche differentiation in a primate community. In Malayan Forest Primates. Edited by: Chivers DJ. New York, London: Plenum Press; 1980:167-191.
Martin P, Bateson P: Measuring Behaviour. Second Edition edn: Cambridge University Press, Cambridge; 1993.
Maruhashi T: Activity patterns of a troop of Japanese monkeys ( Macaca fuscata yakui ) on Yakushima Island, Japan. Primates 1981, 22: 1-14. 10.1007/BF02382552
Meijaard E: Mammals of South-East Asian islands and their Late Pleistocene environments. J Biogeogr 2003, 30: 1245-1257. 10.1046/j.1365-2699.2003.00890.x
Ménard N: Do ecological factors explain variation in social organization? In Macaque societies: A model for the study of social organization. Edited by: Thierry B, Singh M, Kaumanns W. Cambridge: Cambridge University Press; 2004:237-266.
Ménard N, Vallet D: Behavioral responses of Barbary macaques ( Macaca sylvanus ) to variations in environmental conditions in Algeria. Am J Primatol 1997, 43: 285-304. 10.1002/(SICI)1098-2345(1997)43:4<285::AID-AJP1>3.0.CO;2-T
Mendiratta U, Kumar A, Mishra C, Sinha A: Winter ecology of the Arunachal macaque Macaca munzala in Pangchen Valley, Western Arunachal Pradesh, Northeastern India. Am J Primatol 2009, 71: 939-947. 10.1002/ajp.20734
Menon S, Poirier FE: Lion-tailed macaques ( Macaca silenus ) in a disturbed forest fragment: Activity patterns and time budget. Int J Primatol 1996, 17: 969-985. 10.1007/BF02735297
Miettinen J, Shi C, Chin Liew S: Deforestation rates in insular Southeast Asia between 2000 and 2010. Glob Change Biol 2011, 17: 2261-2270. 10.1111/j.1365-2486.2011.02398.x
Min BC, Omar-Hor K, Chow Lin O-Y: 1001 garden plants in Singapore. 2nd edition. Singapore, Singapore: National Parks Board; 2006.
Mitchell AH, Tilson RL: Restoring the balance: Traditional hunting and primate conservation in the Mentawaian Islands, Indonesia. In Primate ecology and conservation. Edited by: Else JG, Lee PC. Cambridge: Cambridge University Press; 1986:249-260.
Mittermeier RA, Gil PR, Hoffmann M, Pilgrim J, Brooks T, Mittermeier CG, Lamoreux J, da Fonseca GAB: Hotspots revisited: Earth's biologically richest and most endangered terrestrial ecoregions. Mexico City: Cemex; 2004.
Mittermeier RA, Myers N, Mittermeier CG: Hotspots: Earth's biologically richest and most endangered terrestrial ecoregions. Mexico City: CEMEX; 1999.
Myers N: Hotspots. In Encyclopedia of Biodiversity. Volume 3. Edited by: Levin SA. USA: Academic Press; 2001:371-381.
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J: Biodiversity hotspots for conservation priorities. Nature 2000, 403: 853-858. 10.1038/35002501
Neville MK: Ecology and activity of Himalayan foothill rhesus monkeys ( Macaca mulatta ). Ecology 1968, 49: 110-123. 10.2307/1933566
Nijman V, Meijaard E: Zoogeography of primates in insular Southeast Asia: Species-area relationships and the effects of taxonomy. Contrib Zool 2008, 77: 117-126.
O'Brien TG, Kinnaird MF: Behavior, diet, and movements of the Sulawesi crested black macaque ( Macaca nigra ). Int J Primatol 1997, 18: 321-351. 10.1023/A:1026330332061
Patou M-L, Wilting A, Gaubert P, Esselstyn JA, Cruaud C, Jennings AP, Fickel J, Veron G: Evolutionary history of the Paradoxurus palm civets - A new model for Asian biogeography. J Biogeogr 2010, 37: 2077-2097. 10.1111/j.1365-2699.2010.02364.x
Pielou EC: Shannon's formula as a measure of specific diversity: its use and misuse. Am Nat 1966, 100: 463-465. 10.1086/282439
Polunin I: Plants and flowers of Malaysia. Singapore: Times Editions; 1988.
Pombo AR, Waltert M, Mansjoer SS, Mardiastuti A, Mühlenberg M: Home range, diet and behaviour of the Tonkean macaque ( Macaca tonkeana ) in Lore Lindu National Park, Sulawesi. In Land use, nature conservation and the stability of rainforest margins in Southeast Asia. Edited by: Gerold G, Fremerey M, Guhardja E. Berlin, Heidelberg, New York: Springer; 2004:313-325.
Putz FE, Chai P: Ecological studies of lianas in Lambir National Park, Sarawak, Malaysia. J Ecol 1987, 75: 523-531. 10.2307/2260431
Quinten MC, Waltert M, Syamsuri F, Hodges JK: Peat swamp forest supports high primate densities on Siberut Island, Sumatra, Indonesia. Oryx 2009, 44: 147-151.
Reynolds TD, Laundre JW: Time intervals for estimating pronghorn and coyote home ranges and daily movements. J Wildl Manage 1990, 54: 316-322. 10.2307/3809049
Riley E: Flexibility in diet and activity patterns of Macaca tonkeana in response to anthropogenic habitat alteration. Int J Primatol 2007, 28: 107-133. 10.1007/s10764-006-9104-6
Roos C, Ziegler T, Hodges JK, Zischler H, Abegg C: Molecular phylogeny of Mentawai macaques: Taxonomic and biogeographic implications. Mol Phylogenet Evol 2003, 29: 139-150. 10.1016/S1055-7903(03)00076-9
Sarkar P, Srivastava A, Dasgupta S, Bhattacharjee PC: Activity profile of free ranging forest group of Assamese macaque. The Clarian 2012, 1: 59-67.
Schneider C, Hodges K, Fischer J, Hammerschmidt K: Acoustic niches of Siberut primates. Int J Primatol 2008, 29: 601-613. 10.1007/s10764-007-9181-1
Schülke O, Pesek D, Whitman BJ, Ostner J: Ecology of Assamese macaques ( Macaca assamensis ) at Phu Khieo Wildlife Sanctuary, Thailand. J Wildl Thailand 2011, 18: 1-15.
Seaman DE, Powell RA: An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology 1996, 77: 2075-2085. 10.2307/2265701
Seth PK, Seth S: Ecology and behaviour of rhesus monkeys in India. In Primate ecology and conservation. Volume 2. Edited by: Else JG, Lee PC. Cambridge: Cambridge University Press; 1986:89-103.
Shanahan M, So S, Compton SG, Corlett R: Fig-eating by vertebrate frugivores: A global review. Biol Rev Cambridge Philos Soc 2001, 76: 529-572. 10.1017/S1464793101005760
Siebert SF: The abundance and distribution of rattan over an elevation gradient in Sulawesi, Indonesia. For Ecol Manage 2005, 210: 143-158. 10.1016/j.foreco.2005.02.015
Silverman BW: Density estimation for statistics and data analysis. London, UK: Chapman & Hall; 1986.
Simberloff DS: Equilibrium theory of island biogeography and ecology. Annu Rev Ecol Syst 1974, 5: 161-182. 10.1146/annurev.es.05.110174.001113
Sinclair J: A revision of the Malayan Annonanceae. Garden's Bulletin, Singapore 1955, 14: 149-516.
Singh M, Vinanthe S: Inter-population differences in the time budgets of bonnet monkeys ( Macaca radiata ). Primates 1990, 31: 589-596. 10.1007/BF02382542
Singh MR, Singh ME, Ananda Kumar M, Kumara HN, Sharma AK, Sushma HS: Niche separation in sympatric lion-tailed macaque ( Macaca silenus ) and Nilgiri langur ( Presbytis johnii ) in an Indian tropical rain forest. Primate Rep 2000, 58: 83-95.
Sodhi NS, Koh LP, Brook BW, Ng PKL: Southeast Asian biodiversity: An impending disaster. Trends Ecol Evol 2004, 19: 654-660. 10.1016/j.tree.2004.09.006
Soepadmo E, Saw LG, Chung RCK, Kiew R: Tree Flora of Sabah and Sarawak, Volume 6. Bhd, Kuala Lumpur: Ampang Press Sdn; 2007.
Srivastava A: Primates of Northeast India. Bikaner: Megadiversity Press; 1999.
Su H-H, Lee L-L: Food habits of Formosan Rock macaques ( Macaca cyclopis ) in Jentse, Northeastern Taiwan, assessed by fecal analysis and behavioral observation. Int J Primatol 2001, 22: 359-377. 10.1023/A:1010799410911
Sugiyama Y: Characteristics of the social life of Bonnet macaques ( Macaca radiata ). Primates 1971, 12: 247-266. 10.1007/BF01730414
Sussman RW, Tattersall I: Behavior and ecology of Macaca fascicularis in Mauritius: A preliminary study. Primates 1981, 22: 192-205. 10.1007/BF02382610
Symington CF, Ashton PS, Appanah S: Forester's manual of dipterocarps. In Malayan Forest Records No. 16. Edited by: Barlow HS. Selangor: Malaysian Nature Society; 2004.
Teas J, Richie T, Taylor H, Southwick C: Population patterns and behavioral ecology of rhesus monkeys ( Macaca mulatta ) in Nepal. In The macaques: studies in ecology, behavior and evolution. Edited by: Lindburg DG. New York: van Nostrand Reinhold Company; 1980:247-262.
Tenaza R, Tilson RL: Human predation and Kloss's gibbon (Hylobates klossii ) sleeping trees in Siberut Island, Indonesia. Am J Primatol 1985, 8: 299-308. 10.1002/ajp.1350080405
Tenaza RR: Territory and monogamy among Kloss' gibbons ( Hylobates klossii ) in Siberut Island, Indonesia. Folia Primatol 1975, 24: 60-80. 10.1159/000155685
Tenaza RR, Fuentes A: Monandrous social organization of pigtailed langurs ( Simias concolor ) in the Pagai Islands, Indonesia. Int J Primatol 1995, 16: 295-310. 10.1007/BF02735483
Thomas SC, Baltzer JL: Tropical forests. In Encyclopedia of Life Sciences. London: Macmillan Publishers Ltd; 2002.
Thorington RW Jr, Koprowski JL, Steele MA, Whatton FF: Squirrels of the world. Baltimore: The John Hopkins University Press; 2012.
Tilson RL: Social organization of Simakobu monkeys ( Nasalis concolor ) in Siberut Island. J Mammal 1977, 58: 202-212. 10.2307/1379577
Tilson RL: Family formation strategies of Kloss's gibbons. Folia Primatol 1981, 35: 259-287. 10.1159/000155979
Tilson RT, Tenaza RR: Interspecific spacing between gibbons ( Hylobates klossii ) and langurs ( Presbytis potenziani ) on Siberut island, Indonesia. Am J Primatol 1982, 2: 355-361. 10.1002/ajp.1350020404
Tsuji Y: Regional, temporal, and interindividual variation in the feeding ecology of Japanese macaques. In The Japanese macaques. Volume 5. Edited by: Nakagawa N, Nakamichi M, Sugiura H. Tokyo: Springer; 2010:99-127.
Ungar PS: Fruit preferences of four sympatric primate species at Ketambe, Northern Sumatra, Indonesia. Int J Primatol 1995, 16: 221-245. 10.1007/BF02735479
van Balgooy MMJ: Malesian seed plants, Volume 1: Spot characters: An aid for identification of families and genera. Leiden: Rijksherbarium/Hortus Botanicus; 1997.
van Balgooy MMJ: Malesian seed plants, Volume 2: Portraits of tree families. Leiden: Rijksherbarium/Hortus Botanicus; 1998.
van Schaik CP, van Noordwijk MA, de Boer RJ, den Tonkelaar I: The effect of group size on time budgets and social behaviour in wild long-tailed macaques ( Macaca fascicularis ). Behav Ecol Sociobiol 1983, 13: 173-181. 10.1007/BF00299920
van Steenis CGGJ: Flora Malesiana: Spermatophyta (Flowering plants), Series 1. Groningen: Wolters-Noordhoof; 1972.
Voris HK: Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. J Biogeogr 2000, 27: 1153-1167. 10.1046/j.1365-2699.2000.00489.x
Wada K: World of the wild Japanese macaques. Tokyo: Kodansha; 1979.
Waltert M, Abegg C, Ziegler T, Hadi S, Priata D, Hodges K: Abundance and community structure of Mentawai primates in the Peleonan Forest, North Siberut, Indonesia. Oryx 2008, 42: 1-5.
Wand MP, Jones MC: Kernel smoothing. London, UK: Chapman and Hall, Ltd.; 1995.
Wang C-P: Activity pattern and diet composition of Formosan macaques ( Macaca cyclopis ) at Mt. Longevity, Taiwan, Unpublished MSc thesis. In Biological Sciences Department. National Sun Yat-sen University: ; 2004:74.
Watanabe K: Variations in group composition and population density of the two sympatric Mentawaian leaf-monkeys. Primates 1981, 22: 145-160. 10.1007/BF02382606
Wheatley BP: Feeding and ranging of East Bornean Macaca fascicularis . In The macaques: Studies in ecology, behavior and evolution. New York: van Nostrand Reinhold Company; 1980:215-246.
White GC, Garrott RA: Analysis of wildlife radio-tracking data. San Diego, CA: Academic Press, Inc.; 1990.
Whitmore TC: Tropical rain forests of the Far East. 2nd edition. Clarendon Press, Oxford: Oxford Science Publications; 1984.
Whittaker DJ: A conservation action plan for the Mentawai primates. Primate Conserv 2006, 20: 95-105.
Whitten AJ: The Kloss gibbon in Siberut rain forest, Unpublished PhD thesis. In Sub-Department of Veterinary Anatomy. Cambridge: University of Cambridge; 1980.
Whitten AJ: Diet and feeding behaviour of Kloss gibbons on Siberut Island, Indonesia. Folia Primatol 1982, 37: 177-208. 10.1159/000156032
Whitten AJ: The ecology of singing in Kloss gibbons ( Hylobates klossii ) on Siberut Island, Indonesia. Int J Primatol 1982, 3: 33-52. 10.1007/BF02693489
Whitten AJ: Home range use by Kloss gibbons ( Hylobates klossii ) on Siberut Island, Indonesia. Anim Behav 1982, 30: 182-198. 10.1016/S0003-3472(82)80253-4
Whitten AJ: A numerical analysis of tropical rain forest, using floristic and structural data, and its application to an analysis of gibbon ranging behavior. J Ecol 1982, 70: 249-271. 10.2307/2259877
Whitten AJ: Possible niche expansion of the spangled drongo Dicrurus hottentotus on Siberut Island, Indonesia. Ibis 1982, 124: 192-193.
Whitten AJ, Whitten JEJ: Preliminary observations of the Mentawai macaque on Siberut Island, Indonesia. Int J Primatol 1982, 3: 445-459. 10.1007/BF02693743
Whitten JEJ: Ecological separation of three diurnal squirrels in tropical rainforest on Siberut Island, Indonesia. Journal of Zoology, London 1981, 193: 405-420.
Wilting A, Sollmann R, Meijaard E, Helgen KM, Fickel J: Mentawai's endemic, relictual fauna: Is it evidence for Pleistocene extinctions on Sumatra? J Biogeogr 2012, 39: 1608-1620. 10.1111/j.1365-2699.2012.02717.x
Woodruff DS: Biogeography and conservation in Southeast Asia: How 2.7 million years of repeated environmental fluctuations affect today's patterns and the future of the remaining refugial-phase biodiversity. Biodivers Conserv 2010, 19: 919-941. 10.1007/s10531-010-9783-3
Worton BJ: A review of models of home range for animal movement. Ecol Modell 1987, 38: 277-298. 10.1016/0304-3800(87)90101-3
Worton BJ: Kernel methods for estimating the utilization distribution in home-range studies. Ecology 1989, 70: 164-168. 10.2307/1938423
Worton BJ: Using Monte Carlo simulation to evaluate kernel-based home range estimators. J Wildl Manage 1995, 59: 794-800. 10.2307/3801959
WWF: Saving Siberut: A conservation master plan. Geneva: World Wildlife Fund; 1980:135.
Yeager CP: Feeding ecology of the long-tailed macaque ( Macaca fascicularis ) in Kalimantan Tengah, Indonesia. Int J Primatol 1996, 17: 51-62. 10.1007/BF02696158
Yoder JB, Clancey E, Des Roches S, Eastman JM, Gentry L, Godsoe W, Hagey TJ, Jochimsen D, Oswald BP, Robertson J, Sarver BAJ, Schenk JJ, Spear SF, Harmon LJ: Ecological opportunity and the origin of adaptive radiations. J Evol Biol 2010, 23: 1581-1596. 10.1111/j.1420-9101.2010.02029.x
Yoneda T: Tree flora of Gn. Gadut, West Sumatra. Japan: Kagoshima University; 2004.
Yotsumoto N: The daily activity rhythm in a troop of wild Japanese monkey. Primates 1976, 17: 183-204. 10.1007/BF02382850
Ziegler T, Abegg C, Meijaard E, Perwitasari-Farajallah D, Walter L, Hodges JK, Roos C: Molecular phylogeny and evolutionary history of Southeast Asian macaques forming the M. silenus group. Mol Phylogenet Evol 2007, 42: 807-816. 10.1016/j.ympev.2006.11.015
Acknowledgements
We are grateful to all Indonesian authorities (LIPI, DEPDAGRI, DIKTI, RISTEK, PKSDA Padang) for granting the necessary research permits (Permit No.: 2921/FRP/SM/XII/08). Thanks to the Siberut Conservation Programme (SCP) for allowance to work on their field station, and to all SCP staff for very valuable field and logistical support, especially to Marcel Quinten for his reliable help and enthusiasm for the project. We thank all the people who helped tremendously during habituation and data collection, namely all field assistants (Adi Nugroho, Marliana Chrismiawati, Rahayu Oktaviani, Azhari Purbatrapsila, Rima Lamhatul Mikrimah, Betty Millyanawati), Pierre Gras who joined for his Master thesis, all field guides (P. Safrizal, P. Mathias, P. Mitchen, Bang Risel, P. Tongam, P. Teiba, P. Bitcar) and Pak Tarzan and especially Pak Nauli who helped with their local plant knowledge with the identification of the vernacular names of all plants in the botanical plots, as well as all sleeping and feeding trees. We are thankful to Arthur R. Rodgers and John Kie for advice with the Home Range Extension Tool. We like to acknowledge 4 anonymous reviewers whose detailed and helpful comments greatly improved this manuscript. CR, JO and OS received funding by the Max-Planck Society and the German Initiative of Excellence to the Georg-August University Göttingen, and CR received funding by the Evangelisches Studienwerk e. V. We acknowledge support by the Open Access Publication Funds of the University Göttingen.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JO, KH and OS conceived the study, CR and AT collected data, CR analyzed the data, AT identified the plant specimen, CR, JO and OS wrote the paper. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Richter, C., Taufiq, A., Hodges, K. et al. Ecology of an endemic primate species (Macaca siberu) on Siberut Island, Indonesia. SpringerPlus 2, 137 (2013). https://doi.org/10.1186/2193-1801-2-137
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-1801-2-137