Abstract
Aryl azides were treated with allenylmagnesium bromide to generate 1,5-disubstituted butynyl 1,2,3-triazoles in a domino fashion, which upon Cu(I) catalyzed 1,3-dipolar cycloaddition with aryl azides afforded novel bis-1,2,3-triazoles in quantitative yields. The final products were analyzed for their antimicrobial activities against a panel of bacterial and fungal strains which revealed the products to be potent antimicrobials.

Similar content being viewed by others
1. Introduction
Most of the nitrogen-containing molecules are pharmacologically very active which can be attributed to the fact that nitrogenous compounds are part and parcel of the biomolecular diversity [1–7]. Amongst the pharmacologically active nitrogenous compounds, a large number of 1,2,3-triazoles and their derivatives attracted considerable attention for the past few decades due to their chemotherapeutical value. Many 1,2,3-triazoles, including bis-triazoles, are found to be potent antimicrobial, analgesic, anti-inflammatory, local anesthetic, anti-convulsant, anti-neoplastic, anti-malarial, and anti-viral agents [8–10]. Some of them exhibited anti-proliferative, anticancer activity, and several are used as DNA cleaving agents and potassium channel activators. Such type of diverse biological functions is also reported with a variety of bis-triazoles. The 'click chemistry' approach has been the most widely used method for the synthesis of libraries of a large number of biologically active molecular frameworks particularly for the regioselective synthesis of 1,2,3-triazoles, which involves the copper(I)-catalyzed cycloaddition reaction between azides and terminal alkynes (CuAAC). This reaction has been termed as the 'cream of the crop' of 'click reactions' and has found application in various facets of drug discovery as it enables a modular approach to generate novel pharmacophores utilizing a collection of reliable chemical reactions [11, 12]. Thus, the development of the copper(I)-catalyzed 'triazole click chemistry' has led to many interesting applications including the synthesis, medicinal chemistry, molecular biology, and material science. The bioorthogonality of azide and alkynes [13] has allowed the use of their [3 + 2] cycloaddition in various biological applications including target guided synthesis [14] and activity-based protein profiling [15]. Of particular interest would be the dimeric heterocycle-based ligands which are designed for specific target interactions. Various approaches reported for the synthesis of biologically relevant bis-triazoles include Cu(I)-catalyzed 1,3-dipolar cycloaddition of monoazides with diacetylenes or that of monoacetylenes with diazides. For example, the synthesis of bis-triazoles is reported by the reactions of bis(azidomethyl)benzenes with several substituted acetylenes [16]. Recently, much attention has been paid toward the synthesis and pharmacological evaluation of triazoles and bis-triazoles as potent HIV-1 protease inhibitors [17, 18] and size-specific ligands for mRNA Hairpin loops [19], respectively. Keeping into consideration the tremendous biological potence of triazoles and bis-triazoles in general and the antimicrobial activity in particular, we, in our continuous endeavor toward the synthesis of pharmacologically active molecules, designed the synthesis of novel unsymmetrical bis-1,2,3-triazoles and then evaluated them for antimicrobial activities. The biological results obtained were very interesting and revealed most of the synthesized molecules to be potent antimicrobials.
2. Experimental
2.1. General methods
Melting points were recorded on Buchi Melting point apparatus D-545; IR spectra (KBr disks) were recorded on Bruker Vector 22 instrument. NMR spectra were recorded on Bruker DPX200 instrument in CDCl3 with TMS as internal standard for protons and solvent signals as internal standard for carbon spectra. Chemical shift values are mentioned in δ (ppm) and coupling constants are given in Hz. Mass spectra were recorded on EIMS (Shimadzu) and ESI-esquire 3000 Bruker Daltonics instrument. The progress of all reactions was monitored by TLC on 2 × 5 cm pre-coated silica gel 60 F254 plates of thickness of 0.25 mm (Merck). The chromatograms were visualized under UV 254-366 nm and iodine.
2.2.1. Chemical synthesis
2.2.1.1. General procedure for the synthesis of bis-1,2,3-triazoles (5)
To a suspension of Mg turnings (1.6 g, 0.66 mol, 10 equiv.) in specially dried THF with HgCl2 (5 mg, 1% w/w of propargyl bromide) was added propargyl bromide (3.05 ml of an 80% wt. soln. in toluene, 4 mmol, 5 equiv.) in small portions while stirring the mixture at r.t. (Note: A small grain of HgCl2 is generally required to promote formation of the reagent.) The mixture was stirred at r.t. for 2 h to give a cloudy light green solution. The allenylmagnesium bromide generated as above was cooled to 0°-5° and added dropwise to a solution of 3-methylphenyl azide (1 g, 0.007 mol) maintaining the temperature between 0 and 5°C. The mixture was allowed to attain r.t., and stirring was continued at ambient temperature for 30 min, followed by quenching with aq. NH4Cl solution (10 mL) and diluting with AcOEt (50 mL). The org. layer was separated and the aq. layer extracted with AcOEt (2 × 20 mL). The combined org. layers were dried (anh. Na2SO4) and evaporated under reduced pressure to afford crude product, which was subjected to chromatography (silica gel, 60-120 mesh, elution; hexane/AcOEt gradient) to afford pure 5-(But-3-yn-1-yl)-1-(3-methylphenyl)-1H-1,2,3-triazoles 3 as a colorless liquid. 3-Methyl butynyl triazole (10 mmol) was stirred in 5 mL of tert-butanol and H2O (1:1 mixture). CuSO4 (12 mmol) and sodium ascorbate (50 mmol) were charged into the reaction mixture. After 15 min, 3-methylphenyl azide (10 mmol) was added to the above mixture, and stirred for 8 h. The mixture was diluted with AcOEt, the org. layer was separated, and the aq. layer extracted with AcOEt (2 × 20 mL). The combined org. layers were dried (anh. Na2SO4) and evaporated under reduced pressure to afford crude product 5 (Scheme 1), which was subjected to precipitation in hexane--AcOEt, affording pure bis-triazole 5 as an amorphous brown solid (only entries 2 and 13, see Table 1) (Scheme 2).
The analytical data of all the isolated bis-triazoles is given as under.
1-(4-methoxyphenyl)-5-(2-(1-m-tolyl-1H-1,2,3-triazol-4-yl)ethyl)-1H-1,2,3-triazole (5a)
Syrupy brownish liquid. IR (KBr) cm-1: 3453, 2913, 2865, 1593, 1212, 1080, and 685; 1H NMR (CDCl3): δ 2.44 (s, 3H); 3.12 (m, 4H); 3.87 (s, 3H); 7.02 (d, 2H, J = 8.89 Hz); 7.35 (m, 6H); 7.50 (s, 1H), 7.60 (s, 1H); 13C NMR (500 MHz, CDCl3): δ 21.40, 23.44, 24.53, 55.63, 114.74, 117.53, 119.41, 121.14, 126.71, 128.00, 129.53, 138.11, 140.03, 148.22, 161.12.; ESI-MS: 383 (M+ + Na); Anal. Calcd. for C20H20N6O: C, 66.65; H, 5.59; N, 23.32; Found: C, 66.83; H, 5.38; N, 23.51.
4-(4-(2-(3-(4-methoxyphenyl)-3H-1,2,3-triazol-4-yl)ethyl)-1H-1,2,3-triazol-1-yl)benz-oic acid (5b)
Amorphous white solid. m. p. 195-197°C; IR (KBr) cm-1: 3413, 2922, 2860, 1593, 1234, 1017, and 690; 1H NMR (CDCl3): δ δ 3.10 (t, 2H, J = 5.97); 3.12 (t, 2H, J = 5.97); 3.84 (s, 3H); 7.08 (d, 2H, J = 8.90 Hz); 7.37 (d, 2H, J = 8.90 Hz); 7.72 (s,1H), 7.93 (d, 2H, J = 8.63 Hz), 8.21 (d, 2H, J = 8.90 Hz); 8.33 (s, 1H); 13C NMR (500 MHz, CDCl3): δ 22.50, 23.59, 54.45, 114.15, 115.43, 119.17, 119.21, 120.13, 126.36, 127.43, 130.78, 131.06, 147.23, 200.12; ESI-MS: 391 (M+ + H); Anal. Calcd. for C20H18N6O3: C, 61.53; H, 4.65; N, 21.53; Found: C, 61.71; H, 4.82; N, 21.79.
1-(4-methoxyphenyl)-5-(2-(1-(3-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethyl)-1H-1,2,3-triazole (5c)
Syrupy grayish semisolid. IR (KBr) cm-1: 3393, 2897, 2867, 1582, 1244, 1018, and 689; 1H NMR (CDCl3): δ 3.10 (t, 2H, J = 5.78); 3.23 (t, 2H, J = 5.78); 3.85 (s, 3H); 7.05 (d, 2H, J = 8.94 Hz); 7.38 (d, 2H, J = 8.94 Hz); 7.73-7.88 (m, 2H); 8.22-8.32 (m, 2H); 8.42 (s, 1H); 8.68 (m, 1H); 13C NMR (500 MHz, CDCl3): δ 22.20, 23.69, 55.45, 113.15, 116.43, 118.17, 119.41, 120.13, 127.36, 127.43, 130.78, 132.06, 142.33, 148.25; ESI-MS: 392 (M+ + H); Anal. Calcd. for C19H17N7O3: C, 58.31; H, 4.38; N, 25.05; Found: C, 58.54; H, 4.5; N, 25.21.
1-(4-methoxyphenyl)-5-(2-(1-p-tolyl-1H-1,2,3-triazol-4-yl)ethyl)-1H-1,2,3-triazole (5d)
Syrupy grayish semisolid. IR (KBr) cm-1: 3423, 2932, 2876, 1593, 1234, 10179, and 694; 1H NMR (CDCl3): δ 2.45 (s, 3H); 3.15 (m, 4H); 3.87 (s, 3H); 7.02 (d, 2H, J = 8.89 Hz); 7.18 (d, 2H, J = 8.89 Hz), 7.37 (d, 2H, J = 8.90 Hz); 7.47 (d, 2H, J = 8.90 Hz); 7.50 (s,1H), 7.80 (s, 1H); 13C NMR (500 MHz, CDCl3): δ 18.50, 22.23, 23.59, 54.45, 114.15, 115.43, 119.17, 119.51, 120.13, 126.36, 127.33, 130.78, 135.06, 147.23; ESI-MS: 383 (M+ + Na); Anal. Calcd. for C20H20N6O: C, 66.65; H, 5.59; N, 23.32; Found: C, 66.42; H, 5.68; N, 23.54.
1-(3-chlorophenyl)-5-(2-(1-p-nitrophenyl-1H-1,2,3-triazol-4-yl)ethyl)-1H-1,2,3-triazole (5e)
Syrupy grayish semisolid. IR (KBr) cm-1: 3411, 2945, 2898, 1597, 1265, 1067, and 702; 1H NMR (CDCl3): δ 3.17-3.40 (m, 4H); 7.34-7.57 (m, 2H); 7.77 (m, 2H), 7.86 (s, 1H); 8.17 (d, 2H, J = 7.85 Hz); 8.30 (d, 2H, J = 7.85), 8.55 (s, 1H); 13C NMR (500 MHz, CDCl3): δ 19.25, 21.23, 23.56, 114.15, 115.43, 119.17, 119.51, 120.13, 126.36, 128.33, 130.87, 136.06, 146.23; ESI-MS: 418 (M+ + Na); Anal. Calcd. for C18H14ClN7O: C, 54.62; H, 3.57; N, 24.77; Found: C, 54.85; H, 3.38; N, 24.86.
1-(3-nitrophenyl)-5-(2-(1-m-tolyl-1H-1,2,3-triazol-4-yl)ethyl)-1H-1,2,3-triazole (5f)
Syrupy brownish semisolid. IR (KBr) cm-1: 3417, 2954, 2856, 1587, 1235, 1079, and 698; 1H NMR (CDCl3): δ 2.44 (s, 3H); 3.17-3.25 (m, 4H); 7.39 (d, 2H, J = 7.40 Hz); 7.50 (s, 2H), 7.70-7.82 (m, 4H); 8.36 (m, 2H); 13C NMR (500 MHz, CDCl3): δ 19.54, 21.23, 23.59, 114.15, 116.41, 119.17, 119.51, 120.13, 128.32, 127.35, 130.88, 136.06, 146.23, 153.22.; ESI-MS: 398 (M+ + Na); Anal. Calcd. for C19H17N7O2: C, 60.79; H, 4.56; N, 26.12; Found: C, 60.95; H, 4.72; N, 26.34.
1-(3-nitrophenyl)-5-(2-(1-(3-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethyl)-1H-1,2,3-triazole (5g)
Syrupy brownish semisolid. IR (KBr) cm-1: 3419, 2965, 2857, 1580, 1265, 1099, and 714; 1H NMR (CDCl3): δ 3.21 (t, 2H, J = 5.44); 3.30 (t, 2H, J = 5.44); 7.79 (m, 4H, J = 7.40 Hz); 7.87 (s, 1H), 8.15 (d, 2H, J = 7.82 Hz); 8.38 (m, 2H), 8.54 (s, 1H); 13C NMR (500 MHz, CDCl3): δ 19.47, 21.34, 22.89, 114.15, 116.67, 119.77, 118.52, 120.13, 127.32, 128.35, 130.32, 136.18, 146.56, 154.31; ESI-MS: 407 (M+ + H); Anal. Calcd. for C18H14N8O4: C, 53.20; H, 3.47; N, 27.57; Found: C, 53.11; H, 3.62; N, 27.34.
5-(2-(1-(3-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethyl)-1-o-tolyl-1H-1,2,3-triazole (5h)
Syrupy brownish semisolid. IR (KBr) cm-1: 3418, 2954, 2856, 1587, 1235, 1079, and 698; 1H NMR (CDCl3): δ 2.36 (s, 3H); 3.09 (m, 4H); 7.22 (m, 2H); 7.43 (m, 4H), 7.68 (s, 1H); 7.71-7.84 (m, 2H), 8.17 (d, 1H, J = 7.66); 13C NMR (500 MHz, CDCl3): δ 18.54, 21.23, 23.59, 114.15, 117.41, 119.17, 119.51, 120.13, 128.32, 127.35, 130.88, 136.06, 146.23, and 155.23; ESI-MS: 398 (M+ + Na); Anal. Calcd. for C19H17N7O2: C, 60.79; H, 4.56; N, 26.12; Found: C, 60.95; H, 4.72; N, 26.34.
1-o-tolyl-5-(2-(1-m-tolyl-1H-1,2,3-triazol-4-yl)ethyl)-1H-1,2,3-triazole (5i)
Syrupy brownish semisolid. IR (KBr) cm-1: 3417, 2944, 2856, 1587, 1235, 1079, and 695; 1H NMR (CDCl3): δ 2.07 (s, 3H); 2.15 (s, 3H); 3.12 (m, 2H); 7.54 (m, 2H); 7.63 (s, 1H); 7.74 (m, 3H), 8.08-8.23 (m, 3H); 8.57 (s,1H); 13C NMR (500 MHz, CDCl3): δ 19.68, 19.19, 21.58, 23.38, 116.10, 121.14, 122.22, 124.7, 129.82, 130.30, 131.31, 132.0, 135.80, 136.80, 137.83, 140.01, 148.20; ESI-MS: 367 (M+ + Na); Anal. Calcd. for C20H20N6: C, 69.75; H, 5.85; N, 24.40; Found: C, 69.90; H, 5.52; N, 24.61.
5-(2-(1-(2-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethyl)-1-p-tolyl-1H-1,2,3-triazole (5j)
Syrupy greyish semisolid. IR (KBr) cm-1: 3427, 2966, 2865, 1576, 1235, 1079, and 687; 1H NMR (CDCl3): δ 2.45 (s, 3H); 3.21-3.23 (m, 4H); 7.38-7.45 (d, 2H, J = 8.00 Hz); 7.70 (d, 2H, J = 8.00 Hz), 7.81 (m, 1H); 7.90 (m, 3H), 8.14 (m, 2H); 13C NMR (500 MHz, CDCl3): δ 19.85, 23.00, 23.54, 125.19, 125.27, 127.31, 129.71, 129.97, 130.89, 133.80, 134.97, 140.39, 144.60; ESI-MS: 367 (M+ + Na); Anal. Calcd. for C19H17N7O2: C, 60.79; H, 4.56; N, 26.12; Found: C, 60.61; H, 4.72; N, 26.29.
5-(2-(1-(3-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethyl)-1-p-tolyl-1H-1,2,3-triazole (5k)
Syrupy greyish semisolid. IR (KBr) cm-1: 3417, 2986, 2865, 1576, 1233, 1089, and 677; 1H NMR (CDCl3): δ 2.45 (s, 3H); 3.08-3.22 (m, 4H); 7.33 (s, 4H); 7.50 (s, 1H), 7.52-7.72 (m, 4H), 7.89 (d, 1H, J = 7.89 Hz); 13C NMR (500 MHz, CDCl3): δ 15.82, 21.24, 23.33, 118.90, 122.73, 125.56, 130.23, 132.51, 133.69, 133.80, 135.61, 136.81, 139.97, 142.41, 144.43, 151.16; ESI-MS: 398 (M+ + Na); Anal. Calcd. for C19H17N7O2: C, 60.79; H, 4.56; N, 26.12; Found: C, 60.92; H, 4.30; N, 26.26.
1-m-tolyl-5-(2-(1-m-tolyl-1H-1,2,3-triazol-4-yl)ethyl)-1H-1,2,3-triazol (5l)
Amorphous brown solid; m.p. 175°C. IR (KBr) cm-1: 3429, 3138, 2922, 2860, 1612, 1593, 1549, 1494, 1383, 1234, 1165, 1089, 1047, 1017, 980, 873, 849, 786, 690, and 618; 1H NMR (CDCl3): 2.33 (s, 3H); 2.43 (s, 3H); 3.05 (t, 2H, J = 6.2 Hz); 3.20 (t, 2H, J = 6.2 Hz); 7.25-7.59 (m, 8H); 7.73 (s, 1H); 8.36 (s, 1H). 13C NMR (500 MHz, CDCl3): 19.8, 19.9, 22.8, 23.8, 117.0, 120.4, 122.2, 125.7, 129.1, 129.8, 130.3, 132.0, 135.8, 136.8, 137.8, 140.0, 146.2. ESI-MS: 367 (M+ + Na). Anal. calc. for C20H20N6 : C, 69.75; H, 5.85; N, 24.40; Found: C, 69.80; H, 5.82; N 24.51.
2.2.2. Biology
The bacterial strains used for the analysis were Bacillus subtilis (MTCC 619), Staphylococcus epidermidis (MTCC 435), Proteus vulgaris (MTCC 426), and Pseudomonas aeruginosa (MTCC 424). The fungal strains used were Aspergillus niger (MTCC 1344) and Penicillium chrysogenum (MTCC 947). All the bacterial and fungal strains were obtained from The Microbial Type Culture Collection and Gene Bank (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, India. Kenamycin and flucanazole were used as standard antibacterial and antifungal substances, respectively, under similar conditions for comparison. Dimethyl sulfoxide (DMSO) was used as negative control.
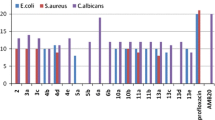
The test organisms were cultured on agar slants, incubated 24 h at 37 ± 0.5°C and 24-48 h at 27 ± 0.2°C for bacteria and fungi, respectively, to get the freshly prepared cultures. The steroidal derivatives were evaluated for antimicrobial activity against these freshly prepared strains of test organisms by agar diffusion method [20, 21]. Muller Hinton Agar (MHA) and Potato Dextrose Agar (PDA) were used as nutrient media for bacterial and fungal strains, respectively. The media (MHA &PDA) were prepared using distilled water and 20 mL of it was transferred into 50-mL test tubes, the test tubes were tightly plugged with cotton and sterilized in autoclave at 15 lb/in2 for 15 min as directed by the manufacturer. After sterilization, the medium was inoculated with freshly cultured bacterial strains under sterile condition, i.e., under Laminar Flow. The inoculation was done when the temperature of the medium reached 50-40°C, so that test organism may not die at higher temperature. The medium inoculated with test microorganisms was transferred into the plates of 90-mm size under sterile conditions. The medium was allowed to solidify and the wells (4/plate) of 6-mm-diameter and 50 μL volume were bored on it using sterile cork borer. The solution of test compound 1000 μg/mL was prepared in DMSO and the wells bored on the medium were each filled (50 μg) with test compound using micropipette (20-200 μL). Four wells were bored on the plates and each filled with same compound and two plates for each test compound were taken and the experiment was repeated twice. The disks of Kenamycin and Flucanazole were also incorporated into the medium for comparison (10-30 μg). The plates containing test organism and test material in contact were incubated at 37 ± 0.5°C for 24 h. Same procedure was employed for antifungal activity; however, the culture strains of fungi were maintained on PDA and spores were transferred into the PDA medium and the plates were incubated at 27 ± 0.2°C for 24-48 h. Inhibition of growth of test organisms (bacterial & fungal) in presence of test material and standard was measured with the help of standard scale and the mean values of inhibition zones are reported in Table 2.
Table 2 gives the antimicrobial screening data obtained after treating different microbial strains with test doses of the different bis-triazolyl derivatives and the values are reported in terms of zone of inhibition in "mm".
It is clear from the above data that all the compounds 3a-l showed significant antimicrobial activity against all microbial strains used for testing. It is evident from the data that even the position of substituent on the aromatic ring influences the relative activity which can be attributed to their differences in either the bioavailability or the protein-binding properties.
3. Results and discussions
The wide range of pharmacological activities especially the antimicrobial potential [14, 22–27] of triazole and bis-triazole systems prompted us to design the synthesis of a library of unsymmetrical bis-1,2,3-triazoles based on a stepwise synthetic route involving domino addition of allenylmagnesium bromide to aryl azides resulting in a serendipitous formation of 5-butynylated triazoles in good yields (> 70%) instead of 4-butynylated triazoles. 5-Butynylated triazoles upon Cu(I) catalyzed 1,3-dipolar cycloaddition with aryl azides generated bis-1,2,3-triazoles in quantitative yields. The products together with the approach for their synthesis being novel, the intermediate 5-butynylated triazoles and the final product, the bistriazole, were characterized by IR, 1H/13C NMR, and mass spectral analysis. The intermediate 3 undergoes a high yielding regioselective Cu(I) catalyzed 1,3-dipolar cycloaddition with aryl azides (click reaction) to afford quantitative yields of the product, i.e., bis-1,2,3-triazoles, which were isolated in pure form after precipitation. The isolated products were evaluated for their antimicrobial activities against a panel of bacterial and fungal cell lines. The biological results were highly encouraging paving a way for the futuristic medicinal chemistry work based on these scaffolds.
4. Conclusion
In conclusion, we have developed an unprecedented, convenient strategy for the synthesis of novel, biologically important bis-1,2,3-triazoles employing a domino reaction followed by the copper catalyzed 'click' protocol. The products thus obtained were found to be potent antimicrobial agents.
References
Wahe H, Asobo PF, Chekasov RA, Fomum ZT, Doepp D: Heterocycles of biological importance: Part 8.1. Formation of pyrimido[1,2- a ] benzimidazoles and oxazolo[3,2- a ]benzimidazoles by conjugate addition of 2-aminobenzimidazoles to 4-hydroxy-2-alkynenitriles. ARKIVOC 2004,2004(i):130–137.
Chauhan PM, Srivastava SK: Recent developments in the combinatorial synthesis of nitrogen heterocycles using solid phase technology. Comb Chem High Throughput Screen 2001,4(1):35–51.
Larock RC, Babu S: Synthesis of nitrogen heterocycles via palladium-catalyzed intramolecular cyclization. Tetrahedron Lett 1987, 28: 5291. 10.1016/S0040-4039(00)96710-8
Banday AH, Bhupinder SA, Alam MS, kumar HMS: A novel 'Domino-Click approach' to unsymmetrical bis-triazoles. Helv Chem Acta 2007,90(12):2368–2374. 10.1002/hlca.200790242
Butler MS: The Role of Natural Product Chemistry in Drug Discovery? J Nat Prod 2004, 67: 2141. 10.1021/np040106y
Martin R, Rivero MR, Buchwald SL: A general, highly flexible Cu-catalyzed domino C-N coupling/hydroamination reaction constitutes a straightforward alternative to existing methodology for the preparation of pyrroles and pyrazoles. Angew Chem Int Ed 2006, 45: 7079–7082. 10.1002/anie.200602917
Srinivasan M, Perummal S: (l)-Proline-catalysed novel tandem reactions of 1-substituted piperidin-4-ones with (E)-4-arylbut-3-en-2-ones: N-substituent mediated product selectivity and synthesis of novel nitrogen heterocycles. Tetrahedron 2007,63(13):2865–2874 and the references cited therein. 10.1016/j.tet.2007.01.038
Modzelewska BB, Jagiello WE: Synthesis and biological activity of BIS-1,2,4-triazole and BIS-1,3,4-thiadiazole derivatives. Acta Pol Pharm 2000,57(3):199–204.
Jin JY, Zhang LX, Chen XX, Zhang AJ, Zhang HL: Syntheses and Biological Activities of 6-Aryl-3-(3-hydroxy- propyl)-7H-1,2,4-triazolo[3,4-b][1,3,4]thiadiazines. Molecules 2007, 12: 297–303. 10.3390/12030297
Sanghvi YS, Bhattacharya BK, Kini GD, Matsumoto SS, Larson SB, Jolley WB, Robins RK, Revankar GR: Growth inhibition and induction of cellular differentiation of human myeloid leukemia cells in culture by carbamoyl congeners of ribavirin. J Med Chem 1990, 33: 336. 10.1021/jm00163a054
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB: A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective Ligation of Azides and Terminal Alkynes. Angew Chem Int Ed 2002, 41: 2596. 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4
Wang Q, Chan RC, Hilgraf R, Fokin VV, Sharpless KB, Finn MG: Bioconjugation by Copper(I)-Catalyzed Azide-Alkyne [3 + 2] Cycloaddition. J Am Chem Soc 2003, 125: 3192. 10.1021/ja021381e
Kolb HC, Sharpless KB: The growing impact of click chemistry on drug discovery. Drug Discov Today 2003, 8: 1128. 10.1016/S1359-6446(03)02933-7
Lewis WG, Green LG, Grynszpan F, Radic Z, Carlier PR, Taylor P, Finn MG, Sharpless KB: Click Chemistry In Situ: Acetylcholinesterase as a Reaction Vessel for the Selective Assembly of a Femtomolar Inhibitor from an Array of Building Blocks. Angew Chem Int Ed 2002, 41: 1053.
Speers AE, Adam GC, Cravatt BF: Activity-Based Protein Profiling in Vivo Using a Copper(I)-Catalyzed Azide-Alkyne [3 + 2] Cycloaddition. J Am Chem Soc 2003, 125: 4686. 10.1021/ja034490h
Abu-Orabi TS, Atfah MA, Jibril I, Marii F, Ali AS: Dipolar Cycloaddition Reactions of Organic Bisazides with Some Acetylenic Compounds. Gazz Chim Ital 1991, 121: 397.
Whiting M, Tripp JC, Lin YC, Lindstorm W, Olson AJ, Elder JH, Sharpless KB, Fokin VV: Rapid Discovery and Structure?Activity Profiling of Novel Inhibitors of Human Immunodeficiency Virus Type 1 Protease Enabled by the Copper(I)-Catalyzed Synthesis of 1,2,3-Triazoles and Their Further Functionalization. J Med Chem 2006, 49: 7697. 10.1021/jm060754+
Whiting M, Muldoon J, Lin YC, Silverman SM, Lindstrom W, Olson AJ, Kolb HC, Finn MG, Sharples KB, Elder JH, Fokin VV: Inhibitors of HIV-1 Protease by Using In Situ Click Chemistry. Angew Chem Int Ed 2006, 45: 1435. 10.1002/anie.200502161
Thomas JR, Liu X, Hergenrother PJ: Size-Specific Ligands for RNA Hairpin Loops. J Am Chem Soc 2005, 127: 12434. 10.1021/ja051685b
Dawane BS, Konda SG, Shaikh BM, Chobe SS, Khandare NT, Kamble VT, Bhosale RB: Synthesis and in vitro antimicrobial activity of some new 1-thiazolyl-2-pyrazoline derivatives. Int J Pharm Sci Rev Res 2010, 1: 2.
Gurubasavaraja PMS, Agasimundin YS: Synthesis and antimicrobial activity of some novel chalcones containing 3-hydroxy benzofuran. Acta Pharmaceutica Sciencia 2008, 50: 197–202.
Brik A, Alexandratos J, Lin YC, Elder JH, Olson AJ, Wlodawer A, Goodsell DS, Wong CH: 1,2,3-Triazole as a Peptide Surrogate in the Rapid Synthesis of HIV-1 Protease Inhibitors. ChemBioChem 2005, 6: 1167. 10.1002/cbic.200500101
Shafi S, Banday AH, Ismail T, Kumar HMS: Domino addition/N-C heterocyclization of Azides with allenyl magnesium bromide: Rapid synthesis of 5-butynyl- 1,2,3-triazoles. Synlett 2007, 7: 1109–1111.
Talekar RR, Wightman RH: Synthesis of some pyrrolo[2,3-d]pyrimidine and 1,2,3-triazole isonucleosides. Tetrahedron 1997, 53: 3831. 10.1016/S0040-4020(97)00102-6
Bertelli L, Biagi G, Giorgi I, Manera C, Livi O, Scartoni V, Betti L, Giannaccini G, Trincavelli L, Barili PL: 1,2,3-Triazolo[1,5-a]quinoxalines: synthesis and binding to benzodiazepine and adenosine receptors. Eur J Med Chem 1998, 33: 113. 10.1016/S0223-5234(98)80036-6
Contelles JM, Fernandez MR: Novel synthesis of 2-thiazolines. Tetrahedron Lett 2000, 41: 381. 10.1016/S0040-4039(99)01981-4
Fox PG, Lewis G, Boden PJ: Some chemical aspects of the corrosion inhibition of copper by benztriazole. Corros Sci 1979, 4: 425.
Acknowledgements
The authors thank the Principal, ICSC, for his interest and encouragement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Banday, A.H., Shameem, S.A. & Ganai, B.A. Antimicrobial studies of unsymmetrical bis-1,2,3-triazoles. Org Med Chem Lett 2, 13 (2012). https://doi.org/10.1186/2191-2858-2-13
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2191-2858-2-13