Abstract
Background
The characterization of fast-decaying radiotracers that are labeled with carbon-11 (t 1/2 = 20.38 min), including critical measurement of specific radioactivity (activity per mole at a specific time) before release for use in positron-emission tomography (PET), has relied heavily on chromatographic plus radiometric measurements, each of which may be vulnerable to significant errors. Thus, we aimed to develop a mass-specific detection method using sensitive liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) for identifying 11C-labeled tracers and for verifying their specific radioactivities.
Methods
The LC-MS/MS was tuned and set up with methods to generate and measure the product ions specific for carbon-11 species and M + 1 carrier (predominantly the carbon-13 isotopologue) in four 11C-labeled tracers. These radiotracers were synthesized and then analyzed before extensive carbon-11 decay. The peak areas of carbon-11 species and M + 1 carrier from the LC-MS/MS measurement and the calculated abundances of carbon-12 carrier and M + 1 radioactive species gave the mole fraction of carbon-11 species in each sample. This value upon multiplication with the theoretical specific radioactivity of carbon-11 gave the specific radioactivity of the radiotracer.
Results
LC-MS/MS of each 11C-labeled tracer generated the product ion peaks for carbon-11 species and M + 1 carrier at the expected LC retention time. The intensity of the radioactive peak diminished as time elapsed and was undetectable after six half-lives of carbon-11. Measurements of radiotracer-specific radioactivity determined solely by LC-MS/MS at timed intervals gave a half-life for carbon-11 (20.43 min) in excellent agreement with the value obtained radiometrically. Additionally, the LC-MS/MS measurement gave specific radioactivity values (83 to 505 GBq/μmol) in good agreement with those from conventional radiometric methods.
Conclusions
11C-Labeled tracers were characterized at a fundamental level involving isolation and mass detection of extremely low-abundance carbon-11 species along with the M + 1 carrier counterpart. This LC-MS/MS method for characterizing fast-decaying radiotracers is valuable in both the development and production of PET radiopharmaceuticals.
Similar content being viewed by others
Background
Positron-emission tomography (PET), as an expanding biomedical research and diagnostic imaging technique, relies on the use of radiotracers labeled with a positron emitter, often either carbon-11 (t 1/2 = 20.38 min) or fluorine-18 (t 1/2 = 109.7 min) [1–3]. The short physical half-lives of these radiotracers demand rapid techniques for establishing the quality of each production batch before release. A suitable technique for this purpose must conclusively identify the radiotracer and also provide specific radioactivity (SA), the ratio of the amount of radioactive compound (GBq) to the amount of compound (mol) in radioactive and non-radioactive forms (isotopologues) at a specific measurement time. High-performance liquid chromatography (HPLC) is almost universally applied to assure radiotracer identity and to measure radiochemical purity and SA at the end of each radiotracer synthesis [4, 5]. Typically, 11C-labeled tracers are produced with only a low degree of 11C enrichment (<0.1%) [1], representing a SA value on the order of 100 GBq/μmol. Nevertheless, an accurate determination of SA is critical in many applications especially where the radiotracer is intended to bind to low-density protein targets, such as neurotransmitter receptors, enzymes or transporters, or in specific cases where the radiotracer is pharmacologically very potent, such as some radiotracers for dopamine, nicotinic, or opiate receptors [6–8].
The identification of a PET radiotracer with HPLC relies on comparison of its retention time (t R) measured with a radioactivity detector with that of the reference non-radioactive compound measured with another detector, often an absorbance detector. In conventional measurement of the SA of a radiotracer, the detector giving the mass response is calibrated for compound mass, and the radioactivity associated with a particular carrier mass peak is measured in a calibrated detector, invariably a dose calibrator (an ionization detector). Surrogate radioisotopes (e.g., 137Cs, 57Co) are often used to calibrate ionization detectors for measuring positron emitters. The accuracy of measurements is highly vulnerable to errors in instrument settings, and to changes in sample containers and geometries, as demonstrated in several studies with fluorine-18 [9–14]. Similar issues will pertain to shorter-lived carbon-11, although this has not been well studied [15]. In addition, the presence of unknown inadvertent impurities in the radiotracer may compromise the mass measurements and hence the accuracy of derived SA. Hitherto, no non-radiometric method for verifying conventional HPLC-radiometric measurements of 11C-labeled tracer SA has been reported.
We recognized the potential of a highly sensitive mass spectrometric technique for developing a reliable method to characterize fast-decaying PET radiotracers. Such a method seemed practical using triple quadrupole liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) because its specificity, sensitivity, and detection range were envisaged to be adequate for measuring both the radioactive species and the carrier counterpart. Additionally, the MS/MS can generate, isolate, and measure ions specifically for the positron-emitting species in the presence of a thousandfold excess carrier. Here, we describe the successful use of sensitive LC-MS/MS alone to identify and measure t 1/2 and the SA of 11C-labeled tracers. The SA values obtained by this new method are compared with those from HPLC-radiometric methods. The LC-MS/MS method was found especially useful for verifying the validity of HPLC-radiometric methods.
Methods
Radiotracers
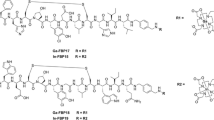
The PET radiotracers used were 11C]PBR28 (radioligand for translocator protein (18 kDa)) [16, 17], 11C]dLop (radiotracer of P-glycoprotein function) [18, 19], 11C]MePPEP (cannabinoid subtype 1 receptor radioligand) [20, 21], and 11C](R)-rolipram (radioligand for cAMP phosphodiesterase IV) [22] (Figure 1A,B,C,D). Each radiotracer was prepared in high radiochemical purity (>99%) and formulated in sterile saline solution, as described previously [16–22].
HPLC-radiometric measurement of SA
Freshly prepared and formulated radiotracer solution (100 μL), contained in a syringe, was measured for radioactivity in a calibrated ionization chamber (Atomlab 300, Biodex, Shirley, NY, USA) and then injected onto HPLC (Beckman, Fullerton, CA, USA). The radiotracer was eluted isocratically (acetonitrile(aq) 10 to 100 mM HCO2NH4 or 0.1% CF3CO2H) on a reverse-phase column (Onyx, Prodigy, or Luna, Phenomenex, Torrance, CA, USA) equipped with radioactivity and absorbance detectors. The mass of the carrier in the injectate was determined from a linear calibration curve generated from injections of known amounts of authentic non-radioactive standards. The SA was calculated from the decay-corrected activity present in the same volume of injectate, as measured in the calibrated ionization chamber.
LC-MS/MS
Methods for radiotracer analyses were developed on an API 5000 LC-MS/MS system (AB Sciex, Foster City, CA, USA), consisting of Shimadzu LC (Columbia, MD, USA) interfaced via electrospray with triple quadrupole MS/MS operated in positive ionization mode. The technique principally involves quantitative measurement of two ions that differ by two mass-to-charge ratio (m/z) units, one for the 11C-labeled species, denoted [11C]species, and another for the carrier, denoted [M + 1]carrier. [M + 1]carrier ions are generated from isotopologues having predominantly one carbon-13 atom or another heavy stable isotope (2H, 15N, or 17O) of very low abundance. Thus, for example, a solution of PBR28 was infused into the MS/MS, and, upon recording the product ions, compound-dependent and gas parameters were optimized for the m/z 349 [M + H]+ → 121 transition for monitoring [12C]species. An acquisition method for [11C]PBR28 was set up using the same instrument parameters and with the multiple-reaction monitoring table being edited to perform transitions, m/z 348 → 120 and m/z 350 → 122 for measuring [11C]species and [M + 1]carrier, respectively (Figure 1A).
Similarly, MS/MS was tuned with dLop, MePPEP, and (R)-rolipram involving transitions m/z 463 [M + H]+ → 252, m/z 455 [M + H]+ → 351, and m/z 276 [M + H]+ → 191, respectively. Methods were set up to acquire product ions to measure [11C]species and [M + 1]carrier in these radiotracers. The dwell time in these methods ranged between 100 and 175 ms. In each method, the possible cross-talk between transitions was verified by inserting a dummy [M + H]+ → product ion transition between those for [11C]species and [M + 1]carrier. The masses for the dummy transition differed by 5 to 10 amu from those for target ions. Each compound's product ion (Figure 1) was further verified by LC-MSn (n = 2, 3) analysis in an ion-trap mass analyzer (LCQ Deca, Thermo Scientific, San Jose, CA, USA).
Each radiotracer sample was diluted four- to tenfold, and 2 to 5 μL of solution (<500 kBq) was injected with an autosampler (n = 6 or 12) at about 5-min intervals onto LC-MS/MS. Samples of [11C]PBR28, [11C]dLop, and [11C]MePPEP were chromatographed at 40°C on a C18 column (2 × 20 mm, 3 μm; Phenomenex) with a gradient of binary solvents (A/B; 400 μL/min), where A was 10 mM MeCO2NH4 in water/acetonitrile (90:10 v/v) and B was 10 mM MeCO2NH4 in acetonitrile/water (90:10 v/v). In the analysis of [11C]PBR28, for example, the pumps ran 70% A:30% B for 0.1 min and then a gradient reaching 20% A:80% B over 1 min. After 3.5 min, the mobile-phase composition was returned to the initial condition. [11C](R)-Rolipram was chromatographed on the same column eluted with a gradient of water (A) and acetonitrile (B) (A/B; 0.2% acetic acid): 80% A:20% B for 0.1 min, reaching 20% A:80% B over 2 min.
The relative abundance of the M + 1 peak was measured by injecting a solution of PBR28 onto the LC-MS/MS setup to perform m/z 349, 350 [M + H]+ → 121, 122 (12C, M + 1) transitions and thereby to provide m/z 122 to 121 peak area ratio (%). Other compounds were similarly analyzed using respective acquisition methods edited to monitor product ions of 12C and M + 1 species. The measured abundance (%) of M + 1 species relative to 12C was used for converting M + 1 into 12C peak area for radiotracer carrier. The peak area ratio (%) of M + 2 to M + 1 was measured by injecting 40 and 400 pg of PBR28 and acquiring transitions m/z 350, 351 → 122, 123.
Calculations
The SA of the radiotracer (Bq/mol) was calculated as
where (1) A* M,M+1 is the sum of the measured 11C, M]species peak area and 11C, M + 1]species area calculated from the relative abundances [23] of 13C, 2H, 15N, and 17O in the product ion; (2) A M+1,M is the sum of the measured [M + 1]carrier peak area and 12C]carrier area calculated from the measured M + 1 abundance in the non-radioactive tracer; and (3) SATheoretical is the theoretical SA of carbon-11 (3.413 × 1020 Bq/mol), the product of ln2/t 1/2 and Avogadro's number. The SA of the radiotracer was decay-corrected to give the SA at the end of radiosynthesis (SA0) as SAeλt, where t is the time between the end of synthesis and peak elution in LC-MS/MS and λ is 0.034 min-1 (ln2/20.38). A plot of lnSA (n = 12) versus clock time of peak elution in Prism 5.02 (GraphPad, La Jolla, CA, USA) gave the value for λ and thus t 1/2 (t 1/2 = ln2/λ).
Results
In order to exemplify the non-radiometric LC-MS/MS method that we developed for identifying and measuring the SA of 11C-labeled tracers, we report results on four radiotracers applied in human PET studies, namely [11C]PBR28, [11C]dLop, [11C]MePPEP, and [11C](R)-rolipram (Figure 1). We illustrate the development of the procedure with the example of [11C]PBR28. In the MS/MS mode of a triple quadrupole analyzer, the [M + H]+ ion of non-radioactive PBR28 was dissociated and then one of the transitions, m/z 349 [M + H]+ → 121, suitable for monitoring [11C]species and [M + 1]carrier in [11C]PBR28 was selected, and instrument parameters were optimized. The MS/MS of PBR28 in an ion-trap analyzer also yielded the product ion, m/z 121, in high abundance. In the same mass analyzer, another dissociation (MS3) involving this target ion generated secondary product ions that were consistent with the genesis of the m/z 121 ion and thus the respective [11C]species and [M + 1]carrier ions by benzylic cleavage, as shown in Figure 1A.
LC-MS/MS of non-radioactive PBR28 showed transitions m/z 349 [M + H]+ → 121 (12C) and 350 [M + H]+ → 122 (M + 1) associated with a peak eluting at 1.61 min on a short reverse-phase LC column. The measured relative abundance (8.88%) of the m/z = 122 for M + 1 species was close to the value (8.83%) calculated for the product ion formed by the benzylic cleavage of the [M + H]+ ion of PBR28 (Figure 1A). Subsequently, the MS/MS acquisition method was edited to monitor the m/z 348 → 120 transition for [11C]species in [11C]PBR28. As expected, a similar LC-MS/MS analysis of non-radioactive PBR28 showed no peak for an m/z 348 → 120 transition. Furthermore, analysis of a fully decayed preparation of [11C]PBR28 showed no peak for this transition. Thus, carrier PBR28 did not generate a peak due to the m/z 120 ion or any other spurious peak when performing the m/z 348 → 120 transition. Moreover, a dummy transition, entered between the transitions for [11C]species and [M + 1]carrier, showed no cross-talk during the acquisition.
The possibility that coeluting carrier PBR28 might affect the ionization of [11C]PBR28 was tested by injecting high (400 pg) and low (40 pg) amounts of PBR28 into the LC-MS/MS and acquiring the product ions from [M + H]+ of the M + 1 and M + 2 species. The peak area ratio (%; mean ± standard deviation (SD); n = 3) of M + 2 to M + 1 was 6.64 ± 0.02 at 400 pg and 6.52 ± 0.09 at 40 pg of PBR28. These ratios agreed well with the calculated M + 2 to M + 1 ratio of 6.56%. Thus, excess [12C]carrier did not cause either [11C]species or [M + 1]carrier to ionize differently, and their ion currents were therefore expected to represent their respective concentrations.
MS/MS of a sample of freshly prepared [11C]PBR28 (approximately 400 kBq) detected an m/z 348 → 120 transition specific for the [11C]species and m/z 350 → 122 for the constituent [M + 1]carrier. The LC peaks (Figure 2A,B) due to the product ions, m/z 120 and 122 for [11C]species and [M + 1]carrier, respectively, appeared in the ion chromatogram at the same t R as reference PBR28. As shown with ion chromatogram overlays, the peak for the [11C]species with m/z = 120 diminished in intensity and was undetectable after elapse of about six t 1/2 of carbon-11. The intensity of the peak for [M + 1]carrier remained constant throughout the analysis, as expected. Mass detection by MS/MS of [11C]species along with [M + 1]carrier as LC peaks at the t R of PBR28 confirmed the identity of the [11C]species including the position of the carbon-11 atom as being in the methoxy group of the methoxybenzyl moiety (Figure 1A).
LC-MS/MS methods were similarly set up for the characterization of [11C]species and [M + 1]carrier in [11C]dLop, [11C]MePPEP, and [11C](R)-rolipram preparations. Analysis of [11C]dLop showed LC peaks at t R = 2.02 min due to the product ions m/z 251 and 253 of [11C]species and [M + 1]carrier, respectively (Figure 1). [11C]MePPEP showed LC peaks at t R = 2.19 min arising from the acquisition of product ions m/z 350 and 352 of [11C]species and [M + 1]carrier, respectively. Similarly, analysis of [11C](R)-rolipram showed LC peaks at t R = 1.96 min upon acquiring the product ions m/z 190 and 192 of [11C]species and [M + 1]carrier, respectively.
The LC-MS/MS measurements of [11C]species and carrier gave the mole fraction of the [11C]species, which, upon multiplication with the theoretical SA of carbon-11, gave the SA of the radiotracer (Equation 1). In calculating the mole fraction of [11C]species, the measured peak area for [M + 1]carrier was converted into that for [12C]carrier using the previously measured [M + 1]/12C ratio in PBR28, dLop, MePPEP, or (R)-rolipram. This indirect measurement had the benefit of neatly circumventing possible saturation of the detector by [12C]carrier at the concentration of the radiotracer needed for measuring [11C]species. Also, in this method, the masses of acquired ions differed by two units, and any contribution of [11C, M + 2]species to the [M + 1]carrier abundance would therefore have been negligible.
As examples, the measured peak areas of [11C]species and [M + 1]carrier and the derived peak areas used for calculating SA are shown for [11C]PBR28 and [11C](R)-rolipram in Table 1. These data show that the peak area and area ratio for [11C]species and the SA were halved after elapse of one t 1/2 of carbon-11 while the peak area for [M + 1]carrier remained constant, as expected. The measurement of [11C]species and [M + 1]carrier is quantitative as reflected in the decay-corrected SA values. The area ratio 7.9240 × 10−4 for [11C]PBR28 implies that 1 out of 1,262 molecules was labeled with carbon-11 at the time of MS/MS detection. This ratio is consistent with the low degree of radionuclide enrichment found in 11C-labeled preparations. Also, in Table 1, the peak areas for [11C](R)-rolipram are for a preparation injected onto LC-MS/MS after elapse of 30.88 and 52.38 min from the end of synthesis. The comparable decay-corrected SA values indicate good accuracy of the measurement even after elapse of two t 1/2 of the radiotracer.
The accuracy of the LC-MS/MS method for measuring the SA of PET radiotracers was further supported through monitoring the loss of mass of 11C]species as the radionuclide decayed. Three preparations each of 11C]PBR28 and 11C](R)-rolipram and two of 11C]dLop were analyzed by injecting each 12 times at known intervals of about 5 min. The SA of the radiotracer was calculated for each injection at the clock time of the peak elution in the ion chromatogram. Plots of the natural logarithm of SA versus measurement time gave straight lines (r2 > 0.99) as shown, for example, for a 11C]PBR28 preparation (Figure 3). In each case, the decay constant λ for carbon-11 was obtained from the negative slope of the regression equation (y = −λx + b). The t 1/2 (t 1/2 = ln2/λ) of carbon-11 was estimated from these decay constants to be 20.43 ± 0.24 min (mean ± SD; n = 8), which is in excellent agreement with the accepted value of 20.38 min ascertained by measuring β+ emission [24]. The accuracy of these t 1/2 measurements with LC-MS/MS implies that the measured peak areas of decaying 11C]species and the stable [M + 1]carrier truly represent their concentrations.
Decay-corrected SA was measured with the LC-MS/MS method on three preparations of each of the four radiotracers (Table 2). Each preparation was measured six times over a period of 30 min. These measurements were remarkably reproducible (<5% RSD). Before LC-MS/MS analysis, each radiotracer was also analyzed by the calibrated radiometric method (activity using an ionization chamber dose calibrator and mass using HPLC apparatus) regularly used in our laboratory for quality control. Each analysis required at least 10 min for completion and was performed only once because of the time constraint imposed by the short half-life. The SA measurements obtained with LC-MS/MS deviated by ±7.4% overall from the mean of both radiometric and LC-MS/MS methods (Table 2). The possible sources of error in the radiometric method include both the HPLC apparatus and the dose calibrator used for measuring carrier and radioactivity, respectively. The HPLC method used a linear calibration curve (r2 > 0.99) generated with authentic non-radioactive compounds, such as, for example, PBR28 (>99% pure; RTI International, Research Triangle Park, NC, USA) or (R)-rolipram (>98%; Tocris, Ellisville, MO, USA). The quantification of the carrier may not always be accurate due to lack of an internal standard and possible presence of a coeluting impurity. Measurements of the radioactivity of 11C-labeled preparations (n = 6) in one ionization chamber were compared with measurements in another to assess their reliability. The two ionization chambers showed 10% to 12% differences in the radioactivity measurements even though both detectors had been identically calibrated. The lack of accuracy and reproducibility in measurements with ionization chambers [10–14] may at least partially explain the difference in SA measured by radiometric-HPLC and LC-MS/MS methods. Other radiochemical detectors likely suffer similar deficiencies, especially as surrogate isotopes are also used for their calibration.
Discussion
Here, we demonstrated the capability of electrospray ionization (ESI)-MS/MS to ionize and isolate [M + H]+ of [11C]species and then generate and detect the product ion-bearing carbon-11. The mass-specific detection of [11C]species along with [M + 1]carrier at the expected t R allowed unambiguous identification of the radiotracer. By monitoring [M + 1]carrier, instead of the carbon-12 counterpart, we avoided saturation of the MS/MS detector during simultaneous measurement of trace concentrations of [11C]species. The measurement of both species gave the SA and t 1/2 of the radiotracer while also confirming radiotracer identity. The results from multiple analyses showed that the LC-MS/MS method is fast, accurate, and reproducible enough for characterizing low-enriched, fast-decaying radiotracers. This mass-specific detection method is suitable for validating the conventional method of characterization of PET radiotracers.
We verified that the excess carrier does not impact the ionization and detection of coeluting [11C]species in our LC-MS/MS system. The peak for [11C]species declined at an expected rate and then disappeared from the ion chromatogram as carbon-11 in the product ion decayed. Secondly, the ratio of M + 2 to M + 1 isotopologues was not altered when the radiotracer's non-labeled standard was injected at a concentration comparable to that of the carrier in the radiotracer. Also, the concentrations of [11C]species and [M + 1]carrier in radiotracers were within the dynamic range of detection of the triple quadrupole MS/MS system. Before being injected into LC-MS/MS, the radiotracer preparations were diluted adequately to avoid possible saturation of the ionization and detection systems. Furthermore, no cross-talk was observed between the [M + H]+ → product ion transition for [11C]species and that for [M + 1]carrier.
Carbon-11 decays to a stable nuclide, boron-11. This energetic decay event would instantly transform any molecule in which the carbon-11 had been incorporated. The product of carbon-11 decay would have a t R and mass different from those of the [11C]species or the [M + 1]carrier. Accordingly, the LC-MS/MS acquisition detected no product or chemical impurity from the decay of any of these PET radiotracers. The measurement showed exponential loss of mass of [11C]species as the radionuclide decayed thereby gave the same expected t 1/2 value for each radiotracer.
The radiometric method of SA measurement involves two detection systems, and both are prone to errors. For example, we observed a significant difference between two dose calibrators in recording the radioactivity of the same radiotracer preparation. The sources of error in the HPLC measurement of mass may include the weighing of standard, preparation of the calibration curve solutions, injection, chromatography, and UV detection. In contrast, the LC-MS/MS technique measures the mole fraction of [11C]species in a radiotracer similar to the isotope ratio in a compound. Thus, SA was obtained simply by entering into a spreadsheet: (1) the measured LC-MS/MS peak areas of [11C]species and [M + 1]carrier, (2) the abundance of [M + 1]carrier measured in the reference standard, (3) the area for the [11C, M + 1]species ion calculated from the natural abundance of heavy stable isotopes in [11C]species, and (4) the theoretical maximum SA of a compound labeled with one carbon-11 atom, as detailed under the ‘Calculations’ subsection.
The rapid LC-MS/MS method described here enables definitive characterization of radiotracers used in human PET imaging. A mass-specific detection method such as this is not susceptible to errors commonly associated with the use of the radio-HPLC method, as discussed. It may not be feasible to use triple quadrupole MS/MS regularly; however, it provides a wholly independent non-radiometric means for confirming identity and verifying SA of a radiotracer when needed. A less expensive and more commonly used single quadrupole MS instrument may offer the sensitivity to measure 11C]species and [M + 1]carrier by selected ion monitoring of respective [M + H]+ following ESI. However, such a less specific MS method can work only if no [M − 1]+ ion is generated during ionization. Other mass analyzers such as time-of-flight and linear ion traps may also be suitable for the characterization of PET radiotracers. Finally, the LC-MS/MS method should be applicable to all 11C-labeled tracers, except in instances where no 11C-containing product ion is obtained or where interfering ions are generated. If a labeled product ion is not available, selected ion monitoring of a precursor ion ([M + H]+) with a high-resolution MS can substitute the MS/MS method for measuring 11C]species and [M + 1]carrier. Also, a high-resolution MS is likely to resolve any interfering ions of isobaric species from the product ion of 11C]species in 11C-labeled tracers. In principle, LC-MS/MS may be used for characterizing PET radiotracers labeled with other short-lived positron emitters. Although an isotope separator has been used to identify the position of 18F in labeled 1,1,1,2-tetrafluoroethane [25], no parallel mass spectrometric method has yet been reported for measuring the SA of 18F-labeled tracers. The LC-MS/MS method should also be suitable for this purpose, as shown with the analysis of an 18F-labeled tracer in our preliminary report [26].
Conclusions
A simultaneous mass detection of [11C]species and [M + 1]carrier in 11C-labeled PET tracers was achieved using the LC-MS/MS technique. A single rapid analysis can verify the identity including the position of label in a 11C-labeled tracer preparation as well as provide a fully independent measure of SA. The LC-MS/MS method is a valuable adjunct to other techniques used in the quality control of PET radiopharmaceuticals.
Abbreviations
- [11C]species :
-
carbon-11 species
- [M + 1]carrier :
-
13C, 2H, 15N, or 17O carrier isotopologue
- ESI:
-
electrospray ionization
- HPLC:
-
high-performance liquid chromatography
- LC-MS/MS:
-
liquid chromatography-mass spectrometry/mass spectrometry
- PET:
-
positron-emission tomography
- SA:
-
specific radioactivity
- t R :
-
HPLC or LC retention time.
References
Fowler JS, Wolf AP: Working against time: rapid radiotracer synthesis and imaging the human brain. Acc Chem Res 1997, 30: 181–188. 10.1021/ar960068c
Miller PW, Long NJ, Vilar R, Gee AD: Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chem Int Ed 2008, 47: 8998–9033. 10.1002/anie.200800222
Cai L, Lu S, Pike VW: Chemistry with [18F]fluoride ion. Eur J Org Chem 2008, 2008: 2853–2873. 10.1002/ejoc.200800114
Meyer G-J: Some aspects of radioanalytical quality control of cyclotron-produced short-lived radiopharmaceuticals. Radiochim Acta 1982, 30: 175–184.
Passchier J: Fast high performance liquid chromatography in PET quality control and metabolite analysis. Quart J Nucl Med Mol Imaging 2009, 53: 411–416.
Kung M-P, Kung HF: Mass effect of injected dose in small rodent imaging by SPECT and PET. Nucl Med Biol 2005, 32: 673–678. 10.1016/j.nucmedbio.2005.04.002
Mizrahi R, Houle S, Vitcu I, Ng A, Wilson AA: Side effects profile in humans of [11C]-(+)-PHNO, a dopamine D2/3 agonist ligand for PET. J Nucl Med 2010, 51: 496–497. 10.2967/jnumed.109.072314
Madsen K, Marner L, Haahr M, Gillings N, Knudsen GM: Mass dose effects and in vivo affinity in brain PET receptor studies - a study of cerebral 5-HT4 receptor binding with [11C]SB207145. Nucl Med Biol 2011, 38: 1085–1091. 10.1016/j.nucmedbio.2011.04.006
Zimmerman BE, Cessna JT: Development of a traceable calibration methodology for solid 68Ge/68Ga sources used as a calibration surrogate for 18F in radionuclide activity calibrators. J Nucl Med 2010, 51: 448–453. 10.2967/jnumed.109.070300
Mo L, Reinhard MI, Davies JB, Alexiev D, Baldock C: Calibration of the Capintec CRC-712M dose calibrator for 18F. Appl Radiat Isot 2006, 64: 485–489. 10.1016/j.apradiso.2005.09.006
Zimmerman BE, Kubicek GJ, Cessna JT, Plascjak PS, Eckelman WC: Radioassays and experimental evaluation of dose calibrator settings for 18F. Appl Radiat Isot 2001, 54: 113–122. 10.1016/S0969-8043(99)00260-2
Zimmerman B, Kinahan P, Galbraith W, Allberg K, Mawlawi O: Multicenter comparison of dose calibrator accuracy for PET imaging using a standardized source [abstract]. J Nucl Med 2009,50(Suppl 2):472P.
Tyler DK, Baker M, Woods MJ: NPL secondary standard radionuclide calibrator. Syringe calibration factors for radionuclides used in nuclear medicine. Appl Radiat Isot 2002, 56: 343–347. 10.1016/S0969-8043(01)00212-3
Cessna JT, Schultz MK, Leslie T, Bores N: Radionuclide calibrator measurements of 18F in a 3 ml plastic syringe. Appl Radiat Isot 2008, 66: 988–993. 10.1016/j.apradiso.2008.02.046
Woods DH, Baker MI, Keightley JD, Keightley LJ, Makepeace JL, Pearce AK, Woodman AP, Woods MJ, Woods SA, Waters S: Standardisation of 11C. Appl Radiat Isot 2002, 56: 327–330. 10.1016/S0969-8043(01)00209-3
Briard E, Zoghbi SS, Imaizumi M, Gourley JP, Shetty HU, Hong J, Cropley V, Fujita M, Innis RB, Pike VW: Synthesis and evaluation in monkey of two sensitive 11C-labeled aryloxyanilide ligands for imaging brain peripheral benzodiazepine receptors in vivo. J Med Chem 2008, 51: 17–30. 10.1021/jm0707370
Fujita M, Imaizumi M, Zoghbi SS, Fujimora Y, Farris AG, Suhara T, Hong J, Pike VW, Innis RB: Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage 2008, 40: 43–52. 10.1016/j.neuroimage.2007.11.011
Lazarova N, Zoghbi SS, Hong J, Seneca N, Tuan E, Gladding RL, Liow J-S, Taku A, Innis RB, Pike VW: Synthesis and evaluation of [ N-methyl-11C ] N -desmethyl-loperamide as a new and improved PET radiotracer for imaging P-gp function. J Med Chem 2008, 51: 6034–6043. 10.1021/jm800510m
Kreisl WC, Liow J-S, Kimura N, Seneca N, Zoghbi SS, Morse CL, Herscovitch P, Pike VW, Innis RB: P-glycoprotein function at the blood–brain barrier in humans can be quantified with the substrate radiotracer 11C- N-desmethyl -loperamide. J Nucl Med 2010, 51: 559–566. 10.2967/jnumed.109.070151
Donohue SR, Krushinski JH, Pike VW, Chernet E, Phebus L, Chesterfield AK, Felder CC, Halldin C, Schaus JM: Synthesis, ex vivo evaluation, and radiolabeling of potent 1,5-diphenylpyrrolidin-2-one cannabinoid subtype-1 receptor ligands as candidates for in vivo imaging. J Med Chem 2008, 51: 5833–5842. 10.1021/jm800416m
Terry GE, Liow J-S, Zoghbi SS, Hirvonen J, Farris AG, Lerner A, Tauscher JT, Schaus JM, Phebus L, Felder CC, Morse CL, Hong JS, Pike VW, Halldin C, Innis RB: Quantitation of cannabinoid CB1 receptors in healthy human brain using positron emission tomography and an inverse agonist radioligand. Neuroimage 2009, 48: 362–370. 10.1016/j.neuroimage.2009.06.059
DaSilva JN, Lourenco CM, Meyer JH, Hussey D, Potter WZ, Houle S: Imaging cAMP-specific phosphodiesterase-4 in human brain with R-[11C]rolipram and positron emission tomography. Eur J Nucl Med 2002, 29: 1680–1683. 10.1007/s00259-002-0950-y
Lide DR: CRC Handbook of Chemistry and Physics. 83rd edition. Boca Raton: CRC; 2002.
Azuelos G, Kitching JE: Half-lives of some T = ½ mirror decays. Phys Rev C 1975, 12: 563–566. 10.1103/PhysRevC.12.563
Pike VW, Waters SL, Aigbirhio FI, Makepeace J, Tanner RJN: Novel use of an isotope separator to determine the position of fluorine-18 in labelled 1,1,1,2-tetrafluoroethanes. Org Mass Spectrom 1994, 29: 499–504. 10.1002/oms.1210290910
Shetty HU, Morse CL, Zhang Y, Pike VW: Characterization of PET radiotracers through LC-MS/MS measurement of the radiotracer and accompanying [13C]carrier [abstract]. In Proceedings of the 58th ASMS Conference on Mass Spectrometry and Allied Topics: May 23–27 2010; Salt Lake City. Santa Fe: ASMS; 2010. MP24:519
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health (NIMH). We thank our colleagues for their helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HUS and VWP designed the experiments and wrote the manuscript. HUS and CLM performed the LC-MS/MS and processed the data. CLM and YZ prepared the radiotracers and performed the HPLC measurements. HUS conceived the study and interpreted the data. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Shetty, H., Morse, C.L., Zhang, Y. et al. Characterization of fast-decaying PET radiotracers solely through LC-MS/MS of constituent radioactive and carrier isotopologues. EJNMMI Res 3, 3 (2013). https://doi.org/10.1186/2191-219X-3-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2191-219X-3-3