Abstract
Background
Bisphosphonates possess strong affinity to bone. 99mTc bisphosphonate complexes are widely used for bone scintigraphy. For positron emission tomography (PET) bone imaging, Ga-68-based PET tracers based on bisphosphonates are highly desirable.
Findings
Two trimeric bisphosphonate conjugates of the triazacyclononane-phosphinate (TRAP) chelator were synthesized, labeled with Ga-68, and used for microPET imaging of bone in male Lewis rats. Both Ga-68 tracers show bone uptake and, thus, are suitable for PET bone imaging. Surprisingly, Ga-71 nuclear magnetic resonance data prove that Ga(III) is not located in the chelating cavity of TRAP and must therefore be bound by the conjugated bisphosphonate units.
Conclusion
The intrinsic Ga-68 chelating properties of TRAP are not needed for Ga-68 PET bone imaging with TRAP-bisphosphonate conjugates. Here, TRAP serves only as a trimeric scaffold. For preparation of Ga-68-based bone seekers for PET, it appears sufficient to equip branched scaffolds with multiple bisphosphonate units, which serve both Ga-68-binding and bone-targeting purposes.
Similar content being viewed by others
Background
Geminal bisphosphonates possess strong affinity to bone [1, 2]. In living organisms, administration of bisphosphonates leads to inhibition of osteoclasts (bone resorbing cells), which results in a lower rate of bone resorption [3, 4]. Therapy with bisphosphonate drugs is thus performed to prevent decrease of bone density caused by osteogenesis imperfecta (brittle bone disease) [5] or osteoporosis [3, 6]. In addition, bisphosphonate complexes of 99mTc (e.g., of medronic acid, '99mTc-MDP'; see Figure 1) are the mainstay of bone imaging by scintigraphy and SPECT. However, as positron emission tomography (PET) offers higher resolution and sensitivity, PET bone-imaging agents are of high interest. Direct utilization of the β+-emitting radionuclide 18 F (t 1/2 = 110 min, E max,β+ = 634 keV) is the most simple and straightforward approach because [18 F]fluoride 18 F- inherently possesses a high affinity to bone. However, 18 F is cyclotron-produced, and therefore, a full geographical coverage, comparable to the supply of generator-produced 99mTc, cannot be guaranteed. Thus, bisphosphonate mono-conjugates of the currently most popular radiometal chelators 1,4,7,10-tetraazacyclododecane-tetraacetic acid [7–9] and 1,4,7-triazacyclononane-triacetic acid [10] have been prepared to utilize generator-produced 68 Ga (t 1/2 = 68 min, E max,β+ = 1.9 MeV) for PET bone imaging. Advancing this approach, this pilot study describes preclinical PET imaging results for trimeric bisphosphonate conjugates of the recently introduced chelator triazacyclononane-phosphinate (TRAP) (see Figure 2) [11–13].
Methods
General procedures and instrumentation (nuclear magnetic resonance (NMR), electrospray mass spectroscopy (ESI-MS), ultrafiltration/diafiltration, PET) have been described before [13]. [18 F]fluoride formulation for injection was prepared by adding 100 MBq of 18 F (obtained from routine cyclotron production at Klinikum rechts der Isar, Technische Universität München, München, Germany) to phosphate buffered saline (PBS) (1 mL).
Synthesis of bisphosphonate conjugates (Figure 2): TRAP·2H2O (0.3 mmol, 185 mg), diisopropylethylamide (3 mmol, 388 mg, 510 μL), and the amino-bisphosphonate (1.5 mmol); for TRAP(MDP)3, tetraethyl(aminomethylene)bisphosphonate 455 mg; and for TRAP(PDP)3, tetraethyl(1-aminopropylene)bisphosphonate 500 mg, were dissolved in DMSO (2 mL). Then, HATU (2.4 mmol, 921 mg) was added with stirring. After 25 min, the reaction mixture was diluted with water (50 mL) and subjected to diafiltration (membrane with 500 Da MWCO). After 250 mL of water had passed, the cell content was concentrated in vacuo and subjected to preparative HPLC (column: YMC C18 ec 250 × 30 mm; detection wavelength, 220 nm; eluent A, MeCN with 0.1% TFA; eluent B, water with 0.1% TFA; gradient 25% to 50% B in 20 min, t R(dodecaethyl-TRAP(MDP)3) = 12 min, t R(dodecaethyl-TRAP(PDP)3) = 16 min). After evaporation of the solvents, the remaining viscous oil was dissolved in HBr/glacial acetic acid (33%) and stirred for 3 days. Addition of methanol to the reaction mixture yielded the products as colorless, crystalline solids. Data for TRAP(MDP)3: yield 201 mg (61%); MW (calculated for C21H51N6O27P9) 1,098.43; ESI-MS negative m/z = 1,097 (M-H+) and 548 (M-2 H+); 1 H NMR (600 MHz, D2O) δ = 2.13 (m, 6 H), 2.67 (m, 6 H), 3.48 (d, 3 J HH = 5.4 Hz, 6 H), 3.56 (s, broad, 12 H), and 4.71 (t, J PH = 21.3 Hz, 3 H) ppm; 13 C NMR (151 MHz, D2O) δ = 26.11 (d, 1 J PC = 93.18 Hz), 28.65, 52.13, 54.74 (d, 1 J PC = 89.07 Hz), 47.45 (t, 1 J PC = 139.28 Hz), and 174.54 (dt, 2 J PC = 12.28 and 3 J PC = 4.34 Hz) ppm; and 31P NMR (121 MHz, D2O) δ = 14.10 (d, 2 J PP = 15.7 Hz) and 39.90 ppm. Data for TRAP(PDP)3: yield 195 mg (55%); MW (calculated for C27H63N6O27P9) 1,182.59; ESI-MS negative m/z = 1,181 (M-H+), 590 (M-2 H+), and 393 (M-3 H+); 1 H NMR (600 MHz, D2O) δ = 2.07 (m, 6 H), 2.10 (m, 6 H), 2.36 (tt, J PH = 23.52 Hz, 3 J HH = 5.97 Hz, 3 H), 2.53 (m, 6 H), 3.44 (t, 3 J HH = 6.3 Hz, 6 H), 3.45 (t, broad, 3 J HH = 6.6 Hz), and 3.52 (s, broad, 12 H) ppm; 13 C NMR (151 MHz, D2O) δ = 25.36 (t, 2 J PC = 4.2 Hz), 26.29 (d, 1 J PC = 93.5 Hz), 28.63 (d, 2 J PC = 3.9 Hz), 35.77 (t, 1 J PC = 128.5 Hz), 39.48 (d, 3 J PC = 7.4 Hz), 52.14, 54.82 (d, 1 J PC = 88.6 Hz), and 175.41 (d, 3 J PC = 13.1 Hz) ppm; and 31P NMR (121 MHz, D2O) δ = 21.53 (d, 2 J PP = 15.5 Hz) and 39.69 ppm.
68 Ga for labeling was obtained from a SnO2-based 68Ge/68 Ga generator (iThemba LABS, Somerset West, South Africa), eluted with 1.0 M HCl. A 1.25 mL fraction of the eluate containing ca. 80% of the total activity (ca. 1.3 GBq) was adjusted to pH 3.3 by adding a solution of 600 mg 2-[4-(2-hydroxyethyl)-1-piperazinyl]-ethanesulfonic acid (HEPES) in 0.5 mL water. To a 90 μL aliquot of this mixture, 10 μL of 10-4 M stock solution of the ligand was added. After heating for 5 min to 95°C, the solution was passed over a cation exchanger SPE cartridge (Chromafix HR-XC M, Macherey-Nagel, Düren, Germany) and purged with water (1 mL). This procedure removed non-complexed 68 Ga as well as HEPES, which was confirmed by processing of blank samples. Radiochemical yields, determined by measuring the activity on the cartridge and in the eluate, were > 85%. Formulation was done by adjusting the pH of the eluate to 7.4 by adding approximately 50 μL of a solution of NaOH (1 g) and NaH2PO4 (483 mg) in water (20 mL) while monitoring the pH with a pH meter. 'Free' 68 Ga formulation was prepared by addition of the generator eluate (40 μL, ca. 50 MBq) to PBS (1 mL), resulting in pH 7.2.
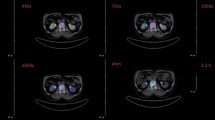
All animal experiments were carried out in accordance with the current animal welfare regulations in Germany. Five male Lewis rats (age 7 weeks, ca. 200 g) were kept under standard laboratory conditions (12 h light/12 h dark) and given standard diet and water ad libitum. For PET, ca. 35 MBq of tracer was injected into the tail vein under isoflurane anesthesia. Two subsequent scans of 15 min were recorded 60 min post injection, using two different axial bed positions in order to image the entire animals. Images were reconstructed using a OSEM3D algorithm without scatter and attenuation correction. For each full-body maximum intensity projection (MIP), two part-body MIPs were stitched together manually using graphics software. PET images are from representative animals reflecting the group.
Results and discussion
Figure 3 shows that free 68 Ga(III) (we use this generalized term since 68 Ga species in PBS solutions are not well defined) provides almost no contrast of the skeleton over other tissues, as intravenous injection of free 68 Ga(III) predominantly results in transferrin-bound activity [14–17]. In contrast, both bisphosphonate tracers 68 Ga-TRAP(MDP)3 and 68 Ga-TRAP(PDP)3 bind to bone while showing low levels in blood and soft tissues. Apparently, PET image quality achieved therewith cannot compete with that of [18 F]fluoride. 18 F possesses a lower positron energy than 68 Ga, resulting in lower tissue penetration (FW20H of 0.54 mm and 2.12 mm in soft tissue for 18 F and 68 Ga, respectively [18]), and therefore in a lower degree of image blurring. However, as the difference in resolution for a clinical 3-mm PET camera is small (3.05 mm for 18 F and 3.57 mm for 68 Ga [18]), a successful application of 68 Ga bone-imaging agents in patients is not precluded.
Upon investigation of the mode of gallium binding, we found that an equimolar mixture of 69,71 Ga3+ and either 68 Ga-TRAP(MDP)3 or 68 Ga-TRAP(PDP)3 does not yield any signal in 71 Ga NMR spectra, not even after heating to 95°C for hours. However, the octahedral N3O3 coordination usually found for 'in-cage' Ga(III) complexes of TRAP ligands generally yields sharp 71 Ga NMR resonances at δ = 130 to 142 ppm [11, 12]. Obviously, Ga(III) ion is not located in the TRAP cavity and, therefore, must be complexed in an 'out-of-cage' manner by the bisphosphonate groups. Although this result is quite unexpected, PET images nevertheless prove that the degree of kinetic stability of these complexes is sufficiently high to carry 68 Ga to the bone and retain it there. However, Figure 3 also shows a slightly higher background uptake for 68 Ga-TRAP(MDP)3, most likely caused by partial decomplexation in vivo due to lower complex stability. Clearance of both 68 Ga tracers occurred faster than 18 F- and exclusively via the kidneys.
Conclusion
68 Ga-labeled trimeric bisphosphonate conjugates of TRAP were successfully applied for bone imaging in rats. Surprisingly, 71 Ga NMR investigation revealed that Ga(III) ion is not located in the macrocyclic cavity of TRAP and, therefore, must be complexed by one or more side chain bisphosphonates. Although the primary chelation site of TRAP possesses excellent Ga(III) complexing properties [12], it apparently cannot compete with the bisphosphonates. In 68 Ga-TRAP(MDP)3 and 68 Ga-TRAP(PDP)3, TRAP thus merely serves as a scaffold, and its ability for 68 Ga binding is not required. We therefore conclude that in designing bisphosphonate tracers for 68 Ga-based PET bone imaging, the introduction of chelating moieties other than the bisphosphonates themselves might be unnecessary. Rather, it appears to be sufficient to equip suitable branched scaffolds with multiple bisphosphonate units which serve both 68 Ga-binding and bone-targeting purposes.
Abbreviations
- ESI-MS:
-
electrospray mass spectroscopy
- HEPES:
-
2-[4-(2-hydroxyethyl)-1-piperazinyl]-ethanesulfonic acid
- MIP:
-
maximum intensity projection
- NMR:
-
nuclear magnetic resonance
- PBS:
-
phosphate buffered saline
- PET:
-
positron emission tomography
- TRAP:
-
triazacyclononane-phosphinate.
References
Mukherjee S, Huang C, Guerra F, Wang K, Oldfield E: Thermodynamics of bisphosphonates binding to human bone: a two-site model. J Am Chem Soc 2009, 131: 8375–8376.
Mukherjee S, Song Y, Oldfield E: NMR investigations of the static and dynamic structures of bisphosphonates on human bone: a molecular model. J Am Chem Soc 2008, 130: 1264–1273. 10.1021/ja0759949
Ebetino FH, Hogan AML, Sun ST, Tsoumpra MK, Duan XC, Triffitt JT, Kwaasi AA, Dunford JE, Barnett BL, Oppermann U, Lundy MW, Boyde A, Kashemirov BA, McKenna CE, Russell RGG: The relationship between the chemistry and biological activity of the bisphosphonates. Bone 2011, 49: 20–33. 10.1016/j.bone.2011.03.774
Roelofs AJ, Thompson K, Ebetino FH, Rogers MJ, Coxon FP: Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr Pharm Design 2010, 16: 2950–2960. 10.2174/138161210793563635
Shapiro JR, Sponsellor PD: Osteogenesis imperfecta: questions and answers. Curr Opin Pediatr 2010, 21: 709–716.
Le Goff B, Guillot P, Glemarec J, Berthelot JM, Maugars Y: A comparison between bisphosphonates and other treatments for osteoporosis. Curr Pharm Design 2010, 16: 3037–3044. 10.2174/138161210793563563
Kubíček V, Rudovský J, Kotek J, Hermann P: Vander Elst L, Muller RN, Kolar ZI, Wolterbeek HT, Peters JA, Lukeš I: A bisphosphonate monoamide analogue of DOTA: a potential agent for bone targeting. J Am Chem Soc 2005, 127: 16477–16485. 10.1021/ja054905u
Vitha T, Kubíček V, Hermann P: Vander Elst L, Muller RN, Kolar ZI, Wolterbeek HT, Breeman WAP, Lukeš I, Peters JA: Lanthanide(III) complexes of bis(phosphonate) monoamide analogues of DOTA: bone-seeking agents for imaging and therapy. J Med Chem 2008, 51: 677–683. 10.1021/jm7012776
Fellner M, Baum RP, Kubíček V, Hermann P, Lukeš I, Prasad V, Rösch F: PET/CT imaging of osteoblastic bone metastases with 68 Ga-bisphosphonates: first human study. Eur J Nucl Med Mol Imaging 2010, 37: 834. 10.1007/s00259-009-1355-y
Suzuki K, Satake M, Suwada J, Oshikiri S, Ashino H, Dozono H, Hino A, Kasahara H, Minamizawa T: Synthesis and evaluation of a novel 68 Ga-chelate-conjugated bisphosphonate as a bone-seeking agent for PET imaging. Nucl Med Biol 2011, 38: 1011–1018. 10.1016/j.nucmedbio.2011.02.015
Notni J, Hermann P, Havlíčková J, Kotek J, Kubíček V, Plutnar J, Loktionova N, Riss PJ, Rösch F, Lukeš I: A triazacyclononane-based bifunctional phosphinate ligand for the preparation of multimeric 68 Ga tracers for positron emission tomography. Chem Eur J 2010, 16: 7174–7185.
Šimeček J, Schulz M, Notni J, Plutnar J, Kubíček V, Havlíčková J, Hermann P: Complexation of metal ions with TRAP (1,4,7-triazacyclononane phosphinic acid) ligands and NOTA: phosphinate-containing ligands as unique chelators for trivalent gallium. Inorg Chem 2012, 51: 577–590. 10.1021/ic202103v
Notni J, Šimeček J, Hermann P, Wester HJ: TRAP, a powerful and versatile framework for gallium-68 radiopharmaceuticals. Chem Eur J 2011, 17: 14718–14722. 10.1002/chem.201103503
Clausen J, Edeling CJ, Fogh J: 67 Ga binding to human serum proteins and tumor components. Cancer Res 1974, 34: 1931–1937.
Chikh Z, Ha-Duong NT, Miquel G: El Hage Chahine JM: Gallium uptake by transferrin and interaction with receptor 1. J Biol Inorg Chem 2007, 12: 90–100.
Otsuki H, Brunetti A, Owens ES, Finn RD, Blasberg RG: Comparison of iron-59, indium-111, and gallium-69 transferrin as a macromolecular tracer of vascular permeability and the transferrin receptor. J Nucl Med 1989, 10: 1676–1685.
Bernstein LR: Mechanisms of therapeutic activity for gallium. Pharmacol Rev 1998, 50: 665–682.
Sánchez-Crespo A, Andreo P, Larsson SA: Positron flight in human tissues and its influence on PET image spatial resolution. Eur J Nucl Med Mol Imaging 2004, 31: 44–51. 10.1007/s00259-003-1330-y
Acknowledgements
The authors thank E. Weidl for the animal handling, and Sibylle Reder, Marco Lehmann, and Markus Mittelhäuser for the assistance with PET imaging.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JN developed the study concept; performed synthesis, radiolabeling, PET imaging, and PET data analysis; and wrote the manuscript. JP performed all NMR measurements and evaluated the data. HJW provided important advice in the conception of the study, gave advice in the interpretation of the data, and critically reviewed the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Notni, J., Plutnar, J. & Wester, HJ. Bone-seeking TRAP conjugates: surprising observations and their implications on the development of gallium-68-labeled bisphosphonates. EJNMMI Res 2, 13 (2012). https://doi.org/10.1186/2191-219X-2-13
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2191-219X-2-13