Abstract
Background
Phytostabilization has been advocated as a promising approach to reduce mine tailings' adverse effects to surrounding environment. With many years of efforts in both laboratory and field trials, organic amendments are found to be essential in tailing revegetation. Yet, the associate geochemical dynamics caused by different amendments has rarely been examined. As reactive minerals are usually rich in tailings, geochemical changes induced by amendments would influence seepage management and revegetation strategies. The present study aimed to investigate geochemical dynamics in Cu-Au tailing leachate, in response to amendments with biochar produced from hardwood timber at high charring temperature or woodchips of mixed native tree species in a column leaching experiment under laboratory conditions.
Results
Results showed that the Cu-Au tailings tested in this study were relatively stable after natural weathering, with little resilience of peak salinity, stable pH and low levels of metals in leachate against the six cycles of leaching over 20 weeks. In comparison with the control without any amendments, biochar treatment did not cause any substantial changes in most examined properties of leachate, except for the reduction in dissolved organic C and NO2-. In contrast, woodchip treatment had a reduced leachate pH and a strong resilience in leachate salinity (and thus major saline ions). The geochemical changes in the woodchip treatment may be related to the active decomposition of woodchips, as indicated by the sharp increases in microbial biomass and activity as well as labile organic C at the end of leaching.
Conclusions
The present results suggest that dynamic hydrogeochemical changes may be induced by amendment of fresh biomass like woodchips, which can increase the load of salts and metals in tailing pore water. This may affect seepage water quality at least in the short-term and thus plant survival if tailings are immediately revegetated after amendment. In contrast, biochar with highly stable carbon may help alleviate geochemical environment in the tailings.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Organic matter amendment is a common practice in tailing rehabilitation. The addition of various organic materials is expected to increase soil carbon capacity, stimulate biological activities and also improve structural factors [1–4]. As tailings are usually rich in reactive minerals, particularly pyrites and carbonates, the lifecycle of organic amendment in tailings will have to be associated with the hydrogeochemical processes driven by active mineral weathering and possibly impact seepage quality [5]. Meanwhile, hydrogeochemical stability is a critical factor affecting the fitness of plants established in tailings for phytostabilization [6]. As a result, it is useful to understand what geochemical changes can be induced by different organic matters in mine tailings in the short/medium term.
Mine tailings are slurry wastes from ore processing. For base metal mines (Pb/Zn/Cu/Ni), tailings are mainly composed of gangue and residue ore minerals normally rich in pyrites. For example, the Pb-Zn mine tailings from Mt. Isa of Queensland contain 6% to 35% of pyrite depending on the ore chemistry over the past several decades [7]. Similarly, the pyrite contents in the Cu/Zn tailings of Boliden (Sweden) were found to be up to 48.7% [8], and up to 29% in the tailings of Quebec, Canada [9]. It is well known that pyrite oxidation is a major factor responsible for the acidification of mine wastes, which cause extreme salinity and metal toxicity, and generate acid mine drainage when carbonates are depleted [10]. Thus, mine tailings rich in pyrites are generally unstable in hydrogeochemistry. Recently, there has been an increasing awareness that such hydrogeochemical instability can be the primary constrain for plants' initial establishment in tailings and thus merits a systematic assessment for tailing rehabilitation [6], especially when various amendments are used.
Plant biomass-based organic matter such as woodchips are commonly used in soil and mine waste remediation, while the application of biochar in mined land remediation has just recently been proposed [11, 12]. Woodchips are mainly composed of carbohydrates (65% to 75%) and lignin (18% to 35%) [13]. Therefore, as well as biosolids, manure and various industry organic wastes (e.g. paper mill), woodchips are supposed to ameliorate tailing environment by providing labile carbon and promoting microbial activity [11]. However, some studies have also implied that organic matter amendment may induce unwanted changes of tailing pore water chemistry, such as increased metal release by municipal biosolids [14] and cattle manure [15, 16]. These findings implied that organic matters, including woodchips, may be able to reactively interact with tailing geochemistry and impact seepage/leachate chemistry. Biochar is the pyrolysis residue of plant biomass and mainly composed of aromatic C at high charring temperature [17, 18]. As a popular soil ameliorator [19], biochar has also been introduced into mine land rehabilitation recently. Based on a short-term (15 days) test [20], biochar was found to be an ideal inert ameliorant for tailing remediation. Yet, how biochar amendment impacts tailing hydrogeochemistry in a longer term remains to be shown.
Therefore, the primary aim of the present study was to characterize the effects of woodchips and biochar on the geochemical dynamics of tailing leachate through a column leaching experiment. The specific objective was to determine the dynamics of leachate chemistry, indicated by acidity, salinity, common toxic elements and labile C, in neutral Cu-Au tailings with and without woodchips or biochar amendment under laboratory leaching conditions over 20 weeks. Column leaching has been used as a routine approach for assessing the geochemistry and toxicity of porous materials for many years [21–23], and it was particularly useful for mine wastes as supposed to better simulate field conditions than the batch methods [24–27]. Despite the significant deviation in leachate chemistry among replicate columns over time [23, 28], column leaching was still thought to be a valuable approach to assess the medium- to long-term dynamics of mine tailings' toxicity and hydrogeochemistry.
Results
Leachate geochemistry
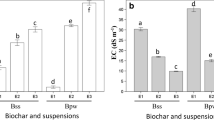
Electrical conductivity (EC) decreased sharply in the pure tailings in the first cycle and tended to be quasi-stable after three cycles of leaching (about 30 pore volumes of water). No substantial recovery of EC was observed after either 4- or 8-week extended weathering. Leachate pH of the EHM tailings kept relatively stable after 20-week leaching (Figures 1 and 2).
A box plot showing the change of pH of the three treatments in column leaching. EHM-Char, EHM tailings amended with 10% biochar; EHM-Wood, EHM tailings amended with 20% woodchips. The number following the treatment labels indicates for the number of leaching cycle. Only the first and sixth cycles were compared here.
The dynamics of leachate EC and pH in biochar-amended tailings was similar to that of the pure tailings. In contrast, woodchip treatment had a substantial recovery of EC after each cycle and a lower median pH maintained over the six cycles.
The dominant anions were found to be sulphate (SO42-), and major cations (Ca2+, Mg2+, K+ and Na+) were all found to be important in all the leachate. The dynamics of these ions was consistent with the pattern for EC of the corresponding treatments (Additional file 1: Figure S1).
Nitrite was only detected in the leachate of EHM, with a relatively stable concentration of around 2 mg L-1, but not biochar and woodchip treatments (Additional file 1: Figure S2). Nitrate decreased sharply in all the treatment after the first cycle of leaching, and it kept a little higher in EHM than woodchip and biochar treatments.
The levels of common toxic elements in all the leachate solutions were not of concern, like Ni, Hg, Pb, Zn, and Cr (data not shown), except for As and Cu. The dynamics of Cu and As in the leachate was shown in Figure S3 in Additional file 1 and Figure 3. Woodchip treatment sharply increased the release of Cu, and extended weathering obviously increased Cu release, which was not found for the pure tailings and the biochar treatment, as in the leachate was above Australian drinking water guideline for the first four cycles of leaching for all the treatments.
Dynamic of As in the first 50-ml leachate over six cycles of leaching. EHM, pure tailings; EHM + 20% woodchips, tailings with woodchip; EHM + 10% biochar, tailings with biochar amendment. ADWG stands for Australian Drinking Water Guideline (for more information, please refer to NHMRC website http://www.nhmrc.gov.au/guidelines/publications/eh34).
The dynamics of DOC and Fe was distinct from the patterns of saline and trace elemental ions (Figures 4 and 5). DOC was nearly negligible at the first four cycles in the biochar treatment and had an obvious increase after extended weathering. The EHM tailings had much higher DOC than the biochar treatment throughout the leaching. The DOC of the woodchip treatment was at similar levels as the pure tailings at the first two cycles, but increased substantially in a wavy manner and kept highest then. Fe in all the treatments kept around 0.2 mg L-1 in the first two cycles, and after a sharp decrease in the third cycle, a steady increase was observed. After the third cycle, the concentrations of Fe in leachate were highest in the woodchip treatment.
Tailing mineralogy
The profiles of major crystal minerals were similar among all the samples, based on the XRD spectra shown in Figure 6. Dominate species identified included quartz (diagnostic d-spacings, 3.34, 4.26, 1.82), kaolinite (7.17), microcline (3.24), calcite (3.03), dolomite (2.88), pyrite (1.63, 2.71) and magnetite (2.53, 1.48, 1.61), according to the standard values of minerals [29].
X-ray diffraction (XRD) patterns for tailing samples used in this study. Prominent peaks were assigned as shown in the figure. EHM_weathered, tailings collected from the tailings impoundment weathered for several years and used for column leaching; EHM_fresh, fresh tailings from ore processing; EHM_leached, tailings after 6-month leaching in the column leaching experiment; EHM + 10% biochar, tailings with 10% biochar amendment and after 6-month leaching in the column leaching experiment; EHM + 20% woodchips, tailings with 20% woodchip amendment and after 6-month leaching in the column leaching experiment.
Due to the similar origination, it allowed a semi-quantitative comparison of mineralogy among the samples of different treatments and sampling time, following the intensity ratio method described by Harris W [29]. As shown in Figure 6, no apparent change can be detected between the original weathered tailings and tailings with and without organic amendment after six-cycle leaching. However, a substantial difference in carbonate (calcite and dolomite) content between the weathered and fresh tailings was found. The intensity ratio of carbonates to quartz was more than five times higher in the fresh tailings than that in the weathered tailings.
Organic amendment chemistry
The composition of C functional groups was analysed using solid 13C NMR for biochar and woodchips before and after leaching. The NMR spectra were shown in Figure S4 in Additional file 1. Based on the peak assignment described above, the biochar used in this study is practically composed of mainly unsubstituted aromatic C, with a single broad NMR peak of around 120 ppm and remained unchanged after six-cycle leaching. The woodchips were composed of mainly cellulose and hemicellulose C with a small part of lignin C, and also remained largely unchanged after leaching, except that an obvious increase in aromatic C (peak around 138), though very little, was observed.
Carbon fraction and microbial respiration at the end of leaching
Soluble (SOC) and microbial biomass C (MBC) and soil basal respiration (SBR) were determined at the end of the column leaching. The results showed that SOC, MBC and SBR were comparable in the pure tailings and the biochar treatment, while they were relatively high in the woodchip treatment. Especially, SOC and SBR were more than an order of magnitude higher in the woodchip treatment than the pure tailings and the biochar treatment (Figure 7).
Discussion
Overall, the results in this study showed that (1) biochar was basically an inert amendment to tailing geochemistry, and (2) woodchips can impact tailing hydrogeochemistry immediately and substantially in a short/medium term. An interesting finding is that woodchip amendment, though beneficial for tailing revegetation by increasing labile C and microbial biomass, may have the potential to accelerate the weathering of tailings' reactive minerals, acidifying the pore water and increasing the release of saline ions and toxic elements.
High salinity has been reported to be one of the major constrains for tailing revegetation in many tailing impoundments [6], especially in Queensland [30, 31]. Thus, knowledge on the dynamics of salinity in tailings is of primary importance for tailing revegetation. For the pure tailings, salts can be leached to a level of normal soil salinity after two cycles of leaching (1,400 ml, approximately 20 pore volumes), and salt recovery was negligible after prolonged weathering. The high EC at the beginning of leaching and its low resilience thereafter basically implied that the EHM tailings had ever experienced a highly reactive stage, which accumulated salts, and now are tending to be temperate in geochemical reactivity. This is in agreement with the results from the long-term on-site monitoring of EHM hydrogeochemistry [6], and quite different from the pattern of hydrogeochemical stability of some coal mine tailings which had a high and rapid resilience of salinity even after 60-pore-volume leaching [32]. Quasi-stable hydrogeochemistry is a good sign for tailing revegetation as surface layer is the essential zone for plants' root development.
The salinity of EHM tailings is dominated by gypsum type salts (Ca2+-SO42-) (Additional file 1: Figure S1). In soils and coal mine tailings of the Australian continent, the dominant salt is usually chloride (Na+-Cl-) mainly accumulated through rainfall [33, 34]. Thus, the sulphate dominance can be a signature of historical pyrite weathering [35]. Both the leachate chemistry and mineralogy (Figure 6) pointed to an active coupled dissolution of carbonates and pyrite at the early stage of EHM tailings since disposal. Pyrite is a ubiquitous iron mineral in metal ores and a well-known source for acid generation in mine land [10]. Ca/Mg carbonate minerals are also common gangue materials in mine tailings, with dissolution rates 3-order-of-magnitude faster than sulphide oxidation [36]. Consequently, in sulphidic metal mine tailings abundant in carbonates, pH can be well buffered as shown in this (Figure 2) and previous studies [20, 37–39], resulting in extreme salinity inevitably. This mechanism controlling tailing hydrogeochemistry has been well demonstrated elsewhere [35, 36] and again reflected in this study.
The biochar used in this study was found to be inert in terms of both tailing leachate chemistry and microbial activity (microbial respiration). We found little difference in leachate EC and pH, microbial biomass and soil respiration between the biochar treatment and the pure tailings. The biochar is practically composed of only unsubstituted aromatic carbon, which is consistent with recent findings that at higher production temperature (>500°C), wood chars only had aromatic structures left [18]. Biochar is generally hard to be used by soil microorganisms [40, 41]. The resistance of biochar to degradation has been well demonstrated in recent publications, like Kuzyakov et al. [42] who estimated that the mean residence time of black carbon in soils under natural conditions is about 2,000 years, and Olivier [41] who found that biochar cannot be directly utilized by soil microbial consortia as sole carbon source. Thus, the short- to medium-term effects of biochar on tailing geochemistry are normally through physical modification of tailing environment, like water-holding capacity and adsorption capacity [20]. The effect of biochar on the adsorption capacity of tailings was reflected by the retention of DOC in the biochar treatment, but not by metal release, as toxic elements were mostly not of concern in the EHM tailing leachate.
Different from biochar, woodchips substantially impacted the tailing leachate chemistry. The persistence of lower pH in woodchip treatment may be also related to the active microbial activity indicated by the sharp increase in DOC release, labile C, microbial biomass and soil respiration, as well as the slight modification of woodchip chemistry (Figures 4 and 7, and Additional file 1: Figure S4), as inputs of organic acids from litter decomposition and carbonate acid from microbial respiration were two important mechanisms responsible for soil acidification [43]. Meanwhile, Cu and Fe release was significantly enhanced by woodchip amendment. Mobility of Cu in soil is well known to be controlled by DOC and pH [44], and we did find that there is a positive correlation between DOC and Cu concentration in all the leachate. It is not surprising that organic amendments can increase metal release into mine drainage [15, 16]. The increase of Fe release in woodchip treatment may be a response to enhanced pyrite weathering. Pyrite weathering in mine tailings was a factor of not only chemical environment but also microbial activity. Some tolerant microorganisms were found to able to survive tailing environment by oxidizing pyrite [45, 46], generating acid and soluble iron. Therefore, the input of labile C from woodchips was also possible to enhance pyrite weathering and then increase Fe release. Taken together, woodchip amendment had the potential to enhance tailing weathering and lead to lower pH and increase in elemental release.
The steady release of nitrite in pure tailings and its total disappearance in the other treatments may imply that biochar and woodchips changed the N cycling of tailings by altering the redox environment or/and the microbial composition. Nitrite is an intermediate of soil nitrification/denitrification process and can easily react with O2[47]. Thus, nitrite dynamics in soil is related to both soil redox environment and microbial functional groups. Nitrite is normally low in oxic layer and may only accumulate where oxygen is depleted by organic matter decomposition [48] or microscale consolidation and nitrification is depressed [49]. Previous studies have revealed an unexpected diversity of microbes present in Cu mine tailings [50, 51], and many of them are able to fix gaseous N [39] into tailing N pool. Reasonably, N-cycling microorganisms may have played important roles in controlling the N dynamics in the tailing leachate. For the pure tailings, the microscale consolidation was also possible, which can reduce O2 availability and is favourable for nitrite accumulation.
It is worth to point out that the column leaching used in this study may be of limitations in predicating the real processes in the field. The actual weathering in the field can be much stronger due to dry-wet and heat-cold cycles, while the actual salt loss can be largely knocked off due to poor drainage conditions and topsoil salinization effects, which is quite common in tailing landscape [6].
Conclusions
The Cu-Au tailing used in this study was shown to be relatively stable after decades of natural weathering. Biochar and woodchips had different effects on EHM geochemistry. Biochar was chemically and microbially inert, while woodchips can continue to degrade over time, buffer the release of major saline ions and even mobilize toxic elements in the long run. We speculate that fresh biomass amendment like woodchips may pose challenges for seepage management in a short/medium term since amending and the induced geochemical changes need to be considered in the strategy of tailing revegetation. Considering the distinct impacts of biochar and woodchips on tailing hydrogeochemistry, the combination of biochar and woodchips is of potential to be an ideal amendment for tailing rehabilitation.
Methods
Materials used in column leaching
Copper-Au tailings were sampled from the tailing storage facility of Ernest Henry Mine (EHM) in northwest Queensland in October in 2010. The primary minerals in the tailings comprised pyrite, magnetite and non-sulphide gangue such as carbonates and silicates [52]. The tailing samples for column leaching were collected from the weathered section. For comparison purpose, bulk fresh tailings were sampled in October in 2012 from the newly deposited section. The ratio of carbonates to pyrites (the company used this value to calculate potential acidification risks of tailings' long-term storage) in the fresh tailings has not changed substantially in recent years, within a factor of 1.5 (unpublished data). The tailings used have a pH of 8.1, EC of 8.4 mS/cm, 12.8% of Fe, 1.1% of S, 1.0% of Ca and 6.0% of Mg.
Woodchips were mixed biomass from tree pruning. After oven dried at 60°C over 48 h, the woodchips were ground and sieved through 2-mm mesh. The woodchips used have a pH of 4.3 and EC of 0.5 mS/cm. Biochar was produced from Jarrah timber wastes at about 700°C with 0.5-h residence time. The biochar has a pH of 10.3, C content of 91.5%, ash content of 2.35% and exchangeable cations (Ca2+, Mg2+, K+, and Na+) of 11.3 cmol(+)/kg. Biochar was also ground and sieved through 2-mm mesh before use.
Column leaching
The column setup (Additional file 1: Figure S5) and leaching procedure referred an OECD method [53] with some modifications described in our previous study [32]. Each column contained 400-g tailings. Tailings were loosely packed into the column without compaction. Double-layer filter papers (110 mm, Filtech®, Australia; pore size 2.3 μm, 0.06% ash) were placed on the bottom of the funnel (Buchner 110 mm, Kartell®, Italy) before loading the samples.
Tailing amendment treatments were 20% woodchips (80-g woodchips + 400-g tailings) and 10% biochar (40-g biochar + 400-g tailings). The rates of woodchips and biochar amendment referred to those used in the field trial and laboratory studies [6, 20]. Leaching DI water was added from the top of the column gently through a plastic container with multi-hole at the bottom which was to prevent preferential flow. At each time, 70-ml water (approximately the pore volume of the tailings) was added first and was allowed to stand for 1 min to saturate the samples in the column [54]. Leachate was collected consecutively, and the tubes were changed every 50 ml. A water head greater than 0.5 cm was maintained during the periods of leachate collection to maintain nearly constant dripping rate, which was monitored manually. In total, 700 ml water (39.6 mm in height in the column, equal to long-term average monthly precipitation at Mt. Isa) was used for each cycle leaching. After leaching, the columns were left to air dry. Leaching was done every 2 weeks for the first four cycles, and then 4 weeks for the fifth cycle and 8 weeks for the sixth cycle to test the recovery of leachate salinity. Ten lots of 50-ml leachate were collected in each cycle for EC and pH analyses. The first 50-ml leachate of each cycle was stored in 4°C for elemental analysis. Aliquots of the first 50-ml leachate of each cycle were kept in -20°C for the analyses of anion and dissolved organic carbon (DOC). At the end of the column leaching, the columns were destructively sampled for NMR, X-ray diffraction, C fraction and soil respiration analyses.
Leachate chemistry
All the EC and pH were measured electrically immediately after the collection of leachate. Elemental and DOC analyses were done using ICP-AES in the Analytical Services centre within the School of Agriculture and Food Science, the University of Queensland. All the leachate was filtered through 0.45 μm (Minisart®, Germany) before analysis. Elements screened in the leachate included Al, As, Au, B, Ba, Be, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, P, Pb, Sb, Se, Si, Sr, Ti, U, V, and Zn. DOC was determined using ICP after the removal of inorganic C through acidifying the solution, as described by Stefansson et al. [55].
13C CP/MAS solid-state NMR
Solid state 13C NMR for woodchips and biochar (raw samples and at sixth month) was done in the Centre for Advanced Imaging, the University of Queensland, using a Bruker Advance 300 high-resolution NMR spectrometer (MA, USA) interfaced to a 7.05 Tesla ULTRASHIELD bore magnet system. Material was placed in the 4-mm zirconium rotor and rotated at 7 kHz. Usual parameters included 42-ms acquisition time with sweep width of 30 kHz; 2 K data points were collected. Cross-polarization time was between 1 and 4 ms. High-power decoupling was applied using tppm15 scheme. The scans were collected between 4 and 10 K. For char samples HPdec (single pulse with high-power decoupling) was used in some instances due to low amount of protons. Decoupling power was 55 KHz; between 500 and 2000 scans were collected with recycling time of 40 s. The spectra were plotted between -15 and 265 ppm, and the peaks in the spectrum were assigned to acetyl-CH3 in hemicellulose (21 ppm), methoxyl-CH3 in lignin (56 ppm), C1-C6 carbon in carbohydrates (63, 73, 89, and 105 ppm), sugars in hemicelullose (82 ppm), aromatic carbon atoms (127 to 167 ppm), esters and carboxylic acids (169 to 195 ppm) including acetyl groups in hemicellulose at 173 ppm [56].
Mineralogy
X-ray diffraction for tailing mineralogy was done in the Centre for Microscopy and Microanalysis, the University of Queensland. Samples were analysed using a Bruker D8 Advance X-Ray Diffractometer equipped with a scintillation counter, graphite monochromator and Cu target (Bruker Corporation). The XRD operational conditions were as follows: 40-kV generator tension, 30-mA generator current, 2° to 70° 2-Theta, 0.050° step size, 20-mm variable slits and 0.5 s per step. Prominent minerals were identified by EVA software (Palo Alto, CA, USA) with its embedded database and further checked manually according to reported mineralogical data. All the samples were air dried, ground, and sieved through a 1-mm mesh before XRD analysis. The analysed samples included the fresh EHM tailings, original weathering tailings for column leaching, and tailings of three column treatments after 6-month leaching.
Dissolved organic carbon and microbial carbon
Water-soluble organic matter was extracted by shaking pre-incubated soil (adjusted to 50% water holding capacity and incubated for 24 h) at a soil/water ratio of 1:2 on an end-to-end shaker at 20°C for 1 h. The mixture was then centrifuged at 4,000 rpm for 10 min and filtered through a 0.45-μm glass fibre filter. The microbial biomass was determined, following the chloroform fumigation methods [57]. Briefly, a pair of air-dried samples was adjusted to 50% water holding capacity and incubated at 25°C for 7 days. One group of the samples was vacuum fumigated with CHCl3 vapour in darkness for 24 h. Both fumigated and non-fumigated samples were extracted with 0.25 M K2SO4 and filtrated through Whatman No. 42 filter paper. Microbial biomass C was calculated as the difference of organic C in fumigated and non-fumigated samples using an Ec factor of 2.64. Organic C in all the extractions was determined by dichromate digestion method [58].
Soil respiration
Soil respiration was measured using the conventional method by Chen et al. [59]. Briefly, 100-g oven-dried equivalent soil was adjusted to 50% water holding capacity and aerobically incubated at 25°C in a 1-L sealed glass jar for 7 days, and the CO2 that evolved from the soil was trapped in 0.1 M NaOH. The residual NaOH was titrated with 0.05 M HCl to phenolphthalein endpoint. The evolved carbon dioxide was calculated from the difference in normality between NaOH blanks and samples.
Data analysis
All data was processed, and all figures were drawn using Microsoft® Excel (WA, USA). Data collection and primary analysis for NMR and XRD were done by the embedded software of the instruments as described above.
References
Mendez MO, Glenn ER, Maier RM: Phytostabilization potential of quailbush for mine tailings: growth, metal accumulation, and microbial community changes. J Environ Qual 2007, 36: 245–253. 10.2134/jeq2006.0197
Ye ZH, Wong JWC, Wong MH, Lan CY, Baker AJM: Lime and pig manure as ameliorants for revegetating lead/zinc mine tailings: a greenhouse study. Bioresource Technol 1999, 69: 35–43. 10.1016/S0960-8524(98)00171-0
Renault S, Green S Report of Activities 2005, Manitoba Industry, Economic Development and Mines, Manitoba Geological Survey. Phytoremediation and revegetation of mine tailings and bio-ore production: effects of paper mill sludge on plant growth in tailings from Central Manitoba (Au) minesite (NTS 52L13) 2005.
Green S, Renault S: Influence of papermill sludge on growth of Medicago sativa, Festuca rubra and Agropyron trachycaulum in gold mine tailings: a greenhouse study. Environ Pollut 2008, 151: 524–531. 10.1016/j.envpol.2007.04.016
Lindsay MBJ, Blowes DW, Condon PD, Ptacek CJ: Managing pore-water quality in mine tailings by inducing microbial sulfate reduction. Environ Sci Technol 2009, 43: 7086–7091. 10.1021/es901524z
Huang L, Baumgartl T, Mulligan D: Is rhizosphere remediation sufficient for sustainable revegetation of mine tailings? Ann Bot-London 2012, 110: 223–238. 10.1093/aob/mcs115
Forsyth B, Edraki M, Baumgartl T Seventh Australian Workshop on Acid and Metalliferous Drainage (AMD): 2011 June 21–24; Darwin, Australia. Understanding the long-term seepage chemistry of base metal mine tailings in a semi-arid tropical climate, Mount Isa, Australia 2011.
Lars L, Tomas H, Linda M, Torbjörn K: Immobilisation of Trace Metals in Sulfidic Mine Tailings. Granada: IMWA; 2011.
Mermillod-Blondin R, Benzaazoua M, Kongolo M, Donato P, Aubertin M: Multidisciplinary characterization of mine tailings: Application to environmental desulphurization. Montreal: Industrial Chair NSERC Polytechnique-UQAT Environment and Mine Tailings Management; 2003.
Evangelou VP, Zhang YL: A review: pyrite oxidation mechanisms and acid mine drainage prevention. Crit Rev Env Sci Technol 1995, 25: 141–199. 10.1080/10643389509388477
Mendez MO, Maier RM: Phytostabilization of mine tailings in arid and semiarid environments—an emerging remediation technology. Environ Health Persp 2008, 116: 278–283.
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D: Biochar effects on soil biota—a review. Soil Biol Biochem 2011, 43: 1812–1836. 10.1016/j.soilbio.2011.04.022
Pettersen RC: The chemical composition of wood. In The Chemistry of Solid Wood, Advances in Chemistry Series. Volume 207. Edited by: Rowell RM. Washington, DC: American Chemical Society; 1984:76–81.
Pond AP, White SA, Milczarek M, Thompson TL: Accelerated weathering of biosolid-amended copper mine tailings. J Environ Qual 2005, 34: 1293–1301. 10.2134/jeq2004.0405
Garcés DMC, Faz Cano Á, Arocena JLM: Dissolved organic carbon and metals release in amended mine soils. Revista de la Sociedad Española de Mineralogía 2008, 10: 115–117.
Schwab P, Zhu D, Banks MK: Heavy metal leaching from mine tailings as affected by organic amendments. Bioresource Technol 2007, 98: 2935–2941. 10.1016/j.biortech.2006.10.012
Keiluweit M, Nico PS, Johnson MG, Kleber M: Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 2010, 44: 1247–1253. 10.1021/es9031419
Kim KH, Kim JY, Cho TS, Choi JW: Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). Bioresource Technol 2012, 118: 158–162.
Glaser B, Lehmann J, Zech W: Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biol Fert Soils 2002, 35: 219–230. 10.1007/s00374-002-0466-4
Fellet G, Marchiol L, Delle Vedove G, Peressotti A: Application of biochar on mine tailings: effects and perspectives for land reclamation. Chemosphere 2011, 83: 1262–1267. 10.1016/j.chemosphere.2011.03.053
Al-Abeda SR, Jegadeesan G, Purandare J, Allen D: Leaching behavior of mineral processing waste: comparison of batch and column investigations. J Hazard Mater 2008, 153: 1088–1092. 10.1016/j.jhazmat.2007.09.063
Brubaker TM, Stewart BW, Capo RC, Schroeder KT, Chapman EC, Spivak-Birndorf LJ, Vesper DJ, Cardone CR, Rohar PC: Coal fly ash interaction with environmental fluids: geochemical and strontium isotope results from combined column and batch leaching experiments. Appl Geochem 2013, 32: 184–194.
Jackson DR, Garrett BC, Bishop TA: Comparison of batch and column methods for assessing leachability of hazardous-waste. Environ Sci Technol 1984, 18: 668–673. 10.1021/es00127a007
Doye I, Duchesne J: Column leaching test to evaluate the use of alkaline industrial wastes to neutralize acid mine tailings. J Environ Eng-ASCE 2005, 131: 1221–1229. 10.1061/(ASCE)0733-9372(2005)131:8(1221)
Kossoff D, Hudson-Edwards KA, Dubbin WE, Alfredsson MA: Incongruent weathering of Cd and Zn from mine tailings: a column leaching study. Chem Geol 2011, 281: 52–71. 10.1016/j.chemgeo.2010.11.028
Doepker R, O'Connor W: Column leach study II: heavy metal dissolution characteristics from selected lead-zinc mine tailings. Mine Water Environ 1991, 10: 73–92.
Salinas Villafane OR, Igarashi T, Kurosawa M, Takase T: Comparison of potentially toxic metals leaching from weathered rocks at a closed mine site between laboratory columns and field observation. Appl Geochem 2012, 27: 2271–2279. 10.1016/j.apgeochem.2012.08.013
Deopker R, O'Connor W: Column leach study 1: heavy metal dissolution characteristics from selected copper mine tailings. Mine Water Environ 1991, 10: 57–71.
Harris W: X-ray diffraction techniques for soil mineral identification. In Methods of Soil Analysis Part 5 Mineralogical Methods. Edited by: Ulery AL, Drees LR. Madison, USA: Soil Science Society of America; 2007:81–115.
Madsen PA, Mulligan DR: Effect of NaCl on emergence and growth of a range of provenances of Eucalyptus citriodora, Eucalyptus populnea, Eucalyptus camaldulensis and Acacia salicina. Forest Ecol Manag 2006, 228: 152–159. 10.1016/j.foreco.2006.02.044
Gozzard E, Vink S, Nanjappa V, Moran CJ: Salt dissolution dynamics on surface mine spoils. In Proceedings of Water in Mining: September 15–17 2009. Perth, Australia. Carlton: Australasian Institute of Mining & Metallurgy; 2009:233–240.
Park JH, Li X, Edraki M, Baumgartl T, Kirsch B: Geochemical assessments and classification of coal mine spoils for better understanding of potential salinity issues at closure. Environ Sci Process Impacts 2013,15(6):1235–1244. 10.1039/c3em30672k
Bui EN, Moran CJ: Regional-scale investigation of the spatial distribution and origin of soluble salts in central north Queensland. Hydrol Process 2000, 14: 237–250. 10.1002/(SICI)1099-1085(20000215)14:2<237::AID-HYP922>3.0.CO;2-E
Herczeg AL, Dogramaci SS, Leaney FWJ: Origin of dissolved salts in a large, semi-arid groundwater system: Murray Basin, Australia. Mar Freshwater Res 2001, 52: 41–52. 10.1071/MF00040
Dold B, Fontbote L: Element cycling and secondary mineralogy in porphyry copper tailings as a function of climate, primary mineralogy, and mineral processing. J Geochem Explor 2001, 74: 3–55. 10.1016/S0375-6742(01)00174-1
Banwart SA, Malmström ME: Hydrochemical modelling for preliminary assessment of minewater pollution. J Geochem Explor 2001, 74: 73–97. 10.1016/S0375-6742(01)00176-5
Hunter G, Whiteman P: Revegetation of mine wastes at Mt. Isa, Queensland. I. The nature of the material and defining the problems. Aust J Exp Agr 1975, 15: 513–518. 10.1071/EA9750513
Piha MI, Vallack HW, Reeler BM, Michael N: A low-input approach to vegetation establishment on mine and coal ash wastes in semiarid regions. I. Tin mine tailings in Zimbabwe. J Appl Ecol 1995, 32: 372–381. 10.2307/2405103
Huang LN, Tang FZ, Song YS, Wan CY, Wang SL, Liu WQ, Shu WS: Biodiversity, abundance, and activity of nitrogen-fixing bacteria during primary succession on a copper mine tailings. FEMS Microbiol Ecol 2011, 78: 439–450. 10.1111/j.1574-6941.2011.01178.x
Steiner C, Teixeira WG, Lehmann J, Nehls T, de Macedo JLV, Blum WEH, Zech W: Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291: 275–290. 10.1007/s11104-007-9193-9
Olivier CF Masters Thesis. In An investigation into the degradation of biochar and its interactions with plants and soil microbial community. Stellenbosch University, Department of Soil Science, Faculty of Agri Sciences; 2011.
Kuzyakov Y, Subbotina I, Chen HQ, Bogomolova I, Xu XL: Black carbon decomposition and incorporation into soil microbial biomass estimated by C-14 labeling. Soil Biol Biochem 2009, 41: 210–219. 10.1016/j.soilbio.2008.10.016
Jobbagy EG, Jackson RB: Patterns and mechanisms of soil acidification in the conversion of grasslands to forests. Biogeochemistry 2003, 64: 205–229. 10.1023/A:1024985629259
Altaher H, Dietrich A, Novak J: Factors affecting copper sorption and mobility through A and B horizon soils from the eastern shore of Virginia. Yanbu J Eng Sci 2011, 2: 91–105.
Edwards KJ, Bond PL, Gihring TM, Banfield JF: An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 2000, 287: 1796–1799. 10.1126/science.287.5459.1796
Baker BJ, Banfield JF: Microbial communities in acid mine drainage. FEMS Microbiol Ecol 2003, 44: 139–152. 10.1016/S0168-6496(03)00028-X
Cleemput O, Samater A: Nitrite in soils: accumulation and role in the formation of gaseous N compounds. Fertilizer Research 1995, 45: 81–89. 10.1007/BF00749884
Hamilton JL, Lowe RH: Organic-matter and N-effects on soil nitrite accumulation and resultant nitrite toxicity to tobacco transplants. Agron J 1981, 73: 787–790. 10.2134/agronj1981.000219620073000500010x
Smith CJ, Chalk PM: Mineralization of nitrite fixed by soil organic-matter. Soil Biol Biochem 1979, 11: 515–519. 10.1016/0038-0717(79)90011-7
Diaby N, Dold B, Pfeifer HR, Holliger C, Johnson DB, Hallberg KB: Microbial communities in a porphyry copper tailings impoundment and their impact on the geochemical dynamics of the mine waste. Environ Microbiol 2007, 9: 298–307. 10.1111/j.1462-2920.2006.01138.x
De la Iglesia R, Castro D, Ginocchio R, van der Lelie D, Gonzalez B: Factors influencing the composition of bacterial communities found at abandoned copper-tailings dumps. J Appl Microbiol 2006, 100: 537–544. 10.1111/j.1365-2672.2005.02793.x
Davey KJ, Zhu R, Nielsen P, Bruckard WJ: Full Value Recovery from EH Tailings. Clayton: CSIEO Minerals; 2008.
OECD: Leaching in Soil Columns vol. 312. Paris: Organisation for Economic Co-operation and Development; 2004.
Hendrickx JMH, Flury M: Uniform and preferential flow mechanisms in the vadose zone. In Conceptual Models of Flow and Transport in the Fractured Vadose Zone. Edited by: Panel on conceptual models of flow and transport in the fractured vadose zone. Washington, D.C: National Academies; 2001:149–187.
Stefansson A, Gunnarsson I, Giroud N: New methods for the direct determination of dissolved inorganic, organic and total carbon in natural waters by reagent-free (TM) ion chromatography and inductively coupled plasma atomic emission spectrometry. Anal Chim Acta 2007, 582: 69–74. 10.1016/j.aca.2006.09.001
Gilardi G, Abis L, Cass AEG: Carbon-13 CP/MAS solid-state NMR and FT-IR spectroscopy of wood cell wall biodegradation. Enzyme Microb Tech 1995, 17: 268–275. 10.1016/0141-0229(94)00019-N
Jenkinson DS, Powlson DS: Effects of biocidal treatments on metabolism in soil 1 fumigation with chloroform. Soil Biol Biochem 1976, 8: 167–177. 10.1016/0038-0717(76)90001-8
Vance ED, Brookes PC, Jenkinson DS: An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 1987, 19: 703–707. 10.1016/0038-0717(87)90052-6
Chen CR, Condron LM, Davis MR, Sherlock RR: Effects of afforestation on phosphorus dynamics and biological properties in a New Zealand grassland soil. Plant Soil 2000, 220: 151–163. 10.1023/A:1004712401721
Acknowledgments
We would like to thank Dr. Anya Josefa Yago in the Department of Microscopy and Microanalysis, The University of Queensland for mineralogical analysis. This project is financially supported by UQ Postdoctoral Fund, UQ Early Career Researcher Grants (ID ECR122) and Xstrata Copper Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
XL performed all experiments and drafted the manuscript. FY was responsible for carbon and microbial biomass analyses. ES and FY were responsible for NMR analysis. LH and ME participated in the design of the study and were involved in drafting the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
12302_2013_118_MOESM1_ESM.docx
Additional file 1: Figures S1 to S5: Dynamics of major cations and anions, nitrate and nitrite, and Cu, CP/MAS 13C NMR spectra, and schematic diagram of the column leaching setup. (DOCX 363 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Li, X., You, F., Huang, L. et al. Dynamics in leachate chemistry of Cu-Au tailings in response to biochar and woodchip amendments: a column leaching study. Environ Sci Eur 25, 32 (2013). https://doi.org/10.1186/2190-4715-25-32
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2190-4715-25-32