Abstract
Background
The Lung Injury Score (LIS) remains a commonly utilized measure of lung injury severity though the additive value of LIS to predict ARDS outcomes over the recent Berlin definition of ARDS, which incorporates severity, is not known.
Methods
We tested the association of LIS (in which scores range from 0 to 4, with higher scores indicating more severe lung injury) and its four components calculated on the day of ARDS diagnosis with ARDS morbidity and mortality in a large, multi-ICU cohort of patients with Berlin-defined ARDS. Receiver Operator Characteristic (ROC) curves were generated to compare the predictive validity of LIS for mortality to Berlin stages of severity (mild, moderate and severe).
Results
In 550 ARDS patients, a one-point increase in LIS was associated with 58% increased odds of in-hospital death (95% CI 14 to 219%, P = 0.006), a 7% reduction in ventilator-free days (95% CI 2 to 13%, P = 0.01), and, among patients surviving hospitalization, a 25% increase in days of mechanical ventilation (95% CI 9 to 43%, P = 0.001) and a 16% increase (95% CI 2 to 31%, P = 0.02) in the number of ICU days. However, the mean LIS was only 0.2 points higher (95% CI 0.1 to 0.3) among those who died compared to those who lived. Berlin stages of severity were highly correlated with LIS (Spearman’s rho 0.72, P < 0.0001) and were also significantly associated with ARDS mortality and similar morbidity measures. The predictive validity of LIS for mortality was similar to Berlin stages of severity with an area under the curve of 0.58 compared to 0.60, respectively (P-value 0.49).
Conclusions
In a large, multi-ICU cohort of patients with ARDS, both LIS and the Berlin definition severity stages were associated with increased in-hospital morbidity and mortality. However, predictive validity of both scores was marginal, and there was no additive value of LIS over Berlin. Although neither LIS nor the Berlin definition were designed to prognosticate outcomes, these findings suggest that the role of LIS in characterizing lung injury severity in the era of the Berlin definition ARDS may be limited.
Similar content being viewed by others
Background
Mortality in the acute respiratory distress syndrome (ARDS) has declined significantly in the last decade as a result of improved supportive care, treatments for sepsis and multi-organ failure, and the advent of low tidal volume ventilation [1, 2]. Accurate clinical measures of severity of ARDS and mortality prediction are necessary to select appropriate patients for clinical trials to detect the beneficial effect of novel therapies [3–6]. Although not designed to prognosticate outcomes, the recent Berlin definition for ARDS was created, in part, to address the need for a consistent measure of severity of ARDS that corresponded with clinical outcomes [7]. Generated using an experimental method combined with a consensus process to define ARDS severity, the Berlin group considered the degree of hypoxemia (PaO2/FiO2), in combination with ancillary variables for severe ARDS including radiographic severity, respiratory system compliance, positive end-expiratory pressure (PEEP) and corrected expired volume per minute to define lung injury severity. After testing these variables in over 4,000 patients from multiple centers, they found that only hypoxemia contributed to the predictive validity of the definition. These findings raise the question of the utility of these ancillary variables in describing severity of lung injury.
The Lung Injury Score (LIS), proposed in 1988 by Murray and colleagues, [8] has been a commonly utilized measure of acute lung injury (ALI) severity in clinical studies. Derived empirically by expert consensus, the score is composed of four components: 1) chest radiograph; 2) hypoxemia score; 3) PEEP; and 4) static compliance of respiratory system. The LIS preceded the first consensus definition of ALI/ARDS (American-European Consensus Committee (AECC) definition for ALI/ARDS) in 1994, [9] and was designed to measure the pathophysiological features of ARDS; however, it has not been validated as an accurate measure of lung injury severity and its use is not specific to ARDS [10]. Nonetheless, LIS has become a standard measure of ARDS severity that remains widely used. In this capacity, LIS has been presented as a measure of baseline lung injury severity in ARDS clinical studies. In addition, LIS ≥ 3 has been commonly used to identify severe ARDS for consideration of possible rescue therapies, [11–13] and changes in LIS over time have been used as a primary physiologic endpoint to study the efficacy of interventions [12, 14]. The original manuscript [8], cited in over 1,400 scholarly articles, has accumulated over 67 new citations since 2012, many of which were published following the announcement of the Berlin definition of ARDS [15].
Although LIS is frequently employed as a measure of the severity of lung injury, its validity in predicting acute lung injury-related outcomes has not been rigorously studied in the era of lung-protective ventilation. Furthermore, the utility of LIS in the context of the recently described Berlin definition of ARDS, [7] which subdivides ARDS into three levels of severity of hypoxemia, is not known. Thus, it is important to determine whether LIS remains a useful measure of lung injury severity in the broad spectrum of patients treated for ARDS in modern clinical practice. This study was designed to compare the association between LIS and Berlin severity stages and in-hospital mortality and morbidity in a large, prospective, multi-ICU cohort of patients meeting the Berlin definition of ARDS.
Methods
Subjects
We analyzed data drawn from a multi-ICU, prospective cohort study, entitled the Validation of biomarkers in Acute Lung Injury Diagnosis (VALID) study, the primary purpose of which is to identify biomarkers of diagnosis and prognosis in ARDS. Patients at Vanderbilt University Medical Center (VUMC) were enrolled between January 2006 and February 2011, and details of study enrollment and informed consent have been described previously [16–18]. Briefly, enrolled patients were admitted to the medical, surgical, trauma or cardiovascular ICUs at VUMC if they remained in the ICU the morning of day two. Exclusions included ICU stay greater than 48 hours prior to VUMC ICU admission, uncomplicated overdose, severe chronic lung disease, plans to transfer out of ICU on day two and non-mechanically ventilated or post-surgical patients in the cardiovascular ICU.
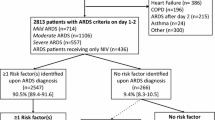
For this analysis, we included patients who met the Berlin definition of ARDS [7]. Patients were classified according to Berlin level of severity as mild (PaO2/FiO2 200 to ≤ 300 mmHg with PEEP ≥ 5 cm H2O or continuous positive airway pressure (CPAP) ≥ 5 cm H2O), moderate (PaO2/FiO2 100 to ≤ 200 mmHg with PEEP ≥ 5 cm H2O), and severe (PaO2/FiO2 ≤ 100 mmHg with PEEP ≥ 5 cm H2O). The diagnosis of ARDS could be established at any time during the first four days in the ICU. For diagnosis, the ratio of pulse oximetric saturation to fraction of inspired oxygen (SpO2/FiO2) was used as a validated surrogate for PaO2/FiO2 among patients without an arterial blood gas measurement at the time of ARDS diagnosis: SpO2/FiO2 = 64 + 0.84 × (PaO2/FiO2) [19]. Among 2,325 patients enrolled in VALID, we included 550 patients who met Berlin definition criteria for ARDS (Flow diagram Figure 1).
The Institutional Review Board (IRB) at Vanderbilt University (IRB#051065) approved the study.
Informed consent
Informed consent was obtained from patients or their surrogates whenever possible. For patients who were unable to provide informed consent due to their clinical condition and for whom no surrogates were available, a waiver of informed consent was granted by the IRB due to the minimal risk of the observational study.
Lung Injury Score
The Lung Injury Score and each of its components [8] and Berlin severity levels were calculated from the most severe measures on the day of ARDS diagnosis or on the initial enrollment day in VALID, whichever came last. For LIS, each of the four components is categorized from 0 to 4, where a higher number is worse (Table 1). The total LIS is obtained by dividing the aggregate sum by the number of components used. For example, if one component is unavailable then the LIS would be the sum of the three other components divided by three.
Outcomes
The primary outcome was in-hospital mortality, defined as death during the incident hospitalization. Secondary outcomes included 28-day ventilator-free days (VFD), defined as the number of days alive and free of mechanical ventilation to day 28, with VFD = 0 for patients who died in the first four weeks, [20] and among survivors to hospital discharge: days of mechanical ventilation; days in ICU; and length of hospital stay.
Other prognostic indices
We compared LIS and Berlin level of severity to two well-validated, general severity of illness scores: (1) Acute Physiology and Chronic Health Evaluation (APACHE) II, [21] and (2) Simplified Acute Physiology Score (SAPS) II [22]. Both scores were calculated on the day of enrollment into VALID on the morning of ICU day two.
Definitions of ARDS risk factors
Sepsis was defined according to consensus definition [23] as evidence of infection and at least two criteria of systemic inflammatory response syndrome. Trauma was defined as major blunt or penetrating traumatic injury necessitating admission to the trauma ICU. To diagnose pneumonia, two or more of the following criteria were required: (1) new infiltrate on chest radiograph; (2) temperature higher than 38°C or lower than 36°C or white blood counts more than 12,000/μl, less than 4,000/μl or band forms more than 10%; (3) positive microbiologic culture. Aspiration was defined as witnessed or suspected aspiration events or retrieval of gastric contents from the airway or endotracheal tube.
Statistical analysis
For all analyses, the LIS score was treated as a continuous variable and LIS components as ordinal variables. Berlin severity levels were treated as ordinal variables. To determine differences in LIS score according to in-hospital mortality, we used the Student’s t-test. For LIS components and Berlin severity levels, P-values were generated using the Wilcoxon-rank sum test. The association between LIS and secondary outcomes was tested using the Spearman correlation test. We compared the discrimination of LIS and Berlin severity to the general severity scores using Receiver Operating Characteristic (ROC) curves and used the test of equality of ROC areas to determine differences across severity indices. Calibration of the model to evaluate the concordance of observed and predicted mortality was evaluated with Hosmer-Lemeshow goodness-of-fit test, and mortality was categorized according to four different LIS categories (0 to 1.0; 1.1 to 2.0; 2.1 to 3.0; > 3.0) to identify whether there was trend between increasing mortality with each increased point in the LIS score.
As a sensitivity analysis, we tested the prognostic value of LIS in two ‘severe ARDS’ subgroups as defined as (1) LIS ≥ 3, a cut-point used to identify ‘refractory ARDS’ for consideration of possible rescue therapies; [11–13] and (2) PEEP values ≥ 10 cm H2O, a cutoff which has been associated with improved consistency in measurement of hypoxemia [24].
Analyses were performed using STATA version 12.1 (STATA Corp, College Station, TX, USA). Statistical significance was defined as a two-tailed P < 0.05 for all analyses.
Results
Table 2 shows baseline characteristics among 550 patients with ARDS included in this study. The sample was 59% male and 85% white. Trauma, sepsis, and pneumonia were the top ARDS risk factors, and mean LIS differed significantly across ARDS risk factor groups, driven by a higher mean LIS score among patients with pneumonia (P < 0.0001) (Figure 2). Patients who died in the hospital were older, more likely to be white (which was accounted for by racial differences in ARDS risk factor), had increased severity of illness on presentation, and were more likely to have sepsis and less likely to have trauma as a risk factor for ARDS (Table 2).
Box plot comparison of Lung Injury Score according to acute respiratory distress syndrome (ARDS) risk factor in 550 patients with Berlin-defined ARDS. The line in the middle of the box represents the median and the lines that form the box correspond to the 25th and 75th percentiles. The LIS differs significantly across ARDS risk factor group, which is driven by an increased LIS among patients with pneumonia, P < 0.0001. Compared to all other causes of ARDS, mean baseline LIS for patients with pneumonia as a primary cause of ARDS is 0.5 points higher (95% CI 0.3 to 0.6).
LIS and clinical outcomes
The mean LIS was 2.7 ± 0.6 in hospital survivors (N = 415) compared to 2.9 ± 0.6 in non-survivors (N = 135) (Table 3). This 0.2 point difference (95% CI 0.1 to 0.3) was statistically significant P = 0.006. The association between LIS and in-hospital death was not modified by the ARDS risk factor (P = 0.19 for heterogeneity) or the presence of sepsis in the first four days after enrollment (P = 0.68). The LIS components most strongly associated with overall mortality were the PaO2/FiO2 (P < 0.001) and the level of PEEP (P = 0.02). The chest radiograph and compliance scores did not differ according to mortality (Table 3). A one-point increase in LIS was associated with a 58% increased odds of in-hospital death (OR 1.58, 95% CI 1.14 to 2.19, P = 0.006).
Among secondary outcomes evaluated, VFDs were inversely associated with LIS (Spearman’s rho = −0.17, P = 0.0001) with a 7% reduction in VFD (95% CI 2 to 13%, P-value = 0.01) for every one-point increase in LIS. Among 415 hospital survivors, the LIS was positively associated with days of mechanical ventilation (Spearman’s rho = 0.17, P = 0.0004) and days in the ICU (Spearman’s rho = 0.13, P = 0.007). For every one-point increase in LIS, there was a 25% increase (95% CI 9 to 43%, P = 0.001) in number of days of mechanical ventilation and a 16% increase (95% CI 2 to 31%, P = 0.02) in number of ICU days. The LIS did not predict total days of hospitalization (Spearman’s rho = 0.07, P = 0.15) among hospital survivors.
LIS and Berlin definition
LIS was highly correlated with the Berlin oxygenation criteria (Spearman’s rho of 0.72, P < 0.0001). LIS increased with each increased level of Berlin severity (Figure 3 and Table 4). Among patients characterized by the Berlin definition as mild, the mean LIS was 2.1 ± 0.4 compared to a mean LIS of 2.5 ± 0.5 in moderate and 3.3 ± 0.4 in severe Berlin definition ARDS (P < 0.001 for trend).
Box plot comparison of Lung Injury Score according to Berlin definition severity in 550 patients with Berlin definition acute respiratory distress syndrome (ARDS). The line in the middle of the box represents the median and the lines that form the box correspond to the 25th and 75th percentiles. The LIS increases with increase Berlin definition severity, P < 0.001 for trend.
An increased Berlin level of ARDS severity was associated with worse clinical outcomes in ARDS including increased in-hospital mortality, decreased ventilator-free days, and increased duration of mechanical ventilation in survivors (Table 4).
Comparison of LIS and Berlin severity to general severity scores
The area under the ROC curve (AUC) of LIS for hospital mortality was 0.58 (95% CI 0.53 to 0.64) compared to an AUC of 0.60 (95% CI 0.55 to 0.65) for the Berlin severity scores. There was no statistically significant difference between these values, P-value = 0.47. Compared to the LIS, the AUC for APACHE II 0.66 (0.61 to 0.71) was significantly higher (P = 0.04); the AUC for SAPS II was also higher (0.63, 95% CI 0.58 to 0.69) but this difference was not statistically significant (P = 0.22) (Figure 4). Calibration was adequate for LIS, Berlin severity and both severity of illness scores, with similar expected to observed mortality across subgroups (data not shown). All scores passed the Hosmer-Lemeshow goodness-of-fit test with P-values > 0.25. Figure 5 demonstrates that mortality increased for each one-point increase in LIS.
Sensitivity analyses
Using a cut-point of a LIS score of ≥ 3, high LIS was present in 45% (N = 249) of patients on the day of ARDS diagnosis, with 30% mortality in the high LIS group compared with 20% mortality in the lower LIS group (P = 0.006). The AUC at this cut-point was 0.57 (95% CI 0.52 to 0.62). Discrimination of LIS was not improved in 342 (62%) patients with PEEP values ≥ 10 cm H2O (AUC 0.58, 95% CI 0.51 to 0.65).
Discussion
Although the four-point LIS was never intended as a prognostic tool, it has been used as a measure of the severity of lung injury that thereby infers prognostic value. The recently developed Berlin definition of ARDS includes a measure of lung injury severity based on three levels of hypoxemia and baseline PEEP of at least 5 cm H2O [7]. Our study tested the predictive validity of LIS on mortality and morbidity in a large, heterogeneous group of patients with Berlin definition ARDS. We found that although LIS at the time of ARDS diagnosis was associated with in-hospital mortality, the difference between mean LIS in those who died and lived was only 0.2 points, a difference of minimal clinical significance. Of the four LIS components, only PaO2/FiO2 and PEEP categories were associated with mortality overall. The discrimination of LIS for in-hospital mortality was comparable to the Berlin severity scale and only marginally better than chance alone. The predictive validity was not improved when evaluated in more severe subgroups such as those with higher PEEP or LIS ≥ 3. We also found that LIS was predictive of the duration of mechanical ventilation and days in the ICU. This finding was important, as LIS may be more suited to discriminate pulmonary-specific outcomes. The Berlin definition severity stages were associated with mortality and also found to be associated with increased mechanical ventilation requirements. The predictive validity of Berlin criteria was similarly marginal and failed to identify three distinct mortality classes with a mortality of 18% in mild versus 19% in moderate ARDS.
Overall, our findings are consistent with the findings presented in the development of the Berlin definition for ARDS [7]. Although several components included in the LIS were considered for the Berlin definition, including severity of radiographic criteria, higher levels of PEEP, and static respiratory compliance, they were ultimately dropped in the final Berlin definition for lack of additive predictive value. Similarly, we found that only PEEP category and level of hypoxemia in the LIS were associated with mortality. We also found that both LIS and the Berlin definition were associated with the duration of mechanical ventilation. Only LIS was significantly associated with duration of ICU stay among survivors, though this correlation was weak and there was a similar, non-statistical trend for the Berlin severity stages.
Clinical measures of severity of lung injury have limitations. First, measures of lung injury will not perform well as prognostic measures because non-pulmonary factors including age, severity of sepsis, co-morbidities and non-pulmonary organ failure remain the most influential predictors of hospital mortality in ARDS, [25–39] and non-resolving respiratory failure accounts for less than 20% of ARDS deaths [34–36]. Also, although the finding that PaO2/FiO2 level is associated with mortality is consistent with the findings reported from cohorts used for empirical analysis in the development of the Berlin ARDS definition, [40–46] this has not been demonstrated consistently, a finding that may be attributable, in part, to practice variability in mechanical ventilation settings, which is known to have a large effect on PaO2/FiO2 levels [5, 6, 24, 47, 48]. Furthermore, post-mortem studies highlight the poor accuracy that clinical definitions such as the Berlin criteria have for histological definitions of diffuse alveolar damage, which are found in only a minority of patients with clinical ARDS [49, 50]. Nonetheless, clinical measures of lung injury severity remain necessary to identify patients for ARDS treatments and clinical trials.
To our knowledge, our study is the first to report a statistically significant association between LIS and mortality in ARDS [5, 24, 26, 28, 31, 41, 51]. Prior studies were limited by small sample size (largest N = 259), mostly considered only a subset of patients with ARDS (PaO2/FIO2 < 200 mmHg,) [26, 28, 31, 51], and were largely conducted prior to recent treatment advancements including low tidal volume ventilation. At least two studies demonstrated a similarly increased LIS in non-survivors of ARDS compared to survivors as observed in the current study (approximately 0.2 points), but did not have the power to detect a statistically significant difference [5, 41]. Furthermore, a study by Villar et al. identified LIS as an independent predictor of developing established AECC-defined ARDS after one day of standardized ventilator management [24], which is consistent with our finding that LIS predicts pulmonary outcomes. The lack of a significant association between LIS and clinical outcomes in these smaller studies underscores our finding that LIS may be a marginal though not clinically relevant predictor of outcomes. Finally, it is also possible that we detected an association between LIS and clinical outcomes as a result of limiting our sample to patients meeting the Berlin definition of ARDS, a definition that was created to improve the validity and reliability of the ARDS diagnosis [7].
It is important to note that the mortality rate in our study was lower than in several other epidemiological studies on ARDS [7, 52–55]. This was in part due to the high proportion of patients with trauma-related ARDS, for whom the in-hospital mortality rate was 13%. Also, our outcome was in-hospital mortality rather than 60- or 90-day mortality in some other studies. However, even with a lower overall mortality, we detected a statistically significant association between both LIS and Berlin severity and both in-hospital death and more prolonged respiratory failure, demonstrating sufficient power. We recently demonstrated that death after discharge from ARDS hospitalization is more related to underlying co-morbid illness and age rather than severity of ARDS and determined that in-hospital mortality would be more sensitive to measures of lung injury severity [56]. Furthermore, using an endpoint of mortality at 90 days did not change our results (data not shown) although overall mortality increased by 10%.
Despite a statistically significant association between LIS and outcomes, these results should be considered in view of the current use of LIS for clinical and research applications in the context of a the new Berlin definition for ARDS. A single LIS measurement on initial diagnosis of ARDS did not provide additive prognostic information over the three severity categories of the Berlin definition alone. In addition, with only marginal discrimination for mortality, our results do not clearly support the use of LIS cut-points used to define refractory ARDS for consideration of experimental approaches such as extracorporeal membrane oxygenation [13].
Several limitations of the study deserve mention. First, it was performed at a single, tertiary care site; therefore, generalizing the results to other settings may be limited. However, using a large, multi-ICU sample, including a broad range of patients with ARDS, is likely to improve generalizability overall. Furthermore, our test of the prognostic value of the Berlin definition severity stages replicated those described in the initial Berlin definition, further suggesting our sample is representative of a broader ARDS population. Second, we did not evaluate the prognostic value of change in LIS over time. Further studies will be required to determine whether measurement of change in LIS is a more useful prognostic indicator as has been previously suggested [57]. Third, our primary outcome was all-cause in-hospital mortality, so we were not able to directly assess the prognostic value of LIS in identifying the minority of patients with ARDS who die of respiratory failure. However, secondary outcomes of VFD and days of mechanical ventilation were assessed as measures of pulmonary-specific outcomes. Lastly, our general severity scores were generated over the first 24 hours of enrollment, whereas LIS was calculated on the day of ARDS diagnosis. This was the same day for the vast majority (N = 419, 76%) of the study cohort. However, the time difference for the 24% should provide an advantage for LIS as a prognostic marker. Therefore, improved test characteristics in the general severity scores compared to LIS may have been an underestimate of this difference.
Conclusions
In conclusion, the LIS remains a widely utilized measure of initial lung injury severity in ARDS but does not provide additional prognostic value for mortality or duration of mechanical ventilation compared to the Berlin definition of ARDS. White the four-point LIS may still have value for research purposes to more completely define abnormal lung physiology, it has limited value for estimating prognosis in ARDS patients.
Abbreviations
- AECC:
-
American-European Consensus Committee
- ALI:
-
acute lung injury
- ARDS:
-
acute respiratory distress syndrome
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- AUC:
-
area under the curve
- CPAP:
-
continuous positive airway pressure
- IRB:
-
Institutional Review Board
- LIS:
-
Lung Injury Score
- PaO2/FiO2:
-
ratio of arterial oxygen concentration to the fraction of inspired oxygen
- PEEP:
-
positive end-expiratory pressure
- ROC:
-
Receiver Operator Characteristic
- SAPS:
-
Simplified Acute Physiology Score
- SpO2/FiO2:
-
ratio of the pulse oximetric saturation to the fraction of inspired oxygen
- VALID:
-
Validation of biomarkers in Acute Lung Injury Diagnosis
- VFD:
-
ventilator-free days
- VUMC:
-
Vanderbilt University Medical Center.
References
Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD: Recent trends in acute lung injury mortality: 1996 to 2005. Crit Care Med 2009,37(5):1574–1579. 10.1097/CCM.0b013e31819fefdf
Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, Jimenez E, Mohan A, Khan RA, Whittle J, et al.: Nationwide trends of severe sepsis in the 21st century (2000 to 2007). Chest 2011,140(5):1223–1231. 10.1378/chest.11-0352
Altman DG: Statistics and ethics in medical research: III how large a sample? Br Med J 1980,281(6251):1336–1338. 10.1136/bmj.281.6251.1336
Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, Gotzsche PC, Lang T: The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001,134(8):663–694. 10.7326/0003-4819-134-8-200104170-00012
Villar J, Perez-Mendez L, Basaldua S, Blanco J, Aguilar G, Toral D, Zavala E, Romera MA, Gonzalez-Diaz G, Nogal FD, et al.: A risk tertiles model for predicting mortality in patients with acute respiratory distress syndrome: age, plateau pressure, and P(aO(2))/F(IO(2)) at ARDS onset can predict mortality. Respir Care 2011,56(4):420–428. 10.4187/respcare.00811
Villar J, Perez-Mendez L, Blanco J, Anon JM, Blanch L, Belda J, Santos-Bouza A, Fernandez RL, Kacmarek RM, Spanish Initiative for Epidemiology S, et al.: A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting – a prospective, multicenter validation study. Intensive Care Med 2013,39(4):583–592. 10.1007/s00134-012-2803-x
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS: Acute respiratory distress syndrome: the Berlin definition. JAMA 2012,307(23):2526–2533.
Murray JF, Matthay MA, Luce JM, Flick MR: An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988,138(3):720–723. 10.1164/ajrccm/138.3.720
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R: The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994,149(3 Pt 1):818–824.
Troche G, Moine P: Is the duration of mechanical ventilation predictable? Chest 1997,112(3):745–751. 10.1378/chest.112.3.745
Diaz JV, Brower R, Calfee CS, Matthay MA: Therapeutic strategies for severe acute lung injury. Crit Care Med 2010,38(8):1644–1650. 10.1097/CCM.0b013e3181e795ee
Meduri GU, Chinn AJ, Leeper KV, Wunderink RG, Tolley E, Winer-Muram HT, Khare V, Eltorky M: Corticosteroid rescue treatment of progressive fibroproliferation in late ARDS. Patterns of response and predictors of outcome. Chest 1994,105(5):1516–1527. 10.1378/chest.105.5.1516
Peek GJ, Clemens F, Elbourne D, Firmin R, Hardy P, Hibbert C, Killer H, Mugford M, Thalanany M, Tiruvoipati R, et al.: CESAR: conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res 2006, 6: 163. 10.1186/1472-6963-6-163
Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, Gibson M, Umberger R: Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 2007,131(4):954–963. 10.1378/chest.06-2100
Murray JF, Matthay MA, Luce JM, Flick MR: Web of science citation report of articles citing: an expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988,138(3):720–723. [http://apps.webofknowledge.com/summary.do?SID=2F4E2Odl57KCeSSIpo4&product=WOS&qid=1&search_mode=GeneralSearch] 10.1164/ajrccm/138.3.720
Siew ED, Ware LB, Gebretsadik T, Shintani A, Moons KG, Wickersham N, Bossert F, Ikizler TA: Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol 2009,20(8):1823–1832. 10.1681/ASN.2008070673
Janz DR, Bastarache JA, Peterson JF, Sills G, Wickersham N, May AK, Roberts LJ 2nd, Ware LB: Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: an observational study. Crit Care Med 2013,41(3):784–790. 10.1097/CCM.0b013e3182741a54
O’Neal HR Jr, Koyama T, Koehler EA, Siew E, Curtis BR, Fremont RD, May AK, Bernard GR, Ware LB: Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med 2011,39(6):1343–1350. 10.1097/CCM.0b013e3182120992
Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB: Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007,132(2):410–417. 10.1378/chest.07-0617
Schoenfeld DA, Bernard GR: Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 2002,30(8):1772–1777. 10.1097/00003246-200208000-00016
Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II: a severity of disease classification system. Crit Care Med 1985,13(10):818–829. 10.1097/00003246-198510000-00009
Le Gall JR, Lemeshow S, Saulnier F: A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 1993,270(24):2957–2963. 10.1001/jama.1993.03510240069035
American college of chest physicians/society of critical care medicine consensus conference:: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med; 1992. 20: 864–874
Villar J, Perez-Mendez L, Lopez J, Belda J, Blanco J, Saralegui I, Suarez-Sipmann F, Lubillo S, Kacmarek RM: An early PEEP/FIO2 trial identifies different degrees of lung injury in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2007,176(8):795–804. 10.1164/rccm.200610-1534OC
Bone RC, Maunder R, Slotman G, Silverman H, Hyers TM, Kerstein MD, Ursprung JJ: An early test of survival in patients with the adult respiratory distress syndrome. The PaO2/FIo2 ratio and its differential response to conventional therapy. Prostaglandin E1 Study Group. Chest 1989,96(4):849–851. 10.1378/chest.96.4.849
Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA: Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med 1995,152(6 Pt 1):1818–1824.
Ely EW, Wheeler AP, Thompson BT, Ancukiewicz M, Steinberg KP, Bernard GR: Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med 2002,136(1):25–36.
Monchi M, Bellenfant F, Cariou A, Joly LM, Thebert D, Laurent I, Dhainaut JF, Brunet F: Early predictive factors of survival in the acute respiratory distress syndrome. A multivariate analysis. Am J Respir Crit Care Med 1998,158(4):1076–1081. 10.1164/ajrccm.158.4.9802009
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA: Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 2002,346(17):1281–1286. 10.1056/NEJMoa012835
Seeley E, McAuley DF, Eisner M, Miletin M, Matthay MA, Kallet RH: Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax 2008,63(11):994–998. 10.1136/thx.2007.093658
Zilberberg MD, Epstein SK: Acute lung injury in the medical ICU: comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med 1998,157(4 Pt 1):1159–1164.
Cooke CR, Shah CV, Gallop R, Bellamy S, Ancukiewicz M, Eisner MD, Lanken PN, Localio AR, Christie JD: A simple clinical predictive index for objective estimates of mortality in acute lung injury. Crit Care Med 2009,37(6):1913–1920. 10.1097/CCM.0b013e3181a009b4
Cooke CR, Kahn JM, Caldwell E, Okamoto VN, Heckbert SR, Hudson LD, Rubenfeld GD: Predictors of hospital mortality in a population-based cohort of patients with acute lung injury. Crit Care Med 2008,36(5):1412–1420. 10.1097/CCM.0b013e318170a375
Estenssoro E, Dubin A, Laffaire E, Canales H, Saenz G, Moseinco M, Pozo M, Gomez A, Baredes N, Jannello G, et al.: Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med 2002,30(11):2450–2456. 10.1097/00003246-200211000-00008
Montgomery AB, Stager MA, Carrico CJ, Hudson LD: Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 1985,132(3):485–489.
Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP: Causes and timing of death in patients with ARDS. Chest 2005,128(2):525–532. 10.1378/chest.128.2.525
Brown LM, Calfee CS, Matthay MA, Brower RG, Thompson BT, Checkley W: A simple classification model for hospital mortality in patients with acute lung injury managed with lung protective ventilation. Crit Care Med 2011,39(12):2645–2651.
Bull TM, Clark B, McFann K, Moss M, National Institutes of Health/National Heart L, Blood Institute AN: Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med 2010,182(9):1123–1128. 10.1164/rccm.201002-0250OC
Cepkova M, Kapur V, Ren X, Quinn T, Zhuo H, Foster E, Liu KD, Matthay MA: Pulmonary dead space fraction and pulmonary artery systolic pressure as early predictors of clinical outcome in acute lung injury. Chest 2007,132(3):836–842. 10.1378/chest.07-0409
Bellani G, Guerra L, Musch G, Zanella A, Patroniti N, Mauri T, Messa C, Pesenti A: Lung regional metabolic activity and gas volume changes induced by tidal ventilation in patients with acute lung injury. Am J Respir Crit Care Med 2011,183(9):1193–1199. 10.1164/rccm.201008-1318OC
Bersten AD, Edibam C, Hunt T, Moran J: Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med 2002,165(4):443–448. 10.1164/ajrccm.165.4.2101124
Britos M, Smoot E, Liu KD, Thompson BT, Checkley W, Brower RG, National Institutes of Health Acute Respiratory Distress Syndrome Network I: The value of positive end-expiratory pressure and Fio(2) criteria in the definition of the acute respiratory distress syndrome. Crit Care Med 2011,39(9):2025–2030. 10.1097/CCM.0b013e31821cb774
Needham DM, Dennison CR, Dowdy DW, Mendez-Tellez PA, Ciesla N, Desai SV, Sevransky J, Shanholtz C, Scharfstein D, Herridge MS, et al.: Study protocol: the Improving Care of Acute lung injury Patients (ICAP) study. Crit Care 2006,10(1):R9. 10.1186/cc3948
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD: Incidence and outcomes of acute lung injury. N Engl J Med 2005,353(16):1685–1693. 10.1056/NEJMoa050333
Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, Faggiano C, Quintel M, Gattinoni L, Ranieri VM: Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology 2009,111(4):826–835. 10.1097/ALN.0b013e3181b764d2
Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, Gandini G, Herrmann P, Mascia L, Quintel M, et al.: Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 2007,175(2):160–166. 10.1164/rccm.200607-915OC
Ferguson ND, Kacmarek RM, Chiche JD, Singh JM, Hallett DC, Mehta S, Stewart TE: Screening of ARDS patients using standardized ventilator settings: influence on enrollment in a clinical trial. Intensive Care Med 2004,30(6):1111–1116. 10.1007/s00134-004-2163-2
Villar J, Perez-Mendez L, Kacmarek RM: Current definitions of acute lung injury and the acute respiratory distress syndrome do not reflect their true severity and outcome. Intensive Care Med 1999,25(9):930–935. 10.1007/s001340050984
Thille AW, Esteban A, Fernandez-Segoviano P, Rodriguez JM, Aramburu JA, Penuelas O, Cortes-Puch I, Cardinal-Fernandez P, Lorente JA, Frutos-Vivar F: Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med 2013,187(7):761–767. 10.1164/rccm.201211-1981OC
Thompson BT, Matthay MA: The Berlin definition of ARDS versus pathological evidence of diffuse alveolar damage. Am J Respir Crit Care Med 2013,187(7):675–677. 10.1164/rccm.201302-0385ED
Heffner JE, Brown LK, Barbieri CA, Harpel KS, DeLeo J: Prospective validation of an acute respiratory distress syndrome predictive score. Am J Respir Crit Care Med 1995,152(5 Pt 1):1518–1526.
Cox proportional hazards sample size calculation [http://cct.jhsph.edu/javamarc/index.htm]
Li G, Malinchoc M, Cartin-Ceba R, Venkata CV, Kor DJ, Peters SG, Hubmayr RD, Gajic O: Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med 2011,183(1):59–66. 10.1164/rccm.201003-0436OC
Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, et al.: Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. Am J Respir Crit Care Med 2009,179(3):220–227. 10.1164/rccm.200805-722OC
Villar J, Blanco J, Anon JM, Santos-Bouza A, Blanch L, Ambros A, Gandia F, Carriedo D, Mosteiro F, Basaldua S, et al.: The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 2011,37(12):1932–1941. 10.1007/s00134-011-2380-4
Wang CY, Calfee CS, Paul DW, Janz DR, May AK, Zhuo H, Bernard GR, Matthay MA, Ware LB, Kangelaris KN: One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med 2014. http://www.ncbi.nlm.nih.gov/pubmed/24435201
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL: Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006,354(24):2564–2575.
Funding sources
At the time the research was conducted Dr. Kangelaris was supported by the Society of Hospital Medicine Young Researchers Award, the NIH National Center for Advancing Translational Sciences through UCSF-CTSI KL2 TR000143, and NHLBI 1K23HL116800-01. Dr. Calfee was supported by NHLBI HL090833 and HL110989. Dr Ware was supported by NHLBI HL081332, and HL103836. This work was supported by the National Institutes of Health. Dr. Matthay was supported by NHLBI HL51856.
We thank the patients and the family members of those who participated in the VALID study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
No author (KNK, AM, HZ, MAM, LBW) reports a conflict of interest. Dr. Calfee has served on medical advisory boards for Cerus Corp and Glaxo Smith Kline.
Authors’ contributions
KNK, CSC, MAM and LBW contributed to study design, data analysis and interpretation and drafting and revising the manuscript critically for important intellectual content. HZ and AKM contributed to acquisition of data, and analysis and interpretation of data and revising the manuscript. All authors approved the manuscript to be published, and KNK is the guarantor of the entire manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kangelaris, K.N., Calfee, C.S., May, A.K. et al. Is there still a role for the lung injury score in the era of the Berlin definition ARDS?. Ann. Intensive Care 4, 4 (2014). https://doi.org/10.1186/2110-5820-4-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2110-5820-4-4