Abstract
Biomarker-guided initiation of antibiotic therapy has been studied in four conditions: acute pancreatitis, lower respiratory tract infection (LRTI), meningitis, and sepsis in the ICU. In pancreatitis with suspected infected necrosis, initiating antibiotics best relies on fine-needle aspiration and demonstration of infected material. We suggest that PCT be measured to help predict infection; however, available data are insufficient to decide on initiating antibiotics based on PCT levels. In adult patients suspected of community-acquired LRTI, we suggest withholding antibiotic therapy when the serum PCT level is low (<0.25 ng/mL); in patients having nosocomial LRTI, data are insufficient to recommend initiating therapy based on a single PCT level or even repeated measurements. For children with suspected bacterial meningitis, we recommend using a decision rule as an aid to therapeutic decisions, such as the Bacterial Meningitis Score or the Meningitest®; a single PCT level ≥0.5 ng/mL also may be used, but false-negatives may occur. In adults with suspected bacterial meningitis, we suggest integrating serum PCT measurements in a clinical decision rule to help distinguish between viral and bacterial meningitis, using a 0.5 ng/mL threshold. For ICU patients suspected of community-acquired infection, we do not recommend using a threshold serum PCT value to help the decision to initiate antibiotic therapy; data are insufficient to recommend using PCT serum kinetics for the decision to initiate antibiotic therapy in patients suspected of ICU-acquired infection. In children, CRP can probably be used to help discontinue therapy, although the evidence is limited. In adults, antibiotic discontinuation can be based on an algorithm using repeated PCT measurements. In non-immunocompromised out- or in- patients treated for RTI, antibiotics can be discontinued if the PCT level at day 3 is < 0.25 ng/mL or has decreased by >80-90%, whether or not microbiological documentation has been obtained. For ICU patients who have nonbacteremic sepsis from a known site of infection, antibiotics can be stopped if the PCT level at day 3 is < 0.5 ng/mL or has decreased by >80% relative to the highest level recorded, irrespective of the severity of the infectious episode; in bacteremic patients, a minimal duration of therapy of 5 days is recommended.
Similar content being viewed by others
Biomarkers and initiation of antibiotic therapy
According to the preset selection criteria (see part I), the panel reviewed four conditions in which the potential clinical role of biomarkers has been studied: acute pancreatitis, respiratory tract infections, meningitis, and sepsis in the ICU.
Acute pancreatitis in adults
The clinical presentation and severity of patients having acute pancreatitis varies considerably, from a mild abdominal discomfort to multiple organ failure and death. The potential role of biomarkers in this condition should thus be twofold: 1) a prognostic value, to help define the most appropriate therapeutic approach, by predicting the severity of the disease and accurately select those patients needing close monitoring in the ICU; and 2) a diagnostic value, to help identify those patients having infected pancreatic necrosis, who might need drainage or surgery. Mofidi et al. [1] have recently reviewed the potential role of PCT in answering these two questions, by analysing 12 observational studies [2-9] totalling 956 patients. The threshold PCT value used in these studies to predict the severity of pancreatitis varied from 0.25 to 1.8 mg/L, with an associated combined sensitivity of 0.72 (0.65-0.78), a specificity of 0.86 (0.83-0.89), and an area under the receiver operating curve (AUROC) of 0.87. In the seven studies (n = 264 patients) examining the value of PCT for predicting the presence of infected necrosis [2–5, 7–9], the threshold value varied across studies between 0.48 and 3.5 mg/L, with an associated sensitivity of 0.8 (0.71-0.88), a specificity of 0.91 (0.87-0.94), and an AUROC of 0.91 (Table 1). In these seven studies, PCT levels were confronted to microbiological results obtained from fine-needle biopsy and culture of intra-abdominal collections, taken as the “gold standard.”
Other less commonly measured biomarkers (IL-6, IL-8, sTREM-1, TNF-α) also have been compared to PCT for their ability to help answer the two questions above. These biomarkers provided AUROC comparable to those of PCT, both in terms of prognostic value and of diagnosis of infected necrosis [2, 4–6, 9, 10]. Conversely, CRP levels appear less discriminatory for the prediction of infected necrosis [2]. No study has evaluated the value of repeated PCT measurements to predict infection, and no study has evaluated the impact on patients’ outcome of the initiation of antibiotic therapy guided by a biomarker level in patients suspected of infected necrosis.
In summary, we suggest that PCT be measured to help predict infection in patients suspected of infected necrosis during acute pancreatitis; it is however difficult from the available literature to define a precise threshold value (0.5-1.0 mg/L). A PCT value above the threshold might reinforce the clinician’s judgment that a fine-needle aspiration and culture is needed to confirm infection, while a value below this threshold might help deferring this intervention and proceed with watchful waiting. There is insufficient data to recommend initiating antibiotic therapy based on biomarker levels: this decision is based on a careful repeated evaluation of the patient and on the results of fine-needle aspiration material, which currently remains the cornerstone for the decision to initiate or maintain antibiotic therapy.
Lower respiratory tract infection in adults
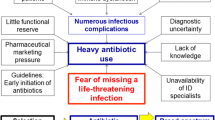
Antibiotics often are prescribed in excess to patients having a clinical syndrome of community-acquired lower respiratory tract infection (LRTI). Despite the usually viral aetiology of their illness, an estimated 75% of patients with acute bronchitis receive antibiotics [11]; indeed, clinical presentation does not allow the distinction between bacterial and viral infection, which encourages physicians to err on the “safe side” and prescribe antibiotics. Communication campaigns inciting primary physicians to limit unnecessary prescriptions for LRTI have a moderate impact, which is difficult to maintain over time [12, 13]. In this context, the addition of biomarker measurements to the clinical evaluation of such patients may have two main potential effects: improve the diagnostic accuracy, and reassure the patient and the physician that antibiotic therapy is unnecessary.
An abundant literature is available on PCT-guided initiation of antibiotic therapy in patients suspected clinically of having LRTI, providing a high-level of evidence. To date, 11 randomised, controlled studies using a similar approach have been published and provide consistent results [14–25]. All these studies have used a similar algorithm [26–29] to help decide on the initiation and continuation of antibiotic therapy, with a lower PCT threshold of <0.25 ng/mL to encourage physicians to withhold antibiotic prescription. The absolute risk reduction of antibiotic administration varies between 11% and 72% across these studies compared with “usual care” based on local recommendations and physicians’ judgment and preferences (Table 2). In one study, however, antibiotic prescriptions increased by 6% with PCT-guided therapy [19]. It also should be noted that the 0.25 ng/mL threshold may be less reliable in the elderly, where an 8% false-positive rate has been reported [30].
Among the 14 studies of PCT-guided therapy for LRTI reviewed by the Cochrane Collaboration [31, 32], only 3 enrolled patients with a nosocomial infection (hospital-acquired or ventilator-associated) [33–35], 2 of which evaluated the impact of PCT-guided therapy on the initiation of treatment [33, 34]. However, nosocomial acquisition of infection is identifiable only in the study by Bouadma et al. [33], and only 5% (n = 141) of all patients enrolled fulfilled this criteria; nearly all patients in this subgroup were administered antibiotics (99% in the PCT-guided therapy group and 100% in controls). Repeated measurements might be helpful for initiating antibiotics in this subgroup; however, the few data available on a limited number of patients (n = 89 patients) [36] do not allow making a recommendation in this regard.
In summary, we suggest withholding antibiotic therapy in adult patients suspected of community-acquired LRTI and having a serum PCT level <0.25 ng/mL; if clinical suspicion is high, it is however recommended to repeat the PCT measurement at a 6-h interval and reassess the therapeutic approach, accounting for new clinical findings. In patients having nosocomial LRTI, data are insufficient to recommend tailoring the therapeutic approach based on a single PCT level or even repeated measurements.
Meningitis
Childhood meningitis
Most acute meningitis in children is of viral aetiology and evolves favourably [37, 38]. Despite their relatively low prevalence, acute bacterial meningitis are severe infections, often resulting in debilitating sequels or even death [39]; thus, antibiotic therapy is recommended in children presenting with acute meningitis, at least until cerebrospinal fluid (CSF) cultures are available, i.e., within the first 48–72 h [40]. The risk-benefit ratio and costs associated with this prudent approach is likely unfavourable, because it involves numerous unnecessary hospitalisations, increased costs, and side effects of treatments, including selection of resistant organisms [41]. Biomarkers might help to reduce these unwanted effects [42, 43]. Ideally, a good biomarker would have 100% sensitivity for the diagnosis of bacterial meningitis, together with an acceptable specificity [38]; however, when used alone, available biomarkers (PCT, CRP, IFN-Υ, etc.) have sensitivities and specificities that do not appear high enough to base a therapeutic decision on their results given the risks incurred in case of a false-negative test [44, 45].
To overcome this problem, several groups have proposed decision rules combining clinical criteria and biomarker results [46–50]. The Bacterial Meningitis Score (BMS) [46] has been reported to have 100% sensitivity and 67% specificity for the detection of bacterial meningitis and is easily applicable at the bedside. This decision rule encourages ambulatory treatment of children having meningitis (i.e., a CSF leucocytes count ≥7/mm3) if none of the following five criteria is present: seizures, blood polymorphonuclear (PMN) cells count ≥10,000/mm3, direct examination of CSF positive, CSF protein level ≥0.8 g/L, or CSF PMN ≥1000/mm3. The BMS has undergone external validation on the large database of the French national registry for childhood bacterial meningitis (ACTIV-GPIP) [51]. Of 889 children with confirmed bacterial meningitis, 884 were correctly identified by the BMS rule (sensitivity = 99.6%; 95% confidence interval (CI) 98.9-99.8) with a specificity >60%. Thus, despite these near-perfect results, a few patients with bacterial meningitis (n = 5) were not detected by the BMS [52]; that the BMS can have a few false-negatives also was confirmed by a recent meta-analysis [53]. The Meningitest® (European patent EP1977244) has been subsequently proposed to refine the BMS and avoid these false-negatives, by omitting some variables of poor discriminatory power and introducing the serum PCT level [45, 54]. The Meningitest® rule suggests initiating antibiotic therapy if at least one of the following criteria is present: seizures, toxic appearance, purpura, PCT level ≥0.5 ng/mL, positive CSF Gram stain, or CSF protein level ≥0.5 g/L. External validation of the Meningitest® has been performed on an European database of 198 patients (including 96 with bacterial meningitis), where its sensitivity and specificity were respectively of 100% (95% CI 96–100) and 36% (95% CI 27–46) for the diagnosis of bacterial meningitis, whereas corresponding values for the BMS were 100% (95% CI 96–100) and 52% (95% CI 42–62) [55]. A single serum PCT level ≥0.5 ng/mL has similar sensitivity and specificity as the BMS, whereas combining CRP with CSF protein levels provided lower performances.
In summary, for children with suspected bacterial meningitis, we recommend using a decision rule as an aid to triage decisions and antibiotic prescribing, such as the BMS (more specific, but with a few false-negatives) or the Meningitest® (less specific, but no false-negative described to date). A single PCT level ≥0.5 ng/mL also may be used, but false-negatives may occur.
Adult meningitis
The potential role of biomarkers in the management of meningitis has been much less studied in adults than in children. Similarly to children, the use of a clinical decision rule to distinguish between viral and bacterial meningitis is recommended in adults [56]. For example, the French 2008 consensus conference on meningitis recommended using one of three decision rules: the rule developed by Hoen et al. [57], the BMS, or the Meningitest® [56]. It should be noted that the former rule has insufficient sensitivity in children (94%), with a risk of false-negatives [45].
Knudsen et al. have examined the impact of various biomarkers in the diagnostic workup of 55 adult patients with meningitis [58]. These authors found an AUROC of 0.91, 0.87, and 0.72 for CRP, PCT, and sCD 163, respectively, and concluded that CRP and PCT levels could be useful when combined with results of CSF examination to help diagnose bacterial meningitis. One recent study [59] included 151 patients admitted to an adult emergency department with suspected of bacterial meningitis and a negative direct examination of CSF, to assess the diagnostic value of CRP, PCT, and CSF leucocytes count. The AUROC of PCT and CRP were 0.98 (95% CI, 0.83-1.0) and 0.81 (95% CI, 0.58-0.92), respectively; however, the small number of patients with confirmed bacterial meningitis (n = 18) limits the inferences from this study. Of note, the CSF leucocytes count appeared to have little discriminatory value (AUC = 0.59) in that study. In another study of 30 patients (including 16 having bacterial meningitis), Schwarz et al. [60] found that PCT had a sensitivity of 69% and a specificity of 100% for diagnosing bacterial meningitis. In another larger prospective study that included 112 adult patients admitted to the hospital for meningitis (90 viral and 22 bacterial), Viallon et al. [61] found that a serum PCT value >0.93 ng/ml was 100% sensitive for the diagnosis of bacterial meningitis; conversely, a CSF lactate level <3.2 mmoles/L had a 100% NPV (Table 3). Low CRP levels have high NPV, but have not been shown to contribute markedly to the diagnostic approach [62]. The 2008 French consensus conference on management of acute bacterial meningitis [56] concluded that these biomarkers could be helpful for diagnosing bacterial meningitis in adults, pointing out that a threshold value for serum PCT of 0.5 ng/mL had a high sensitivity (99%; 95% CI, 97–100) and specificity (83%; 95% CI, 76–90), and that bacterial meningitis could be considered very unlikely when PCT was <0.5 ng/mL or CSF lactate was below 3 mmoles/L.
In summary, we suggest integrating serum PCT measurements in a clinical decision rule for meningitis in adults to help distinguish between viral and bacterial meningitis, using a threshold of 0.5 ng/mL.
Adult intensive care patients
Most controlled studies performed in intensive care patients have examined the value of biomarkers to limit the duration of antibiotic therapy, and few have concentrated on its initiation. Although a recent meta-analysis suggests that PCT is helpful for differentiating sepsis from SIRS [63], the initiation of antibiotic therapy in ICU patients has been assessed in only two randomised open studies testing a PCT-based algorithm (Table 4) [33, 34]. In the study by Layios et al. [34], there was no difference in the rate of initiation of therapy between the control group and the PCT-based group (where antibiotics were strongly discouraged if PCT was lower than 0.25 ng/mL, and strongly encouraged if PCT was higher than 1 ng/mL). In the multicentre study performed by Bouadma et al. [33] and using a similar algorithm, the risk reduction of initiating antibiotic therapy varied between 5% and 13% across centres. However, the small number of patients having CAP (n = 69) and the very low observance of the algorithm for withholding antibiotics when PCT levels were low (6%) in this study do not allow concluding on this point.
Relying on changes in PCT levels might be helpful for the initiation of antibiotic therapy in intensive care patients suspected of ICU-acquired infection; however, currently available data (on a total of 207 patients) are insufficient to base a recommendation on these [36, 64]. One randomized, controlled study that enrolled 604 ICU patients has tested the diagnostic value of daily measurements of serum PCT levels (using a threshold of 1 ng/mL to rapidly initiate a diagnostic workup and protocolised therapy) [65]. The length of ICU stay and of mechanical ventilation were actually higher in the PCT arm (without difference in 28-day mortality), and time to adequate therapy was not lower (except for patients with bacteremia). Of note, antibiotic consumption was significantly higher in the PCT arm, as well as the total number of days spent in the ICU with three or more antibiotics.
In summary, we do not recommend using a threshold serum PCT value to help in the decision to initiate antibiotic therapy in ICU patients suspected of community-acquired pneumonia. There are insufficient data available to recommend using repeated PCT measurements and serum kinetics for the decision to initiate antibiotic therapy in ICU patients suspected of ICU-acquired infection.
When can biomarkers help the decision to stop antibiotic therapy?
Given the number of studies examining this question and the high level of evidence generated, investigating this question was limited to examining randomized, controlled studies having tested a strategy based on biomarker measurement(s), to the exclusion of all other study designs. All studies in hospitalised patients used serum PCT level measurements, as there is no study testing the impact of using another biomarker in this specific indication; whereas several studies have tested the value of CRP for initiating antibiotics in pre-hospital care [24, 66–68], none examined its potential impact on discontinuation of antibiotics, although several studies are ongoing (see http://www.clinicaltrials.gov/ct2/results?term=c+reactive+protein+and+duration&recr=&rslt=&type=&cond=&intr=&outc=&spons=&lead=&id=&state1=&cntry1=&state2=&cntry2=&state3=&cntry3=&locn=&gndr=&rcv_s=&rcv_e=&lup_s=&lup_e=). Accordingly, only studies using PCT levels are considered below.
How can biomarkers be used to help decide on discontinuing antibiotic treatment?
To date, 14 trials have examined the clinical impact of PCT-guided antibiotic therapy and its discontinuation [15–20, 22, 23, 33, 35, 69, 70, 72],[73]. Nine of these focused on the latter objective; four were conducted in prehospital care or emergency room, whereas the remaining five were conducted in ICU patients. Although specific stopping rules may vary across trials and population enrolled, all studies used a PCT-based algorithm to help decide on stopping antibiotics (Table 5).
In outpatients and emergency room patients (excluding ICU patients), a serum PCT level below 0.25 ng/mL obtained 3 days or more after initiation of antibiotics, or a more than 80% decrease from the peak PCT level, allows stopping therapy.
Five studies have enrolled ICU patients with community- or hospital-acquired infection; four used a similar algorithm and the fifth used a different algorithm. It seems reasonable to recommend using the algorithm tested on the largest number of patients, i.e., as in the ProVAP et Prorata studies [33, 69], where stopping therapy was strongly encouraged when the serum PCT level was <0.5 ng/mL at 3 days or more after initiating antibiotics, or an >80% decrease from the maximal serum PCT value was recorded.
Does the site of infection (known, presumed, unknown) influence the utility of biomarkers to help withdrawing antibiotics?
In all studies examining the prognostic and follow-up value of PCT, the site of infection was known, to the exception of a few patients (n = 18) in the PRORATA study (10 and 8 in the PCT arm and control group, respectively). This small number does not allow any conclusion for this subgroup. It should be noted that patients having infective endocarditis, bone and joint infection, acute mediastinitis, intracerebral or intra-abdominal abscess were excluded from the above studies. Therefore, PCT-based algorithms cannot be used in these patients for discontinuing antibiotics.
Therefore, PCT-guided interruption of therapy can be used as indicated above in patients having a clinically documented site of infection to the exception of those sites listed above, which were excluded from clinical trials. When the site of infection is unknown, insufficient data are available to make a recommendation.
Does microbiological documentation influences the clinical utility of biomarkers to help withdrawing antibiotics?
Microbiologically documented infection
In the PRORATA study, most patients enrolled (70%) had microbiologically documented infection (222 and 213 in the PCT and control group, respectively) [33]. In the subgroup of 108 patients having positive blood cultures (55 and 53 in the PCT and control group, respectively), those randomised to the PCT-guided algorithm received 3 days less antibiotics than those enrolled in the control group (IC95%, -6 to 0.1 day, p = 0.06), without difference in mortality rate.
In the ProHosp trial, 72 patients had positive blood cultures [25]; patients enrolled in the PCT-guided therapy group received 5 days less antibiotics (10.3 vs. 15.1 days). Among 237 patients with bacteremic LRTI included in a recent meta-analysis [32], those treated with the aid of a PCT-based algorithm had 3.5 less antibiotic days (95% CI 1.55-5.54, p < 0.001), without significant difference in mortality rate (OR 1.09; 95% CI 0.51-2.31).
Lack of microbiological documentation
Most studies conducted outside of the ICU have enrolled patients in whom microbiological documentation was lacking. Although this specific subgroup has not been examined separately in individual studies or meta-analyses, it seems reasonable to recommend using a PCT algorithm in the non-ICU population to help decide stopping antibiotic therapy.
Most ICU patients enrolled in the above mentioned studies had documented infection. However, in the PRORATA study [33], 186 episodes were nondocumented and those treated in the PCT-guided therapy arm had a nonsignificant reduction in antibiotic days (2.4 less days), with no difference in mortality rate. Therefore, the documentation of infection does not appear to influence the impact of PCT-guided withdrawal of therapy, whether in ICU or non-ICU populations.
Does an immunocompromised status of patients influence the use of biomarkers for stopping antibiotic therapy?
Among the nine trials testing the impact of PCT-guided discontinuation of therapy, only the PRORATA trial [33] enrolled immunocompromised patients in the ICU. This trial enrolled patients having HIV infection or AIDS, organ transplant recipients, patients having haematological malignancy or receiving chemotherapy or radiation therapy, immunosuppressive agents or long-term steroids, to the notable exception of bone marrow transplant recipients or those having severe neutropenia (<500 leucocytes/mm3). About a hundred such immunocompromised patients were included (47 and 51 in the PCT-guided therapy group and control group, respectively). In this subgroup, PCT-guided discontinuation of therapy was associated with a significantly reduced duration of therapy (3.6 days; 95% CI, 0.2-7 days), without apparent effect on morbidity or mortality (control vs. PCT, -7.1%; 95% CI -18.7 to 4.5%).
Therefore, PCT-guided algorithms to reduce the duration of antibiotic therapy can be used safely in immunocompromised patients, to the exception of neutropenic patients (<500 neutrophils/mm3) or bone marrow transplant recipients, which were excluded from trials and in whom PCT-guided therapy cannot be recommended.
Does the impact of PCT-guided therapy vary according to the severity of acute illness?
Systematic reviews and meta-analyses
Six studies were reviewed in the meta-analysis by Tang et al. [74], totalling 1,548 patients. Four of these trials enrolled patients with suspected LRTI, two studies enrolled patients with sepsis, and one focused on severe infections in surgical ICU patients. Algorithms used varied across studies, using 2, 3, or 4 PCT levels for decision-making. There was a nonsignificant trend to a reduced duration of antibiotic therapy among LRTI studies (p = 0.067), which showed significant heterogeneity between trials. Conversely, in the other three studies of sepsis and surgical patients that enrolled more severe patients, no substantial heterogeneity was observed, and PCT-based algorithm for discontinuation of antibiotics were associated with a significant reduction in antibiotic duration, without apparent deleterious effect. This meta-analysis did not, however, stratify trials according to the severity of illness. In the subgroup of four studies having the strongest design (including 3 of the 4 studies on LRTI), there was a significant reduction in the duration of antibiotic therapy, but a significant heterogeneity persisted.
Significant heterogeneity also was evidenced in a recent meta-analysis including eight studies of LRTI [75]. There was one trial in patients with acute exacerbation of COPD, one on patients with CAP, two on patients with upper and lower RTI [16], and three on LRTI. A significant reduction of antibiotic duration was noted in all but one study [16], where a PCT-based algorithm was not used. Similarly to the previous analysis, this meta-analysis did not stratify patients according to their severity.
In the more recent systematic review by Schuetz et al. focusing on LRTI [32], 14 trials totalling 4,221 patients were analysed. A reduced rate of treatment initiation was confirmed in studies performed in primary care and patients having upper or lower RTI or acute bronchitis. Trials performed in the emergency department or the ICU and enrolling patients with LRTI, whether CAP or VAP, also found a reduction in the duration of antibiotic therapy. A sensitivity analysis showed no significant difference in the reduction of antibiotic duration according to the type of LRTI and site of care. It was however noted that the observance of clinical algorithms was lower in the ICU setting than at other sites.
Individual studies
Christ-Crain et al. [18] reported that PCT levels increased with the severity of illness, as assessed by the pneumonia severity index (PSI). However, the duration of antibiotic prescription decreased similarly with PCT-guided therapy in the low-risk (PSI I-III) or high-risk (PSI IV-V) group. In another study in patients with LRTI from the same group [17], only admission PCT levels were recorded but not duration of therapy. In the PROHOSP study [25], the reduction of antibiotic duration with PCT-guided therapy was more marked among patients having acute bronchitis (-65%) than among patients with acute exacerbation of COPD (-50%), and lowest (-32%), but still strongly significant, among those with CAP.
In the trial conducted by Stolz et al. in patients with acute exacerbation of COPD [20], the impact of PCT-guided therapy was not analysed according to the severity of the episode or of the underlying COPD, which included all severity stages.
Briel et al. enrolled patients with RTI from various causes, including upper respiratory tract infection or acute bronchitis, CAP, or acute exacerbation of COPD [15]. The reduction in antibiotic duration was more marked in the former group than in those with CAP or acute exacerbation of COPD.
The proREAL trial also enrolled patients (n = 1,759) with acute bronchitis, exacerbation of COPD, and CAP [27]. The observance of the algorithm was 81%, 70%, and 64% respectively, confirming other observations [32, 33] that the observance decreases with increasing severity of illness. Of note, the algorithm used in that study included both the clinical context and PCT levels (Table 3).
Long et al. also found a reduction in antibiotic duration of patients with CAP [22]. However, this study enrolled only patients with nonsevere pneumonia and cannot inform this assessment according to severity of illness. In trials dealing with the more severe infections (VAP, sepsis) [33, 35, 65, 66], analyses have not been stratified according to the level of severity.
Summary and conclusions
In view of currently available data, PCT is the only biomarker that has been extensively studied so far to help decision-making in discontinuing antibiotic therapy in adults. In clinical practice, an algorithm should be used, based on PCT levels on day 1 (reference value), then at day 2–3, and every 48 h until antibiotic therapy is stopped.
In nonimmunocompromised patients treated for RTI as outpatients or hospitalised in regular wards, the following stopping rule can be used: discontinuation of antibiotic therapy if the PCT level at day 3 is lower than 0.25 ng/mL or has decreased by >80-90% relative to the maximal value initially recorded, whether or not microbiological documentation has been obtained.
For patients hospitalised in ICU, including immunocompromised patients (but not neutropenic patients or bone marrow transplant recipients), the following decision rule can be suggested for nonbacteremic patients with a known site of infection (whether or not microbiological documentation is obtained): stopping antibiotics if the PCT level at day 3 is <0.5 ng/ml or has decreased by >80% relative to the highest level recorded during this episode. In bacteremic patients, a minimal duration of therapy of 5 days is recommended.
Overall, the severity of the infectious episode does not appear to alter substantially the impact of PCT measurements on the reduction of antibiotic duration; however, the magnitude of the reduction is more marked in infections of lesser severity, which likely reflects at least two factors: 1) the less common indications for antibiotic therapy in such conditions, which is in contrast to the high tendency among physicians to initiate therapy when in doubt on the aetiology; 2) the better observance of decision algorithms by physicians, likely related to their greater confidence in the lack of serious risk associated with withholding or withdrawing antibiotics in these low-severity patients.
Abbreviations
- AIDS:
-
Acquired immunodeficiency syndrome
- AUC:
-
Area under the curve
- AUROC:
-
Area under the receiver operating curve
- BM:
-
Bacterial meningitis
- BMS:
-
Bacterial meningitis score
- CAP:
-
Community-acquired pneumonia
- COPD:
-
Chronic obstructive pulmonary disease
- CRP:
-
C-Reactive protein
- CSF:
-
Cerebrospinal fluid
- HIV:
-
Human immunodeficiency virus
- ICU:
-
Intensive care unit
- IFN-Υ:
-
Interferon-gamma
- IL:
-
Interleukin
- LRTI:
-
Lower respiratory tract infection
- PCT:
-
Procalcitonin
- PMN:
-
Polymorphonuclear neutrophil
- PPV:
-
Positive predictive value
- PSI:
-
Pneumonia severity index
- ROC:
-
Receiver Operating Characteristic curve
- RTI:
-
Respiratory tract infection
- SIRS:
-
systemic inflammatory response syndrome
- sTREM-1:
-
soluble Triggering Receptor Expressed on Myeloid cells-1
- TNF:
-
Tumor necrosis factor
- VAP:
-
ventilator-associated pneumonia.
References
Mofidi R, Suttie SA, Patil PV, Ogston S, Parks RW: The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery 2009, 146: 72–81. 10.1016/j.surg.2009.02.013
Rau B, Steinbach G, Baumgart K, Gansauge F, Grunert A, Beger HG: The clinical value of procalcitonin in the prediction of infected necrosis in acute pancreatitis. Intensive Care Med 2000,26(Suppl 2):S159-S164.
Morgenthaler NG, Struck J, Christ-Crain M, Bergmann A, Muller B: Pro-atrial natriuretic peptide is a prognostic marker in sepsis, similar to the APACHE II score: an observational study. Crit Care 2005, 9: R37-R45. 10.1186/cc3015
Rau B, Steinbach G, Gansauge F, Mayer JM, Grunert A, Beger HG: The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut 1997, 41: 832–840. 10.1136/gut.41.6.832
Riche FC, Cholley BP, Laisne MJ, Vicaut E, Panis YH, Lajeunie EJ, Boudiaf M, Valleur PD: Inflammatory cytokines, C reactive protein, and procalcitonin as early predictors of necrosis infection in acute necrotizing pancreatitis. Surgery 2003, 133: 257–262. 10.1067/msy.2003.70
Purkayastha S, Chow A, Athanasiou T, Cambaroudis A, Panesar S, Kinross J, Tekkis P, Darzi A: Does serum procalcitonin have a role in evaluating the severity of acute pancreatitis? A question revisited. World J Surg 2006, 30: 1713–1721. 10.1007/s00268-006-0167-5
Lu Z, Liu Y, Dong YH, Zhan XB, Du YQ, Gao J, Gong YF, Li ZS: Soluble triggering receptor expressed on myeloid cells in severe acute pancreatitis: a biological marker of infected necrosis. Intensive Care Med 2012, 38: 69–75. 10.1007/s00134-011-2369-z
Olah A, Belagyi T, Issekutz A, Makay R, Zaborszky A: Value of procalcitonin quick test in the differentiation between sterile and infected forms of acute pancreatitis. Hepatogastroenterology 2005, 52: 243–245.
Mandi Y, Farkas G, Takacs T, Boda K, Lonovics J: Diagnostic relevance of procalcitonin, IL-6, and sICAM-1 in the prediction of infected necrosis in acute pancreatitis. Int J Pancreatol 2000, 28: 41–49. 10.1385/IJGC:28:1:41
Muller CA, Uhl W, Printzen G, Gloor B, Bischofberger H, Tcholakov O, Buchler MW: Role of procalcitonin and granulocyte colony stimulating factor in the early prediction of infected necrosis in severe acute pancreatitis. Gut 2000, 46: 233–238. 10.1136/gut.46.2.233
Evans AT, Husain S, Durairaj L, Sadowski LS, Charles-Damte M, Wang Y: Azithromycin for acute bronchitis: a randomised, double-blind, controlled trial. Lancet 2002, 359: 1648–1654. 10.1016/S0140-6736(02)08597-5
Poses RM, Cebul RD, Wigton RS: You can lead a horse to water–improving physicians' knowledge of probabilities may not affect their decisions. Med Decis Making 1995, 15: 65–75. 10.1177/0272989X9501500110
Sabuncu E, David J, Bernede-Bauduin C, Pepin S, Leroy M, Boelle PY, Watier L, Guillemot D: Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002–2007. PLoS Med 2009, 6: e1000084. 10.1371/journal.pmed.1000084
Briel M, Christ-Crain M, Young J, Schuetz P, Huber P, Periat P, Bucher HC, Muller B: Procalcitonin-guided antibiotic use versus a standard approach for acute respiratory tract infections in primary care: study protocol for a randomised controlled trial and baseline characteristics of participating general practitioners [ISRCTN73182671]. BMC Fam Pract 2005, 6: 34. 10.1186/1471-2296-6-34
Briel M, Schuetz P, Mueller B, Young J, Schild U, Nusbaumer C, Periat P, Bucher HC, Christ-Crain M: Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med 2008, 168: 2000–2007. discussion 2007–2008 10.1001/archinte.168.18.2000
Burkhardt O, Ewig S, Haagen U, Giersdorf S, Hartmann O, Wegscheider K, Hummers-Pradier E, Welte T: Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur Respir J 2010, 36: 601–607. 10.1183/09031936.00163309
Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, Muller B: Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet 2004, 363: 600–607. 10.1016/S0140-6736(04)15591-8
Christ-Crain M, Stolz D, Bingisser R, Muller C, Miedinger D, Huber PR, Zimmerli W, Harbarth S, Tamm M, Muller B: Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med 2006, 174: 84–93. 10.1164/rccm.200512-1922OC
Kristoffersen KB, Sogaard OS, Wejse C, Black FT, Greve T, Tarp B, Storgaard M, Sodemann M: Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission–a randomized trial. Clin Microbiol Infect 2009, 15: 481–487. 10.1111/j.1469-0691.2009.02709.x
Stolz D, Christ-Crain M, Bingisser R, Leuppi J, Miedinger D, Muller C, Huber P, Muller B, Tamm M: Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007, 131: 9–19. 10.1378/chest.06-1500
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, et al.: Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006, 34: 1589–1596. 10.1097/01.CCM.0000217961.75225.E9
Long W, Deng X, Zhang Y, Lu G, Xie J, Tang J: Procalcitonin guidance for reduction of antibiotic use in low-risk outpatients with community-acquired pneumonia. Respirology 2011, 16: 819–824. 10.1111/j.1440-1843.2011.01978.x
Long W, Deng XQ, Tang JG, Xie J, Zhang YC, Zhang Y, Gao YY, Lu G: The value of serum procalcitonin in treatment of community acquired pneumonia in outpatient. Zhonghua Nei Ke Za Zhi 2009, 48: 216–219.
Cals JW, Butler CC, Hopstaken RM, Hood K, Dinant GJ: Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ (Clinical research ed 2009, b1374.
Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, Neidert S, Fricker T, Blum C, Schild U, et al.: Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009, 302: 1059–1066. 10.1001/jama.2009.1297
Muller B, Becker KL, Schachinger H, Rickenbacher PR, Huber PR, Zimmerli W, Ritz R: Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med 2000, 28: 977–983. 10.1097/00003246-200004000-00011
Albrich WC, Dusemund F, Bucher B, Meyer S, Thomann R, Kuhn F, Bassetti S, Sprenger M, Bachli E, Sigrist T, et al.: Effectiveness and safety of procalcitonin-guided antibiotic therapy in lower respiratory tract infections in "real life": an international, multicenter poststudy survey (ProREAL). Arch Intern Med 2012, 172: 715–722. 10.1001/archinternmed.2012.770
Van Der Meer V, Neven AK, van den Broek PJ, Assendelft WJ: Diagnostic value of C reactive protein in infections of the lower respiratory tract: systematic review. BMJ (Clinical research ed 2005, 331: 26. 10.1136/bmj.38483.478183.EB
Schuetz P, Chiappa V, Briel M, Greenwald JL: Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med 2011, 171: 1322–1331. 10.1001/archinternmed.2011.318
Chenevier-Gobeaux C, Trabattoni E, Elfassy Y, Picard C, Guerin S, Borderie D, Claessens YE: Decisional procalcitonin thresholds are not adapted to elderly patients admitted to the emergency room. Biomarkers 2012, 17: 477–481. 10.3109/1354750X.2012.685953
Schuetz P, Muller B, Christ-Crain M, Stolz D, Tamm M, Bouadma L, Luyt CE, Wolff M, Chastre J, Tubach F, et al.: Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane database of systematic reviews (Online) 2012, 9: CD007498.
Schuetz P, Briel M, Christ-Crain M, Stolz D, Bouadma L, Wolff M, Luyt CE, Chastre J, Tubach F, Kristoffersen KB, et al.: Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin Infect Dis 2012, 55: 651–662. 10.1093/cid/cis464
Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, Schortgen F, Lasocki S, Veber B, Dehoux M, et al.: Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010, 375: 463–474. 10.1016/S0140-6736(09)61879-1
Layios N, Lambermont B, Canivet JL, Morimont P, Preiser JC, Garweg C, Ledoux D, Frippiat F, Piret S, Giot JB, et al.: Procalcitonin usefulness for the initiation of antibiotic treatment in intensive care unit patients. Crit Care Med 2012, 40: 2304–2309. 10.1097/CCM.0b013e318251517a
Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J: Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med 2008, 177: 498–505. 10.1164/rccm.200708-1238OC
Charles PE, Kus E, Aho S, Prin S, Doise JM, Olsson NO, Blettery B, Quenot JP: Serum procalcitonin for the early recognition of nosocomial infection in the critically ill patients: a preliminary report. BMC Infect Dis 2009, 9: 49. 10.1186/1471-2334-9-49
Nigrovic LE, Kuppermann N, Macias CG, Cannavino CR, Moro-Sutherland DM, Schremmer RD, Schwab SH, Agrawal D, Mansour KM, Bennett JE, et al.: Clinical prediction rule for identifying children with cerebrospinal fluid pleocytosis at very low risk of bacterial meningitis. JAMA 2007, 297: 52–60. 10.1001/jama.297.1.52
Dubos F, Lamotte B, Bibi-Triki F, Moulin F, Raymond J, Gendrel D, Breart G, Chalumeau M: Clinical decision rules to distinguish between bacterial and aseptic meningitis. Arch Dis Child 2006, 91: 647–650. 10.1136/adc.2005.085704
Saez-Llorens X, McCracken GH Jr: Bacterial meningitis in children. Lancet 2003, 361: 2139–2148. 10.1016/S0140-6736(03)13693-8
Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ: Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004, 39: 1267–1284. 10.1086/425368
Khetsuriani N, Quiroz ES, Holman RC, Anderson LJ: Viral meningitis-associated hospitalizations in the United States, 1988–1999. Neuroepidemiology 2003, 22: 345–352. 10.1159/000072924
Gendrel D, Raymond J, Assicot M, Moulin F, Iniguez JL, Lebon P, Bohuon C: Measurement of procalcitonin levels in children with bacterial or viral meningitis. Clin Infect Dis 1997, 24: 1240–1242. 10.1086/513633
Marc E, Menager C, Moulin F, Stos B, Chalumeau M, Guerin S, Lebon P, Brunet F, Raymond J, Gendrel D: Procalcitonin and viral meningitis: reduction of unnecessary antibiotics by measurement during an outbreak. Arch Pediatr 2002, 9: 358–364. 10.1016/S0929-693X(01)00793-X
Dubos F, Moulin F, Gajdos V, De Suremain N, Biscardi S, Lebon P, Raymond J, Breart G, Gendrel D, Chalumeau M: Serum procalcitonin and other biologic markers to distinguish between bacterial and aseptic meningitis. J Pediatr 2006, 149: 72–76. 10.1016/j.jpeds.2006.02.034
Dubos F, Korczowski B, Aygun DA, Martinot A, Prat C, Galetto-Lacour A, Casado-Flores J, Taskin E, Leclerc F, Rodrigo C, et al.: Serum procalcitonin level and other biological markers to distinguish between bacterial and aseptic meningitis in children: a European multicenter case cohort study. Arch Pediatr Adolesc Med 2008, 162: 1157–1163. 10.1001/archpedi.162.12.1157
Nigrovic LE, Kuppermann N, Malley R: Development and validation of a multivariable predictive model to distinguish bacterial from aseptic meningitis in children in the post-Haemophilus influenzae era. Pediatrics 2002, 110: 712–719. 10.1542/peds.110.4.712
Hoen B, Viel JF, Paquot C, Gerard A, Canton P: Multivariate approach to differential diagnosis of acute meningitis. Eur J Clin Microbiol Infect Dis 1995, 14: 267–274. 10.1007/BF02116518
Freedman SB, Marrocco A, Pirie J, Dick PT: Predictors of bacterial meningitis in the era after Haemophilus influenzae. Arch Pediatr Adolesc Med 2001, 155: 1301–1306. 10.1001/archpedi.155.12.1301
Oostenbrink R, Moons KG, Twijnstra MJ, Grobbee DE, Moll HA: Children with meningeal signs: predicting who needs empiric antibiotic treatment. Arch Pediatr Adolesc Med 2002, 156: 1189–1194. 10.1001/archpedi.156.12.1189
Bonsu BK, Harper MB: Differentiating acute bacterial meningitis from acute viral meningitis among children with cerebrospinal fluid pleocytosis: a multivariable regression model. Pediatr Infect Dis J 2004, 23: 511–517. 10.1097/01.inf.0000129689.58211.9e
Bingen E, Levy C, de la Rocque F, Boucherat M, Varon E, Alonso JM, Dabernat H, Reinert P, Aujard Y, Cohen R: Bacterial meningitis in children: a French prospective study. Clin Infect Dis 2005, 41: 1059–1063. 10.1086/432944
Dubos F, De la Rocque F, Levy C, Bingen E, Aujard Y, Cohen R, Breart G, Gendrel D, Chalumeau M: Sensitivity of the bacterial meningitis score in 889 children with bacterial meningitis. J Pediatr 2008, 152: 378–382. 10.1016/j.jpeds.2007.07.012
Nigrovic LE, Malley R, Kuppermann N: Meta-analysis of bacterial meningitis score validation studies. Arch Dis Child 2012, 97: 799–805. 10.1136/archdischild-2012-301798
Dubos F, Moulin F, Raymond J, Gendrel D, Breart G, Chalumeau M: Distinction between bacterial and aseptic meningitis in children: refinement of a clinical decision rule. Arch Pediatr 2007, 14: 434–438. 10.1016/j.arcped.2006.12.009
Dubos F, Korczowski B, Aygun DA, Martinot A, Prat C, Galetto-Lacour A, Casado-Flores J, Taskin E, Leclerc F, Rodrigo C, et al.: Distinguishing between bacterial and aseptic meningitis in children: European comparison of two clinical decision rules. Arch Dis Child 2010, 95: 963–967. 10.1136/adc.2010.186056
Société de Pathologie Infectieuse de Langue Française (SPILF): Practice guidelines for acute bacterial meningitidis (except newborn and nosocomial meningitis). Med Mal Infect 2009, 39: 356–367.
Baty V, Viel JF, Schuhmacher H, Jaeger F, Canton P, Hoen B: Prospective validation of a diagnosis model as an aid to therapeutic decision-making in acute meningitis. Eur J Clin Microbiol Infect Dis 2000, 19: 422–426. 10.1007/s100960000287
Knudsen TB, Larsen K, Kristiansen TB, Moller HJ, Tvede M, Eugen-Olsen J, Kronborg G: Diagnostic value of soluble CD163 serum levels in patients suspected of meningitis: comparison with CRP and procalcitonin. Scand J Infect Dis 2007, 39: 542–553. 10.1080/00365540601113685
Ray P, Badarou-Acossi G, Viallon A, Boutoille D, Arthaud M, Trystram D, Riou B: Accuracy of the cerebrospinal fluid results to differentiate bacterial from non bacterial meningitis, in case of negative gram-stained smear. Am J Emerg Med 2007, 25: 179–184. 10.1016/j.ajem.2006.07.012
Schwarz S, Bertram M, Schwab S, Andrassy K, Hacke W: Serum procalcitonin levels in bacterial and abacterial meningitis. Crit Care Med 2000, 28: 1828–1832. 10.1097/00003246-200006000-00024
Viallon A, Desseigne N, Marjollet O, Birynczyk A, Belin M, Guyomarch S, Borg J, Pozetto B, Bertrand JC, Zeni F: Meningitis in adult patients with a negative direct cerebrospinal fluid examination: value of cytochemical markers for differential diagnosis. Crit Care 2011, 15: R136. 10.1186/cc10254
Gerdes LU, Jorgensen PE, Nexo E, Wang P: C-reactive protein and bacterial meningitis: a meta-analysis. Scand J Clin Lab Invest 1998, 58: 383–393. 10.1080/00365519850186364
Wacker C, Prkno A, Brunkhorst FM, Schlattmann P: Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 2013, 13: 426–435. 10.1016/S1473-3099(12)70323-7
Theodorou VP, Papaioannou VE, Tripsianis GA, Panopoulou MK, Christophoridis EK, Kouliatsis GA, Gioka TM, Maltezos ES, Ktenidou-Kartali SI, Pneumatikos IA: Procalcitonin and procalcitonin kinetics for diagnosis and prognosis of intravascular catheter-related bloodstream infections in selected critically ill patients: a prospective observational study. BMC Infect Dis 2012, 12: 247. 10.1186/1471-2334-12-247
Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, Thornberg KJ, Loken J, Steensen M, Fox Z, et al.: Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 2011, 39: 2048–2058. 10.1097/CCM.0b013e31821e8791
Cals JW, Hopstaken RM, Butler CC, Hood K, Severens JL, Dinant GJ: Improving management of patients with acute cough by C-reactive protein point of care testing and communication training (IMPAC3T): study protocol of a cluster randomised controlled trial. BMC Fam Pract 2007, 8: 15. 10.1186/1471-2296-8-15
Diederichsen HZ, Skamling M, Diederichsen A, Grinsted P, Antonsen S, Petersen PH, Munck AP, Kragstrup J: Randomised controlled trial of CRP rapid test as a guide to treatment of respiratory infections in general practice. Scand J Prim Health Care 2000, 18: 39–43. 10.1080/02813430050202541
Gonzales R, Aagaard EM, Camargo CA Jr, Ma OJ, Plautz M, Maselli JH, McCulloch CE, Levin SK, Metlay JP: C-reactive protein testing does not decrease antibiotic use for acute cough illness when compared to a clinical algorithm. J Emerg Med 2011, 41: 1–7. 10.1016/j.jemermed.2008.06.021
Stolz D, Smyrnios N, Eggimann P, Pargger H, Thakkar N, Siegemund M, Marsch S, Azzola A, Rakic J, Mueller B, Tamm M: Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J 2009, 34: 1364–1375. 10.1183/09031936.00053209
Hochreiter M, Kohler T, Schweiger AM, Keck FS, Bein B, Von Spiegel T, Schroeder S: Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care 2009, 13: R83. 10.1186/cc7903
Kopterides P, Siempos II, Tsangaris I, Tsantes A, Armaganidis A: Procalcitonin-guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med 2010, 38: 2229–2241. 10.1097/CCM.0b013e3181f17bf9
Schuetz P, Christ-Crain M, Muller B: Procalcitonin and other biomarkers to improve assessment and antibiotic stewardship in infections–hope for hype? Swiss Med Wkly 2009, 139: 318–326.
Schroeder S, Hochreiter M, Koehler T, Schweiger AM, Bein B, Keck FS, Von Spiegel T: Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg 2009, 394: 221–226. 10.1007/s00423-008-0432-1
Tang BM, Eslick GD, Craig JC, McLean AS: Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis 2007, 7: 210–217. 10.1016/S1473-3099(07)70052-X
Li H, Luo YF, Blackwell TS, Xie CM: Meta-analysis and systematic review of procalcitonin-guided therapy in respiratory tract infections. Antimicrob Agents Chemother 2011, 55: 5900–5906. 10.1128/AAC.00335-11
Acknowledgment
The Expert Panel work was conducted under the auspices of the Maurice Rapin Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Meetings of the expert panel group were supported in part by an unrestricted grant from Thermo-Fisher to the Maurice Rapin Institute. This company had no role in the work of the panel, or in the drafting, revision or final approval of the manuscript. A-MD declared participation as an investigator to the UTAPE study, sponsored by Thermo-Fisher, and her institution received funding for analytic studies of CT-pro AVP using Kryptor. CB-B was an investigator in the Prorata trial. MC holds a patent for the MENINGITEST in Europe (European patent EP1977244), USA and Canada, and has a patent pending for the REFLUTEST (WO2010/109089). C-EL was an investigator for the Prorata trial, and received lectures honoraria from Thermo-Fisher and Biomérieux. NR is coordinating the UTAPE study on biomarkers in COPD exacerbations seen in the emergency department, sponsored by Thermo-Fischer. Y-EC received research funding from Thermo-Fisher, Roche Diagnosis and Nephrotek. P-EC received speaking fees from Thermo-Fisher. CG-LG received funding by Thermo-Fisher for attending a scientific meeting. JP received speaking fees for giving lectures within symposia organized by ThermoFischer. J-PB, RG, SL, BM, J-PQ, FP, YP, and J-PS have declared no competing interest in relation to the subject of this manuscript.
Authors' contributions
All panel members contributed to the panel discussions and analyses. Each panel members contributed to drafting different sections of the manuscript: A-MD, YP, FP, SR, and BM drafted part I; J-PQ, SL, Y-EC, J-PS, CG-L, MC, and RG drafted part II; and C-EL, NR, J-PB, JP, and CB-B drafted part III. A-MD, J-PQ, C-EL, RG, BM, MC, and CB-B extensively reviewed the consolidated manuscript and all authors approved its final version.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Quenot, JP., Luyt, CE., Roche, N. et al. Role of biomarkers in the management of antibiotic therapy: an expert panel review II: clinical use of biomarkers for initiation or discontinuation of antibiotic therapy. Ann. Intensive Care 3, 21 (2013). https://doi.org/10.1186/2110-5820-3-21
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2110-5820-3-21