Abstract
Background
The intra-abdominal pressure (IAP) is an important clinical parameter that can significantly change during respiration. Currently, IAP is recorded at end-expiration (IAPee), while continuous IAP changes during respiration (ΔIAP) are ignored. Herein, a novel concept of considering continuous IAP changes during respiration is presented.
Methods
Based on the geometric mean of the IAP waveform (MIAP), a mathematical model was developed for calculating respiratory-integrated MIAP (i.e. ), where 'i' is the decimal fraction of the inspiratory time, and where ΔIAP can be calculated as the difference between the IAP at end-inspiration (IAPei) minus IAPee. The effect of various parameters on IAPee and MIAPri was evaluated with a mathematical model and validated afterwards in six mechanically ventilated patients. The MIAP of the patients was also calculated using a CiMON monitor (Pulsion Medical Systems, Munich, Germany). Several other parameters were recorded and used for comparison.
Results
The human study confirmed the mathematical modelling, showing that MIAPri correlates well with MIAP (R2 = 0.99); MIAPri was significantly higher than IAPee under all conditions that were used to examine the effects of changes in IAPee, the inspiratory/expiratory (I:E) ratio, and ΔIAP (P < 0.001). Univariate Pearson regression analysis showed significant correlations between MIAPri and IAPei (R = 0.99), IAPee (R = 0.99), and ΔIAP (R = 0.78) (P < 0.001); multivariate regression analysis confirmed that IAPee (mainly affected by the level of positive end-expiratory pressure, PEEP), ΔIAP, and the I:E ratio are independent variables (P < 0.001) determining MIAP. According to the results of a regression analysis, MIAP can also be calculated as
Conclusions
We believe that the novel concept of MIAP is a better representation of IAP (especially in mechanically ventilated patients) because MIAP takes into account the IAP changes during respiration. The MIAP can be estimated by the MIAPri equation. Since MIAPri is almost always greater than the classic IAP, this may have implications on end-organ function during intra-abdominal hypertension. Further clinical studies are necessary to evaluate the physiological effects of MIAP.
Similar content being viewed by others
Introduction
The intra-abdominal pressure (IAP) is an important clinical parameter with major prognostic impact [1, 2]. An unrecognised pathological increase in IAP eventually leads to intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) [3, 4], which result in significant morbidity and mortality [5]. Thus, recognition and reliable measurement of IAP are the first important steps for prevention and management of IAH and ACS in critically ill patients [6].
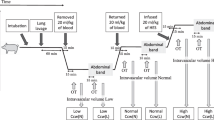
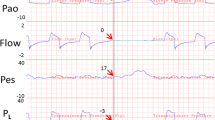
Assuming no respiratory movement, the IAP would be relatively constant and primarily determined by body posture and anthropomorphy (e.g. body mass index) [3, 7]. The IAP may be affected by conditions influencing intra-abdominal volume and abdominal compliance (C ab) [3, 8, 9]. Further, the complex interaction between intra-abdominal volume and C ab during respiration (Figure 1) may significantly [10] and frequently (12 to 40 changes per minute) change the IAP (Figure 2), with more intense effects during positive-pressure mechanical ventilation or the presence of positive end-expiratory pressure (PEEP) [10–12].
Relationship between intra-abdominal volume (IAV), abdominal wall compliance ( C ab ) and intra-abdominal pressure (IAP). The directions of the movement of IAP on the x axis and IAV on the y axis associated with the isolated action of the rib cage inspiratory muscles, abdominal expiratory muscles, and the diaphragm are shown. The direction of the action of the diaphragm depends on the abdominal compliance. Adapted from de Keulenaer et al. [7].
According to the current consensus definitions of the World Society of the Abdominal Compartment Syndrome (WSACS), the IAP should be measured at end-expiration (IAPee) [13], referred to as the 'classic IAP' throughout the text. However, the IAPee is only a single component of an ever-changing trend and thus does not incorporate a considerable portion of this IAP trend (Figure 2). The objectives of this study were to develop and validate a novel IAP measurement concept to consider IAP changes during respiration and to identify independent variables influencing IAP within this novel concept.
Methods
Part A: mathematical model
A set of numerous IAP values occurs for a patient during a single respiratory cycle. The central tendency of a set of values can be calculated by the mathematical function of the 'mean'. In determining the mean IAP, the arithmetic mean for IAPee and the end-inspiratory IAP (IAPei) was described previously [14], calculated by dividing the sum of the values by the number of values. However, employing the arithmetic mean for the IAP waveform is mathematically incorrect. Instead, the mean of a waveform can be calculated by the 'geometric mean' function. The geometric mean is calculated by dividing the area under the waveform in a definite interval (i.e. the definite integral of the waveform) by the value of the definite interval [15]. Therefore, the mean IAP (MIAP) for a sample IAP waveform between the times (T 0) and (T) in Figure 2 can be calculated as follows:
where 'MIAPri' is the respiratory-integrated MIAP, 'T−T 0' is the time interval for a full respiratory cycle, and 'IAP (t) dt' is the IAP at each time point (t). The result would be a time-weighted mean for the IAP waveform. This is closely analogous with the critically important cardiovascular concept of mean arterial blood pressure [16–18], which is the geometric mean of the arterial blood pressure waveform [19, 20]. Equation 1 may be simplified as follows (see the addendum)[21]:
where 'i' is the decimal fraction of the inspiratory time in a respiratory cycle and can be calculated from the inspiratory/expiratory (I:E) ratio (i = I /(I + E); 0 <i < 1) and ΔIAP = IAPei − IAPee. Since IAPee, i, and ΔIAP can be assumed to be independent, a computerised iteration can be used for a set of values for each parameter to determine their effect on MIAPri and to compare the MIAPri with the classic IAP.
The effects of IAPee on MIAPri and the classic IAP were examined through a gradual increase of IAPee from 12 to 25 mmHg, with steps of 1 mmHg (Figure 3). For each IAPee, a range of possible MIAPri values was calculated according to Equation 2 with an I:E ratio of 4:1 and an ΔIAP of 8.16 mmHg for the maximum MIAPri, and an I:E ratio of 1:4 and an ΔIAP of 1 mmHg for the minimum MIAPri. Because previous studies have shown a correlation between ΔIAP and IAPee, the ΔIAP was increased 10% for each 1 mmHg increase in the IAPee.
Mathematical modelling of IAP measurements for various end-expiratory IAP values (IAP ee ). The classic (IAPee) and novel (MIAPri) methods were used to measure the IAP. The dashed line represents the ACS threshold. The lines connecting the Max and Min MIAPri values represent the range of possible MIAPri values.
The effects of the I:E ratio on MIAPri and the classic IAP were examined by a gradual increase in the I:E ratio from 1:4 to 4:1 with steps of 0.5 (Figure 4). The amount of IAPee was held constant (19 mmHg). For each I:E ratio, a range of possible MIAPri values was calculated with an ΔIAP of 7 mmHg for the maximum MIAPri and an ΔIAP of 2 mmHg for the minimum MIAPri.
Mathematical modelling of IAP measurements for a constant 19 mmHg end-expiratory IAP (IAP ee ) and various I:E r atios. The MIAPri values were calculated for various I:E ratios. The classic (IAPee) and novel (MIAPri) methods were compared. For each I:E ratio, a range of possible MIAPri values was calculated according to various ΔIAP values. The dashed line represents the ACS threshold.
The effects of ΔIAP on MIAPri and the classic IAP were examined by a gradual increase in ΔIAP from 1 to 5 mmHg, with steps of 0.5 mmHg (Figure 5). The amount of IAPee was held constant (19 mmHg). For each ΔIAP, a range of possible MIAPri values was calculated with an I:E ratio of 4:1 for the maximum MIAPri and an I:E ratio of 1:4 for the minimum MIAPri.
Mathematical modelling of IAP measurement for a constant 19 mmHg end-expiratory IAP (IAP ee ) and various ΔIAP. The classic (IAPee) and novel (MIAPri) methods were used to measure the IAP. The MIAPri values were calculated for each ΔIAP. A range of possible MIAPri values for each ΔIAP was calculated according to various I:E ratios. The dashed line represents the ACS threshold.
Each of the abovementioned data sets was assumed to be a unique case, and the values shown in Figures 3,4,5 should not be considered as a trend in changes that can be obtained in a single patient.
Part B: human pilot study
In six ICU patients that were mechanically ventilated with Evita XL ventilators (Draeger, Lubeck, Germany), the mean IAP was automatically calculated as the geometrical mean (MIAP) via a balloon-tipped nasogastric tube connected to a CiMON monitor (Pulsion Medical Systems, Munich, Germany). The MIAPri was also calculated according to Equation 2. Data were collected on respiratory settings, plateau and mean alveolar pressures (P plat, P mean), PEEP, and dynamic compliance (calculated as the tidal volume (TV) divided by (P plat - PEEP)). The C ab was calculated as TV divided by ΔIAP. The thoraco-abdominal index of transmission (TAI) was calculated as ΔP alv (= P plat − PEEP) divided by ΔIAP, in which P alv is the alveolar pressure.
The effects of IAPee on MIAPri were examined by a gradual increase in PEEP from 0 to 15 cmH2O, with steps of 5 cmH2O during a best-PEEP manoeuvre (20 measurements at each PEEP level in five patients, resulting in 80 measurements). The effects of ΔIAP on MIAPri were examined by a gradual increase in TV from 250 to 1,000 ml, with steps of 250 ml during a low-flow pressure-volume loop (20 measurements at each TV level in five patients, resulting in 80 measurements). The effects of I:E ratio on MIAPri were examined by a gradual increase in the I:E ratio from 1:2 to 2:1, with steps of 0.5 during a recruitment manoeuvre (9 measurements at each I:E ratio in one patient, resulting in 45 measurements).
Statistical analysis was performed using SPSS software. Pearson correlation analysis and Bland and Altman analysis were performed. For comparisons between MIAPri and IAPee at different levels of IAPee (PEEP), TV, and I:E ratio, a two-tailed paired Student's t-test was performed. Data are expressed as the mean with the standard deviation (SD), unless specified otherwise. A P value below 0.05 was considered statistically significant. The local EC and IRB approved the study, and informed consent was obtained from next of kin.
Results
Part A: mathematical modelling
According to Equation 2, three major independent parameters determine the MIAPri: IAPee, I:E ratio, and ΔIAP. Therefore, for each IAPee, the MIAPri depends on two other factors (Figure 3). For IAPee values between 16 and 20 mmHg, the classic IAP remained below the ACS threshold (dashed line in Figure 3); however, the MIAPri was able to exceed the ACS threshold. Furthermore, as seen in Figures 4 and 5, the classic IAP was continuously below the ACS threshold, but different ranges of probable MIAPri values were above the ACS threshold. By changing the I:E ratio, the MIAPri values changed with dissimilar intensities (e.g. when the I:E ratio decreased from 4:1 to 3.5:1, the intensity of changes in the MIAPri values was less than that when the I:E ratio decreased from 1.5:1 to 1:1; Figure 4). Furthermore, for a constant IAPee, higher values for either the I:E ratio or ΔIAP were found to be capable of causing a wider range of possible MIAPri values (Figures 4 and 5). Mathematically, for all instances in which the ΔIAP was greater than 0 mmHg, the MIAPri was larger than the classic IAP (see the addendum) [21].
Regression plot and Bland and Altman analysis. (A) Regression plot comparing mean IAP measured via the geometric mean (MIAP) versus the respiratory-integrated MIAP (MIAPri). There is an excellent correlation between the two methods. (B) Bland and Altman analysis comparing MIAP with MIAPri. The dashed red lines show the upper and lower limits of agreements.
Part B: human pilot study
Six mechanically ventilated patients (three severely burned patients and three surgical ICU patients) were studied. The male-to-female ratio was 2:1. Table 1 summarises the baseline patient demographics.
Regression analysis and Bland and Altman analysis
In total, 205 paired MIAP and MIAPri measurements were performed with an identical statistical mean of 12.2 ± 3.8 mmHg. Figure 6A shows an excellent correlation between the MIAP and MIAPri (R2 = 0.99, P < 0.001). Analysis according to Bland and Altman showed a bias and precision of 0 and 0.2 mmHg, respectively, with small limits of agreement ranging from −0.4 to 0.5 mmHg (Figure 6B). The percentage error was 3.5%.
Effect of IAPee, I:E ratio, and ΔIAP on MIAPri
Gradually increasing PEEP from 0 to 15 cmH2O resulted in an increase in MIAPri from 11.7 ± 4.1 to 13.1 ± 4.2 mmHg (P < 0.001). Meanwhile, IAPee increased from 9.9 ± 3.4 to 11.9 ± 3.7 mmHg (P < 0.001). Moreover, a gradual increase in the I:E ratio from 0.5 (1:2) to 2 (2:1) caused an increase in MIAPri from 10.8 ± 2.6 to 12.9 ± 2.9 mmHg (P < 0.001), while IAPee increased from 9.7 ± 2.3 to 10.4 ± 2.5 mmHg (P < 0.001). In addition, gradually increasing TV from 250 to 1,000 ml led to an increase in ΔIAP from 2.1 ± 1.1 to 5.7 ± 2.3 (P < 0.001). This increase in ΔIAP resulted in an increase in MIAPri from 11.6 ± 4 to 13.1 ± 4.3 mmHg (P < 0.001), while IAPee increased from 10.7 ± 3.6 to 10.9 ± 3.5 mmHg (P = NS). The MIAPri was significantly higher than IAPee at each PEEP level, I:E ratio, and TV (Figure 7A,B,C; P < 0.001).
The effects of gradual increase of PEEP, I:E ratio, and TV. (A) The effect of gradual increase of PEEP on classic IAP (open circles) and the respiratory-integrated MIAP (MIAPri; closed circles). Both the classic IAP and MIAPri were increased significantly (P < 0.001). The MIAPri was significantly higher than the classic IAP for all PEEP levels (P < 0.001). The dashed line shows the 12 mmHg IAH grade I threshold. (B) The effect of gradual increase of I:E ratio on IAPee (open circles) and MIAPri (closed circles). Both the IAPee and MIAPri were increased significantly (P < 0.001). The MIAPri was significantly higher than IAPee for all I:E ratios (P < 0.001). The dashed line represents the 12 mmHg IAH grade I threshold. (C) The effect of gradual increase of tidal volume (TV; and thus ΔIAP) on IAPee (open circles) and MIAPri (closed circles). The MIAPri was significantly higher than IAPee at all TV values (P < 0.001). The dashed line shows the 12 mmHg IAH grade I threshold.
The classic IAP of patients was below the IAH grade I threshold; however, the MIAPri significantly exceeded the threshold in several instances (P < 0.001; Figure 7).
Univariate analysis
Univariate Pearson regression analysis showed significant correlations between MIAPri and IAPei (R = 0.99), IAPee (R = 0.99), ΔIAP (R = 0.78), and C ab (R = −0.74); between IAPei and IAPee (R = 0.96), ΔIAP (R = 0.86), and C ab (R = −0.73); between IAPee and ΔIAP (R = 0.7) and C ab (R = −0.73); between ΔIAP and ΔP alv (R = 0.79) and C ab (R = −0.58); and finally between TAI and C ab (R = −0.8) (P < 0.001). Figure 8A,B,C shows some regression plots.
Linear regression plots. (A) Linear regression plot showing the respiratory-integrated mean intra-abdominal pressure (MIAPri) in relation to ΔIAP (= IAPei − IAPee, where IAPei is the end-inspiratory IAP and IAPee is the end-expiratory IAP). (B) Linear regression plot showing the respiratory changes of intra-abdominal pressure (ΔIAP) in relation to ΔP alv (= Pplat - PEEP, where P alv is the alveolar pressure, P plat is the plateau alveolar pressure, and PEEP is the positive end-expiratory pressure). (C) Linear regression plot showing the relation between the thoraco-abdominal index of transmission (i.e. TAI = ΔP alv /ΔIAP) and the abdominal wall compliance (i.e. C ab = TV/ΔIAP).
Multivariate regression analysis
Analyses showed that the IAPee (mainly affected by PEEP), ΔIAP, and I:E ratio were independent variables defining the MIAP (Table 2). According to the regression analysis in our sample population, the MIAP can also be calculated from the following simplified formula (P < 0.001), in which 'I' and 'E' are elements of the I:E ratio:
Discussion
A novel concept of IAP measurement based on the geometric mean of the IAP waveform was presented. The independent parameters determining the IAP in this concept were defined. The human pilot study validated the mathematical modelling with an excellent correlation. A significant difference was observed between the classic IAP and the MIAPri in our clinical study.
The human study confirmed that MIAPri is as accurate as an automated geometric MIAP calculation by a CiMON monitor. More importantly, the higher the MIAP or IAPee, the higher the ΔIAP since ΔIAP acts as an indirect marker of C ab. The ΔIAP is correlated with ΔP alv or is thus inversely correlated with dynamic compliance. As well, the higher the C ab, the lower the TAI. The human study confirmed the predictions of the mathematical modelling in which IAPee (affected by different PEEP settings), ΔIAP, and I:E ratio were recognised as the major independent determinants of MIAPri. We also showed that in patients with IAH and under mechanical ventilation, the IAP may be influenced by ventilator settings.
The critical difference between the MIAPri and the classic IAP near the ACS threshold in our mathematical modelling, as well as the significantly higher MIAPri than the IAPee around the IAH threshold in our human study, calls for future studies. The dissimilar intensity in MIAPri changes under changes in the I:E ratio in Figure 4 may implicate the existence of critical points in the I:E ratio, wherein changing this ratio may cause a more intense change in the MIAPri. Furthermore, since MIAPri seems to be almost always larger than the classic IAP, relying only on the classic IAP may place some patients at risk of silent IAH or ACS. Although the aim of the current study was not to address these implications clinically, these findings indicate that further investigations should be performed on respiratory manoeuvres to manage IAH in mechanically ventilated patients (e.g. decreasing the I:E ratio and/or the ΔIAP, or maintaining the I:E ratio in a predefined range).
A limitation of our study was the lack of data to evaluate the physiological difference between the MIAPri and the classic IAP. However, this study only aimed to prove the concept and to set the stage for further studies. Therefore, we believe that the lack of physiological data does not limit our findings. Nonetheless, further studies on the clinical effects of this concept are necessary before it can be introduced in clinical practice.
Conclusions
A novel concept MIAPri was presented to consider the IAP changes during respiration and was based on the geometric mean (MIAP) of the IAP waveform. An excellent correlation was observed between the results of the mathematical modelling and those obtained in real patients. Substantial differences were observed between the two IAP methods (the classic IAP measured at end expiration and the novel MIAP). Based on our findings, we believe that the novel concept of MIAPri may be a better representation for the pressure concealed within the abdominal cavity. Further clinical studies are necessary to reveal the physiological effects of this novel concept.
Authors' information
SA is aveterinary surgeon (DVM, DVSc) and a medical research consultant in laboratory animal researches in the field of trauma, haemorrhage, critical care, and anaesthesia. MLNGM is a former president and treasurer of the World Society of the Abdominal Compartment Syndrome and is the ICU and High Care Burn Unit Director of the Department of Intensive Care in Ziekenhuis Netwerk Antwerpen Stuivenberg.
Addendum
See additional file 1.
Abbreviations
- ACS:
-
abdominal compartment syndrome
- C ab :
-
abdominal compliance
- IAH:
-
intra-abdominal hypertension
- IAP:
-
intra-abdominal pressure
- IAPee :
-
end-expiratory IAP
- IAPei :
-
end-inspiratory IAP
- MIAP:
-
mean intra-abdominal pressure (geometrical mean)
- MIAPri :
-
respiratory-integrated mean intra-abdominal pressure
- P alv :
-
alveolar pressure
- P mean :
-
mean airway pressure
- P plat :
-
plateau airway pressure
- PEEP:
-
positive end-expiratory pressure
- TAI:
-
thoraco-abdominal index of transmission
- TV:
-
tidal volume
- WSACS:
-
World Society of the Abdominal Compartment Syndrome.
References
Cheatham ML, White MW, Sagraves SG, Johnson JL, Block EF: Abdominal perfusion pressure: a superior parameter in the assessment of intra-abdominal hypertension. J Trauma 2000,49(4):621–626. discussion 626–627 10.1097/00005373-200010000-00008
Malbrain ML, De laet IE: Intra-abdominal hypertension: evolving concepts. Clin Chest Med 2009,30(1):45–70. 10.1016/j.ccm.2008.09.003
Ivatury RR, Cheatham ML, Malbrain ML, Sugrue M: Abdominal Compartment Syndrome. Georgetown: Landes Bioscience; 2006.
Malbrain ML, Cheatham ML: Definitions and pathophysiological implications of intra-abdominal hypertension and abdominal compartment syndrome. Am Surg 2011,77(Suppl 1):S6–11.
Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, Del Turco M, Wilmer A, Brienza N, Malcangi V, Cohen J, Japiassu A, De Keulenaer BL, Daelemans R, Jacquet L, Laterre PF, Frank G, de Souza P, Cesana B, Gattinoni L: Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med 2005,33(2):315–322. 10.1097/01.CCM.0000153408.09806.1B
Malbrain ML: Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med 2004,30(3):357–371. 10.1007/s00134-003-2107-2
De Keulenaer BL, De Waele JJ, Powell B, Malbrain ML: What is normal intra-abdominal pressure and how is it affected by positioning, body mass and positive end-expiratory pressure? Intensive Care Med 2009,35(6):969–976. 10.1007/s00134-009-1445-0
Hunter JD: Abdominal compartment syndrome: an under-diagnosed contributory factor to morbidity and mortality in the critically ill. Postgrad Med J 2008,84(992):293–298. 10.1136/pgmj.2007.064212
Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppaniemi A, Olvera C, Ivatury R, D'Amours S, Wendon J, Hillman K, Johansson K, Kolkman K, Wilmer A: Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med 2006,32(11):1722–1732. 10.1007/s00134-006-0349-5
Sturini E, Saporito A, Sugrue M, Parr MJ, Bishop G, Braschi A: Respiratory variation of intra-abdominal pressure: indirect indicator of abdominal compliance? Intensive Care Med 2008,34(9):1632–1637. 10.1007/s00134-008-1155-z
Papavramidis TS, Marinis AD, Pliakos I, Kesisoglou I, Papavramidou N: Abdominal compartment syndrome - intra-abdominal hypertension: defining, diagnosing, and managing. J Emerg Trauma Shock 2011,4(2):279–291. 10.4103/0974-2700.82224
Verzilli D, Constantin JM, Sebbane M, Chanques G, Jung B, Perrigault PF, Malbrain M, Jaber S: Positive end-expiratory pressure affects the value of intra-abdominal pressure in acute lung injury/acute respiratory distress syndrome patients: a pilot study. Crit Care 2010,14(4):R137. 10.1186/cc9193
Malbrain ML, De laet I, Cheatham M: Consensus conference definitions and recommendations on intra-abdominal hypertension (IAH) and the abdominal compartment syndrome (ACS)--the long road to the final publications, how did we get there? Acta Clin Belg 2007,62(Suppl 1(1)):44–59.
Fischbach M, Terzic J, Laugel V, Escande B, Dangelser C, Helmstetter A: Measurement of hydrostatic intraperitoneal pressure: a useful tool for the improvement of dialysis dose prescription. Pediatr Nephrol 2003,18(10):976–980. 10.1007/s00467-003-1199-9
Stewart J: Calculus: Concepts and Contexts. 3rd edition. Belmont: Thomson Brooks/Cole; 2005.
Kanani M, Elliott M: Applied Surgical Physiology Vivas. London: Greenwich Medical Media Ltd; 2004.
Raff H, Levitzky MG: Medical Physiology: A Systems Approach. New York: McGraw-Hill; 2011.
Klabunde RE: Cardiovascular Physiology Concepts. Philadelphia: Lippincott Williams & Wilkins; 2005.
Chemla D, Antony I, Zamani K, Nitenberg A: Mean aortic pressure is the geometric mean of systolic and diastolic aortic pressure in resting humans. J Appl Physiol 2005,99(6):2278–2284. 10.1152/japplphysiol.00713.2005
Moran D, Epstein Y, Keren G, Laor A, Sherez J, Shapiro Y: Calculation of mean arterial pressure during exercise as a function of heart rate. Appl Human Sci 1995,14(6):293–295.
Ahmadi-Noorbakhsh S, Malbrain MLNG: Addendum: Mathematical model for calculation of mean intra-abdominal pressure, taking into account integration of inspiratory and expiratory intra-abdominal pressure. Ann Intensive Care 2012. Supplement 2 (in press)
Acknowledgements
This article has been published as part of Annals of Intensive Care Volume 2 Supplement 1, 2012: Diagnosis and management of intra-abdominal hypertension and abdominal compartment syndrome. The full contents of the supplement are available online at http://www.annalsofintensivecare.com/supplements/2/S1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
MLNGM is a member of the medical advisory board of Pulsion Medical Systems, Munich, Germany.
Authors' contributions
SA and MLNGM planned the study and were responsible for the design, coordination, and drafting the manuscript. SA developed the mathematical model for MIAP calculation and performed the theoretical analyses. MLNGM performed the data collection and statistical analysis for the human pilot study. Both authors read and approved the final manuscript.
Electronic supplementary material
13613_2012_117_MOESM1_ESM.docx
Additional file 1: Mathematical model for calculation of mean intra-abdominal pressure, taking into account integration of inspiratory and expiratory intra-abdominal pressure. (DOCX 35 KB)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ahmadi-Noorbakhsh, S., Malbrain, M.L. Integration of inspiratory and expiratory intra-abdominal pressure: a novel concept looking at mean intra-abdominal pressure. Ann. Intensive Care 2 (Suppl 1), S18 (2012). https://doi.org/10.1186/2110-5820-2-S1-S18
Published:
DOI: https://doi.org/10.1186/2110-5820-2-S1-S18