Abstract
Background
Schistosomiasis is classically described as a rural disease that occurs in areas with poor sanitary conditions. However, over recent decades, there has been an expansion of schistosomiasis foci towards urban areas faced with a rapid and disordered urbanization. In Bamako, Mali, the impact of environmental change on vector-borne diseases such as schistosomiasis is not well known. This study sought to identify the presence of schistosomiasis transmission hotspots in Bamako. Using this perspective, we aimed to describe the risk factors of the endemization and maintenance of schistosomiasis.
Materials and methods
A cross-sectional study was carried out in the six municipalities (communes) in Bamako. Environmental information was obtained from earth observation satellites in order to maximize ecological contrasts. Twenty-nine blocks of 200 m x 200 m were identified. We selected a school inside or nearest to each block for urine and stool samples examination. The study cohort was school children aged between eight and 15 years. The Kato-Katz technique and filtration were used for Schistosoma mansoni and S. haematobium ova research in stools and urine, respectively. The schools and snail breeding sites were georeferenced. Four malacological surveys were conducted between October 2011 and February 2012. Bivariate analysis was used to identify independent predictors of being infected with schistosomiasis.
Results
The prevalence rate of S. haematobium was 14.7% (n = 1,761) and that of S. mansoni 1.5% (n = 1,491). Overall, the urinary form was endemic in 76.6% of schools. The infection significantly varied between the municipalities (p < 0.001). It was also more prevalent on the left side of the Niger River than the right side (17.4% vs. 9.5% respectively; p < 0.001). The vicinity to snail breeding sites (OR = 3.677; 95% IC [2.765–4.889]; p < 10 -3) and parents’ occupations (OR = 7.647; 95% IC [2.406–24.305]; p < 0.001) were the most important risk factors associated with S. haematobium infection exposure. Biomphalaria pfeifferi, Bulinus truncatus, and B. globosus were the intermediate hosts captured. The schistosome natural infection rates (SNIRs), which were low or nil in October and November, rose to 2.8% in January and 8.3% in February for B. pfeifferi and B. truncatus, respectively.
Conclusion
Our findings show that there is a high transmission risk for schistosomiasis in Bamako. Appropriate integrated control measures need to be introduced to control the transmission of this disease in the study area.
Similar content being viewed by others
Multilingual abstracts
Please see Additional file 1 Multilingual abstracts in the six official working languages of the United Nations.
Background
Schistosomiasis is the second most prevalent tropical water-borne disease after malaria, and a leading cause of severe morbidity in many parts of the world. According to a recent estimate, 207 million people were infected by mid-2003. Active transmission is reported in 67 countries, of which 46 are in Africa [1]. In relation to transmission conditions, schistosomiasis is classically described as a rural disease that occurs in areas without potable water and adequate sanitation. Nevertheless, because of migration, and rapid and disordered urbanization, urban areas in Africa and South America are now foci of transmission. In Africa, the disease is endemic in cities such as Ibadan (Nigeria) [2], Addis Ababa (Ethiopia) [3], and Kisumu (Kenya) [4]. One of the major factors noted in the process of “urbanization” of schistosomiasis is the migratory flow of the infected rural population, which occurs due to more attractive employment opportunities in the urban areas [5].
Infections due to Schistosoma haematobium and S. mansoni have existed in Bamako since 1953 [6]. From this period up until now, many surveys have been conducted but they have been geographically limited to one or two quarters [7, 8]. Data collected on disease and snail intermediate hosts were punctual but incomplete, and didn’t reflect the epidemiology of infection in all districts of Bamako. Knowing information on global rates of schistosomiasis and snail distribution could help to identify hotspots in the city and could lead to treatment been given to those who need it most (in terms of prevalence and intensity of infection). It is crucial to describe the risk factors that give rise to occurrences of schistosomiasis in order to identify the mechanisms for transmission and maintenance of this endemic disease in Bamako.
Methods
Study area
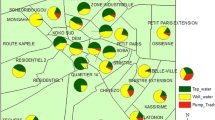
The study was conducted in Bamako (12°39’ N latitude and 8°0’ W longitude), the capital city of Mali (see Figure 1). The surface area of the city is 1420 km2. The town looks like a big basin, surrounded in part by hills, with the Niger River and its tributaries flowing across. The town belongs to the North-Soudanian climatic zone with two major seasons: the wet season from November to May with its beginning and end marked by torrential rains and thunderstorms, and the dry season from April to October. The mean annual rainfall is about 1,400 mm, which occurs mainly during the period from July to September. Temperatures are generally high and almost uniform throughout the year with a mean annual maximum temperature of 33°C and a mean annual minimum temperature of 22°C [9]. The tributaries of the Niger River must be used to collect water from the rain but they have been turned into a refuse dump, which leads to a slow flow or stagnant water, and in turn makes them suitable breeding sites of snail intermediate hosts.
Bamako was founded at the end of the 16th century. In 2009, the population was 1,809,106, with an annual growth of 4.8% [9]. Unfortunately, this rapid and disordered growth has not been followed by improved sanitation, sewage systems, and the right water supplies. The city is partitioned into six municipalities, ranging from M-I to M-VI and more than 50 quarters (see Figure 1). There are four municipalities (I–IV) on the left side and two (V–VI) on the right side of the Niger River. There are about 736,183 inhabitants on the left side compared to 849,727 on the right side. People first occupied the left side, especially the quarter of Niaréla in the CII, near the River. Progressively, and in accordance with the city’s growth due to various factors including migration, other quarters appeared along the Niger River and its tributaries. The quarters on the left side of the River (also named the old town) are characterized by high population densities and poor sanitation conditions compared to those on the right side, which are more spaced out and located further from the River. The municipalities on the right side are newer as they were established after the independence of the country in 1960. The town presents a central zone including the municipalities II, III, and one part of the M-IV called ACI 2000, which contains most of the administrative offices and main industries, and is marked by a high population density.
Study design and sampling techniques
The survey was conducted in twenty-nine (29) blocks, each 200 m × 200 m, in Bamako. These blocks were selected on the basis of the images from the SPOT-5 (Satellite Pour l’Observation de la Terre), part of the National Aeronautics and Space Administration’s (NASA) Earth Observing System [10], at 2.5 m of spatial resolution obtained on March 2, 2009. The non-supervised classification method of the image allows the generation of an earth occupation map. The 29 blocks were selected in order to maximize the ecological (the presence of a permanent water collection system), environmental (how the population manages garbage, and stool and urine residues), and anthropogenic (the relationship between population and water collection) contrasts. According to the distribution of the blocks through the municipalities, three blocks in the M-I, six in the M-II, four in the M-III, five in the M-IV, seven in the M-V, and four in the M-VI were selected. Once the blocks were chosen definitively, a precise mapping was done after collecting GPS points around them.
One school inside or nearest to each of the 29 blocks was chosen for parasitological investigations in order to study the distribution of schistosomes in Bamako. Similarly, all the human water contact sites in the Niger River or its tributaries located near the chosen schools were observed for snail breeding sites.
According to the World Health Organization (WHO) guidelines, 200 to 250 individuals (50 per block or school) from third or fourth year primary school classes (9–10-year-old schoolchildren) should be an adequate sample for an ecologically homogeneous area in order to evaluate prevalence and intensity [11]. The minimum sample size was thus 1,450 (29 blocks × 50). We added 10% (n = 145) to this sample size for the loss of view (n = 1,595). In each school, the children were randomly selected using the class rosters. Figure 2 shows the selection of blocks, schools, classes, and samples for urine and stool examination.
Malacological survey
The survey sites were selected on the basis of their water contact points, that is where people consistently go to collect water, wash clothes, bathe, swim, play (young children), and wash cars. Snail sampling was conducted from October 2011 to February 2012on these major water contact sites on the Niger River or its tributaries. During snail collection, observations were also made on the physical characteristics of the habitat such as vegetation abundance, turbidity, the nature of the substrate, and the speed of the water.
The vegetation abundance was estimated as the coverage rate of each species. The turbidity of the water was classified by observing the water through a test-glass with a black cross on the bottom. The more water there was in turbid, the less of the cross would be visible. The substratum in the breeding sites was described as rocky, muddy, and sandy. The speed of the water was calculated using the following formula: S = D/t (with S, the mean speed in m/s; D, the distance between the two points in meters, and t, the time in seconds).
Two trained field collectors carried out the sampling using standard snail scoops or occasionally just their hands to collect directly snails on supports (aquatic vegetation, wastes, etc.) [12]. The same collectors scooped for snails in all areas to achieve some level of standardized sampling effort. Sampling time was fixed to 15 minutes per location and was performed between 08:30 h and 10:30 h. Each sampling area per location was approximately 10 m2, and lengths of 10 m along streams were used. Snails deeper than one meter in the water were not collected. After each collection, snails from each site were appropriately labeled and transported in separate perforated plastic containers to the Department of Epidemiology and Infectious Diseases (DEAP) of the Faculty of Medicine at the University of Technique and Technologies of Bamako (USTTB) where they were processed. The snail density was expressed as the number of snails captured per collector during the 15 minutes at each site. In our case, where there were two collectors, the total number of snails captured by each collector was divided by two (the number of collectors). At the laboratory, snails were identified to species level based on shell morphological characteristics using standard keys (Bulinus truncatus, B. globosus, and Biomphalaria pfeifferi) [13, 14]. Then snails were rinsed and placed individually in 24-well culture plates containing 1 ml of clear, filtered water (same source as site of collection) and exposed to artificial light for 1–2 h to induce cercarial shedding. The infection was diagnosed and the foci of schistosomiasis transmission were taken to be the breeding sites that presented infected snails [15]. The wells of the plates were then examined for the presence of cercariae under a dissecting microscope. Snails that did not shed cercariae on the first exposure were re-exposed the next day. Following this, the snails were smashed between two glass slides in order to verify the presence of S. haematobium cercariae or sporocyst. Bifurcate cercariae were used to indicate that the cercariae were of mammalian origin. To determine the snails’ natural infection rate, the proportion of the mollusks that were positive for S. mansoni or S. haematobium in relation to the total number of mollusks examined was calculated.
Parasitological survey
One stool and urine examination census survey was conducted among the sample of schoolchildren selected inside or near each of the 29 blocks by using the same methodology: All individuals were asked to register and participate voluntarily; after giving their oral consent, each child was given plastic containers to collect their stool and urine samples; the urine samples were collected on one day and the stool samples were collected the following day. The samples (stool and urine) were examined on fields in situ. To diagnose the number of cases of schistosomiasis and the parasitic load of the parasitized individuals, feces and urine samples were parasitologically diagnosed by means of the Kato-Katz [16] and filtration methods, respectively, with one sample from each participant. The prevalence rate of schistosomiasis in the blocks/schools was defined as the number of positive individuals per block/school per total number of individuals examined per block/school and multiplied by 100. S. haematobium intensity was classified into three categories: i) no egg; ii) slight (1–49 eggs per 10 ml of urine); and iii) heavy (≥50 eggs per 10 ml of urine). S. mansoni intensity was classified into four classes: i) no egg; ii) 1–99 epg (eggs per gram of stool); iii) 100–399 epg; and iv) ≥ 400 epg [17].
Collection of sociodemographic data
Sociodemographic data including gender, age, and parents’ occupations were obtained using a structured questionnaire. Children were invited to answer questions separately. The distance between the school and the snail breeding sites was calculated as a risk factor of schistosomiasis transmission.
Statistical analysis
Data were double entered using Access and Prevalence, and the intensity of infection with 95% confidence intervals were calculated using SPSS (IBM, version 19). Differences in proportions were tested using the chi-square test, either for trend or for independence as appropriate, with a risk of 0.05. A multivariate analysis identified the following factors associated with the infection: Niger River side (left vs. right); gender (male vs. female); age (from 6 to 15 years); parents’ occupations (civil servants, traders vs. working classes, drivers, cleaners, etc.); and distance between the school and snail breeding sites (≤100 m and ≥500 m). In the initial logistic model, all variables associated with the infections in the univariate analysis were included, with p < 0.20. Variables with statistically significant associations (p < 0.05) with S. haematobium infection were kept in the final model.
Ethical approval
The proposal was reviewed and approved by the Institutional Review Board (IRB) of the Faculty of Medicine, Pharmacy and Dentistry, University of Bamako. Community consent was obtained before starting the study. Each study participant signed an assent form.
Results
Environmental characteristics
In the Niger River, the substratum is mainly rocky, sandy, or muddy. The lack of vegetation throughout the riverbed has led to the rise of the water speed (35–40 m/s). In contrast, in the tributary, Woyowayanko, the substratum was mainly rocky with a lot of vegetation, which is associated with snail breeding sites (algae and other immerged and submerged species). The speed of water in the stream was about 30 m/s. On the borders of the tributaries, there are some trees (Mangifera indica, Khaya senegalensis, and Acacia senegalensis), which contribute to the humidity around the snail breeding sites especially favorable for Biomphalaria growth. According to the turbidity, water was more turbid in the Niger River than in the streams except after a rainy period. The speed of water varied from 11 m/s to 28 m/s in the stream (Woyowayanko), whereas it was higher (35–45 m/s) in the River.
Malacological survey
The Niger River and its tributaries (streams) were surveyed for S. haematobium and S. mansoni intermediate hosts from October 2011 to February 2012, and snails were collected using a scoop. The snails have also collected occasionally using big nippers according to the nature of water collection. Bulinus truncatus, B. globosus, and Biomphalaria pfeifferi were collected from both water bodies (see Table 1). They were found attached to leaves falling from surrounding trees, stones, decaying wood, plastic, etc. Other snail species, which are not involved in human schistosome transmission (Bulinus forskalii and Lymnaea natalensis, Bellamya unicolor or Lanistes varicus), were also collected from the breeding sites.
The host snails, Bulinus truncatus, B. globosus, and Biomphalaria pfeifferi, were found with high density in December with 108, 13, and 78 snails collected per person per 15 minutes, respectively (see Table 1).
The foci of transmission were described on the Niger River and the streams (see Figure 3). B. truncatus infected with S. haematobium was found at two points on the tributary of Woyowayanko and two points on each side of the Niger River in CII (see Figure 3). In contrast, Biomphalaria pfeifferi infected with S. mansoni was found at two points of Woyowayanko. No infected B. globosus were found during the survey.
The frequency and the infection rates of Bulinus truncatus and Biomphalaria pfeifferi are shown in Figures 4 and 5. B. truncatus infected with S. haematobium was found from October to February, except for in November. The prevalence of schistosome infection in Bulinus truncatus was found in February. B. pfeifferi infected with S. mansoni was found only in December and January (see Figure 5).
Parasitological survey
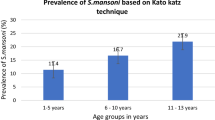
From the 29 schools surveyed, the prevalence rates of S. haematobium, S. mansoni, and those doubly infected were 14.7% (259/1,761), 1.5% (22/1,491), and 1.0% (15/1,459), respectively. Overall, 2.4% (42/1,761) were heavily infected with S. haematobium. For S. mansoni, only 0.5% (7/1,491) of positive children excreted 400 epg or more.
The initial logistic model included the following variables: the River’s sides (p < 10-4), the age (p < 10-4), the gender (p = 0.14), the parents’ occupations (p < 10-4), and the distance between the school and the snail breeding sites (p < 10-4) (see Table 2). Except for gender, the prevalence of S. haematobium varied significantly according to the other variables: the schoolchildren on the left side of the River were significantly more infected than those on the right side; children aged 11–15 years were more infected than those aged 6–10 years; the children whose parents were non officials were more exposed; and the prevalence of the infection was higher in the schools located nearer to the snail breeding sites compared to those located further from the snail habitats. Results of the multivariate analysis showing the relationship between S. haematobium infection and sociodemographic indicators are shown in Table 3. The most important factors associated with the infection were parents’ occupations (officials vs. non officials: OR = 7.647; CI95%: 2.406–24.305) and the distance between the school and the snail breeding sites (≤100 m vs. ≥500 m: OR = 3.67; CI95%: 2.765–4.889).
After the distribution of infection through the district was mapped (see Figure 6), it was evident that S. haematobium was present in all six municipalities. Its prevalence varied from 31.7% in the M-IV to 3.9% in the M-VI. S. mansoni was recorded in four districts where the prevalence was higher (2.6%) in the M-IV and lower (0.6%) in the M-I. Double infection was reported in the M-II (11.5%), -IV (8.5%), and -V (4.2%) districts.
According to the endemicity of the disease throughout the schools, S. haematobium was hyperendemic (P ≥ 50%) in one school of the municipality -II (see Figure 7), mesoendemic (10% ≥ P < 50%) in 12, and hypoendemic in 16 (P < 10%).
Discussion
From October 2011 to February 2012, we captured three intermediate hosts: Bulinus truncatus, B. globosus, and Biomphalaria pfeifferi. During the snail survey, physical characteristics of the water bodies showed that the Woyowayanko stream was moderately turbid, and covered by large amounts of weeds, algae (70% of coverage in some sites), and other garbage such as plastic, clothes, and fallen leaves. It was small and slow flowing (less than 0.6 m/s). The substratum of the water body was rocky (90–100% of coverage of the sites) and sandy (10% of coverage in one site). On the other hand, the Niger River was large, fast flowing, clear, and lacked any vegetation, weeds, or algae. The substratum of the water was sandy and muddy. The snail survey showed a bigger abundance of snails (B. truncatus and B. pfeifferi) in the Woyowayanko stream and relatively few in the Niger River. The reason for the observed difference in the amount of snails in the two water bodies could be explained by the fact that the stream is slow flowing and is abundantly covered with aquatic weeds, whereas the River is not. It has been reported that small rivers with flow rates of 10–30 m/s, with slight turbidity, abundant vegetation at the edge, and that are muddy are potentially favorable habitats for pulmonates snails that could be involved in human schistosome infection [18].
The presence of three intermediate host snail species in Bamako, B. truncatus, B. globosus, and B. pfeifferi, pose a permanent threat to schistosomiasis transmission, as two species were found to be infected by the trematode. This requires attention by health agents because, despite the low prevalence rates of the disease, there is a high risk of schistosomiasis expansion. Other factors contributing to this expansion include poor sewage systems, toilet water thrown in water collections where snails breed, and leisure and domestic activities associated with water contact.
All species involved in human schistosome transmission were described previously in many foci including the rice-irrigated area of Office du Niger, around the Sélingué and Manantali dams, in the Dogon country, and in Bamako [19–21]. In our study, the prevalence of the S. mansoni infection in Biomphalaria pfeifferi was 2.6% in January. This prevalence was lower compared to the 16.9% of the prevalence of the same species in February, but higher than the 0.027% in April in Sanja area, Amhara region, Ethiopia [22].
The endemicity of schistosomiasis now presents a dual picture. In some regions (North Africa, Asia, Caribbean, the Middle East, and Latin America), many programs have been successful in reducing the mortality, morbidity, and transmission, yet schistosomiasis remains a major cause of mortality and morbidity in a number of countries, especially those in Sub-Saharan Africa [23]. For the establishment of schistosomiasis in new transmission foci, the ecologies and environmental conditions, appropriate aquatic snail intermediate hosts, and the human definitive host must converge in space and time in suitable water bodies. In the case of Bamako, the endemic focus might have been established as a result of population movement into the suburban area from other endemic localities (regions of Ségou, Kayes, Mopti, and Koulikoro) [24, 25].
According to the WHO (2000), migrants contribute to the transmission and spread of schistosomiasis in at least three ways: (i) by introducing the parasite into non-endemic areas, (ii) by creating habitats for snail intermediate hosts and water contact points in the areas where they settle, and (iii) by direct moves in which infected people migrate to areas where schistosomiasis has been controlled or eradicated [26]. In any of these cases, the settlement of populations in suburban agglomerations like in Bamako and the lack of sanitation and basic infrastructures result in fecal contamination of aquatic environments, with consequent infection of intermediate hosts and emergence of new foci of schistosomiasis transmission. Concerning the bio-ecological aspect, favorable environmental conditions were detected for vector reproduction (habitat) and parasite survival (pollution of water collections by stool and urine residues thrown in the River and streams) on the left side of the River as compared to the right side.
Our study revealed that there is S. haematobium and S. mansoni transmission in the urban area of Bamako, with the occurrence of infection at 14.7% (259/1,761) and 1.5% (22/1,491), respectively. The predominance of S. haematobium compared to S. mansoni has been reported before in Mali [3, 27]. Adaption of Bulinus truncatus, the major host snail of S. haematobium to a broad range of environmental conditions such as temperature, rainfall, and standing and flowing water bodies could be the reason for the widespread S. haematobium infection [21].
The factors, which were predictive of infection in our study, were the River side, age of child, the sociodemographic status of the parents, and the location of schools in relation to snail breeding sites. Children whose parents were workers were seven times more likely to be infected than children whose parents were civil servants (p < 0.001). The distance between the school where a child lives and the snail-colonized water source was another important exposure risk factor of infection. Children whose schools were located less than 100 m from the stream or River were three times more likely to be infected. According to the diversity of the urban area, children in the peripheral municipalities were likely to be more infected due to the presence and use of Niger River and its tributaries. The results are in agreement with studies conducted in Kenya and Malawi [28, 29]. In studies conducted in Brazil and Zambia, significant risk factors for infection were the male gender [odds ratio (OR) 2.42], and the ages of 9–12 years or 13–17 years (OR 3.33 and 3.26, respectively), compared to 5–8 year olds [30, 31]. In Nigeria, human urinary schistosomiasis appears to be highly endemic in peri-urban/rural areas and closely associated with poverty (a low family income, not living with biological parents). Literacy of the family head was, however, a protective factor [32].
Independently to the vicinity of water bodies, poor sanitation around water sources is a major cause of infection: the prevalence rates of S. haematobium and S. mansoni were 46.7% and 28.2%, respectively, in 1997 around the Farako stream, one of the Niger River tributaries (see Figure 3). During this period, Farako served as a source of water for laundering, bathing, and other domestic and recreational activities. Most of the quarter dwellers, and especially children, excreted their feces throughout the stream, near the water bodies. The stream became a dumping ground propitious for snail growing. But, since 2010, the stream is free from snails and the prevalence rates in the schools bordering it decreased to zero. In general, with the implementation of mass chemotherapy with praziquantel since 2005, prevalence rates and intensities decreased significantly. However, infection persists due to poor sanitation along the other water bodies (streams and River).
Conclusion
The finding of S. haematobium and S. mansoni infected children, and the collection of Bulinus truncatus and B. pfeifferi infected with schistosome cercariae all confirmed the transmission of schistosomiasis in Bamako. Therefore, based on our findings, appropriate integrated control measures (annual mass drug administration with praziquantel for schoolchildren living along the Niger River and streams, and sanitation of water bodies) need to be introduced to control the transmission of this disease in the study area.
Abbreviations
- ACI 2000:
-
Agence de Cession Immobilière
- B Bulinus :
-
Bulinus forskalii
- M-I–M-VI:
-
Mun icipalities
- CI:
-
Confidence interval
- DEAP:
-
Department of Epidemiology and Infectious Diseases
- GPS:
-
Global positioning system
- IBM:
-
International Business Machines Corporation
- ID:
-
Identification number
- IRB:
-
Institutional Review Board
- NASA:
-
National Aeronautics and Space Administration
- SNIR:
-
Schistosome natural infection rate
- OR:
-
Odds ratio
- P:
-
Prevalence
- p :
-
Probability
- SPOT:
-
Satellite Pour l’Observation de la Terre
- SPSS:
-
Statistical Package for the Social science
- vs:
-
versus
- WHO:
-
World Health Organization.
References
WHO - World Health Organization: The social context of schistosomiasis and its control: an introduction and annotated bibliography. 2008. Available from: http://www.dosei.who.int/uhtbin/cgisirsi/tLEqou8myB/26990009/5/0
Olaseha IO, Sridhar MK: Participatory action research: community diagnosis and intervention in controlling urinary schistosomiasis in an urban community in Ibadan, Nigeria. Int Q Community Health Educ 2005–2006,24(2):153–60. 10.2190/CBYM-94N2-E7DH-QRAL
Berhe N, Myrvang B, Gundersen SG: Gastro-intestinal symptoms associated with intense Schistosoma mansoni infection affect class-attentiveness of schoolchildren in Ethiopia. Acta Trop 2009,110(1):52–6. 10.1016/j.actatropica.2009.01.007
Opisa S, Odiere MR, Jura WG, Karanja DM, Mwinzi PN: Malacological survey and geographical distribution of vector snails for schistosomiasis within informal settlements of Kisumu City, western Kenya. Parasit Vectors 2011, 4:226. 10.1186/1756-3305-4-226
Elainne Christine De Souza G, Onicio Batista L-N, Jones A, Hernande Pereira Da S, Constança Simões B: Schistosomiasis transmission and environmental change: a spatio-temporal analysis in Porto de Galinhas, Pernambuco – Brazil. Int J Health Geogr 2012, 11:51. http://www.ij-healthgeographics.com/content/11/1/51 10.1186/1476-072X-11-51
Garrigue M: Enquête générale sur les bilharzioses à Bamako. Bull Soc Path Ex 1953, 46:693–5.
Sangho H, Dabo A, Coulibaly H, Doumbo O: Prévalence et perception de la schistosomose en milieu scolaire périurbain de Bamako au Mali. Bull Soc Path Ex 2002,95(4):292–4.
Dabo A, Sow MY, Sangaré L, Maiga I, Keita A, Bagayoko Y, et al.: Transmission de la schistosomose urbaine et prévalence des helminthoses intestinales à Bamako, Mali. Bull Soc Pathol Exot 2003,96(3):187–90.
Bamako; 2008.http://fr.wikipedia.org/wiki/Bamako
Observation/satellite database. Earth Resources Observation and Science (EROS) Center; 2009. http://gdsc.nlr.nl/gdsc/information/earth
Lwanga KS, Lemeshow S: Sample size determination in health studies. A practical manual. Geneva: World Health Organization; 1991.
Sellin B, Simonkovich E: Les mollusques hôtes intermédiaires des schistosomiases dans la région de Yanfolila-Kangaré (République du Mali). Bamako: Rapport d’enquête. Doc tech OCCGE; 1978:54.
Brown DS: Freshwater snails of Africa and their Medical importance. 2. London: Taylor and Francis; 1994.
DBL-WHO: A field guide to African Freshwater snails. Danish Bilharziasis Laboratory. Denmark: WHO collaborating Centre for Applied Malacology, Charlottenlund; 1998.
Kuntz ER: Effect of light and temperature on shedding of Schistosoma mansoni cercariae. Nav Med Res Inst 1946, 7:16.
Katz N, Chaves A, Pellegrino JP: A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 1972, 14:397–400.
Technical Reports Series, N o 728 In Report of a WHO Expert Committee on the Control of Schistosomiasis (Geneva, 1984). WHO; 1985:113.
Lo CT, Redda A, Gemeda N: Malacological studies of human schistosomiasis in Ethiopia. In Proceedings of Symposium on Human Schistosomiasis in Ethiopia. Edited by: Ayele T, Lo CT. Addis Ababa, Ethiopia: Institute of Pathobiology, Addis Ababa University; 1982.
Madsen H, Coulibaly G, Furu P: Distribution of freshwater snails in the Niger river basin in Mali with special reference to the intermediate hosts of schistosomes. Hydrobiologia 1987, 146:77–88. 10.1007/BF00007580
Coulibaly G, Madsen H: Seasonal density fluctuations of intermediate hosts of schistosomiasis in two streams in Bamako, Mali. J Afr Zool 1990, 104:201–12.
Dabo A, Diop S, Doumbo O: Distribution des mollusques hôtes intermédiaires des schistosomiases humaines à l’Office du Niger (Mali). II. Rôle des différents habitats dans la transmission. Bull Pathol Exot 1994,87(3):164–9.
Getachew A, Berhanu E, Mulugeta A, Beyene P: Epidemiological study on Schistosoma mansoni infection in Sanja area, Amhara region, Ethiopia. Parasit Vectors 2014, 7:15. http://www.parasitesandvectors.com/content/7/1/15 10.1186/1756-3305-7-15
World Health Organization Expert Committee Technical Reports Series N o . 912. In Prevention and control of schistosomiasis and soil-transmitted helminthiasis. Volume 912. WHO; 2002:1–57.
Brinkmann UK, Korte R, Schmidt-Ehry B: The distribution and spread of schistosomiasis in relation to water resources development in Mali. Trop Med Parasit 1988, 39:182–5.
Traoré M, Landouré A, Diarra A, Kanté B, Sacko M, Coulibaly G, et al.: Geographic distribution and epidemiology of urinary schistosomiasis in Mali: implications for a control program. Mali Med 2007,22(3):22–8.
WHO - World Health Organization: Report of the WHO informal consultation on schistosomiasis in low transmission areas: control strategies and criteria for elimination. Geneva: WHO Document WHO/CDS/CPE/SIP/2001.1; 2000:51.
Brinkmann UK, Werler C, Traoré M, Korte R: The National Schistosomiasis Control Programme in Mali: objectives, organization, results. Trop Med Parasitol 1998,39(2):157–61.
Handzel T, Karanaja DM, Addiss DG: Geographic distribution of schistosomiasis and soil transmitted helminthes in western Kenya: implication for anti-helminthic mass treatment. Am J Trop Med Hyg 2003, 69:318–23.
Bowie C, Purcell B, Shaba B, Makaula P, Perez M: A national survey of the prevalence of schistosomiasis and soil transmitted helminths in Malawi. Biomed Central Infect Dis 2004, 10:2334–49.
Enk MJ, Lima AC, Barros Hda S, Massara CL, Coelho PM, Schall VT: Factors related to transmission of and infection with Schistosoma mansoni in a village in the South-eastern Region of Brazil. Mem Inst Oswaldo Cruz 2010,105(4):570–7. 10.1590/S0074-02762010000400037
Agnew-Blais J, Carnevale J, Gropper A, Shilika E, Bail R, Ngoma M: Schistosomiasis haematobium prevalence and risk factors in a school-age population of peri-urban Lusaka, Zambia. J Trop Pediatr 2010,56(4):247–53. 10.1093/tropej/fmp106
Ugbomoiko US, Ofoezie IE, Okoye IC, Heukelbach J: Factors associated with urinary schistosomiasis in two peri-urban communities in south-western Nigeria. Ann Trop Med Parasitol 2010,104(5):409–19. doi:10.1179/136485910X12743554760469 10.1179/136485910X12743554760469
Acknowledgements
We acknowledge the generous support provided by the International Mix Unity (UMI) of the National Scientific Research Centre (CNRS) of France, the Academic Centres of the right and left sides of the Niger River, the directors the selected schools, the schoolchildren, the research assistants for their input and dedication to the study, the staff of the Faculty of Pharmacy, and all other persons without whose support this study would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Paper title: Urban Schistosomiasis and Associated Determinant Factors Among School Children in Bamako, Mali, West Africa. a) AD participated in the conception and design of the study, data analysis, and interpretation. He also contributed to the writing of the paper, and assured the coordination of the trial. He has reviewed the final version. b) AK participated in the design of the study and onsite execution by collecting and analyzing the data. He also had all the clinical responsibility and coordinated the field activities. c) AZD participated in the conception and onsite execution, and collecting and analyzing the data. He also contributed to the writing of the paper. d) DSN participated in the conception and onsite execution, and collecting and analyzing the data. She also assisted in praziquantel distribution and the assessment of side effects. e) OT is a biostatistician who participated in the conception and onsite execution. He also supervised the collection, analysis, and control of the data. f) VM contributed to the selection of the 29 blocks obtained on the basis of the images from SPOT-5, part of NASA’s Earth Observing System of France. She also participated in the analysis and interpretation of the data. g) AO participated in the mapping of the blocks, houses, and snail breeding sites after collecting GPS points around them. h) OD participated in the conception and design of the paper. He contributed to the data analysis, the writing of the paper, and reviewed the final version. All authors read and approved the final paper.
Electronic supplementary material
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Dabo, A., Diarra, A.Z., Machault, V. et al. Urban schistosomiasis and associated determinant factors among school children in Bamako, Mali, West Africa. Infect Dis Poverty 4, 4 (2015). https://doi.org/10.1186/2049-9957-4-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2049-9957-4-4