Abstract
Pulmonary embolism (PE) is diagnosed with increasing frequency nowadays due to advances in the diagnostic methods and the increased awareness of the disease. There is a tendency to use non invasive diagnostic methods for all diseases. D-dimer is a fibrin degradation product. We aimed to detect the relationship between disease severity and the D-dimer levels measured with two different methods. We compared D-dimer levels in cases of massive vs. non-massive PE. A total of 89 patients who were diagnosed between 2006 and 2008 were included in the study. Group 1 included patients whose D-dimer levels were measured with the immunoturbidimetric polyclonal antibody method (D-dimerPLUS®), while Group 2 patients made use of the immunoturbidimetric monoclonal antibody method (InnovanceD-DIMER®). In each group, the D-dimer levels of those with massive and non-massive PE were compared, using the Mann Whitney U test. The mean age of Group 1 (25 F/26 M) was 56.0 ± 17.9 years, and that of Group 2 (22 F/16 M) was 52.9 ± 17.9 years. There was no statistical difference in gender and mean age between the two groups (p > 0.05). In Group 1, the mean D-dimer level of massive cases (n = 7) was 1444.9 ± 657.9 μg/L and that of nonmassive PE (n = 34) was 1304.7 ± 350.5 μg/L (p > 0.05). In Group 2, the mean D-dimer level of massive cases (n = 6) was 9.7 ± 2.2 mg/L and that of non-massive PE (n = 32) was 5.9 ± 1.3 mg/L (p < 0.05). The mean D-dimer levels of massive cases as measured with the immunoturbidimetric monoclonal antibody method were significantly higher. Pulmonary embolism patients whose D-dimer levels are higher (especially higher than 6.6 mg/L) should be considered as possibly having massive embolism. Diagnostic procedures and management can be planned according to this finding.
Riassunto
La diagnosi di embolia polmonare (EP) viene posta oggi giorno con sempre maggiore frequenza grazie ai passi avanti della metodologia diagnostica e alla maggiore consapevolezza di malattia. In tutte le patologie si tende a ricorrere a metodi non invasivi per giungere alla diagnosi. Il D-dimero è un prodotto di degradazione della fibrina. Scopo di questo studio è valutare il rapporto tra gravità di malattia e livelli di D-dimero misurati con due metodiche differenti. Abbiamo comparato i livelli D-dimero in casi di EP massiva e in casi non gravi di EP. Sono stati selezionati 89 pazienti in cui è stata posta diagnosi di EP tra il 2006 e il 2008. Al Gruppo 1 sono stati allocati i pazienti ai quali il D-dimero era stato misurato mediante un metodo immunoturbidimetrico con anticorpi policlonali (D-dimer PLUS®), al Gruppo 2 i pazienti valutati mediante metodo immunoturbidimetrico con anticorpi monoclonali (InnovanceD-DIMER®). La comparazione tra i due gruppi è stata effettuata con il Mann Whitney U test. L’età media del gruppo 1 (25F/26M) era 56,0 ± 17,9 anni, nel Gruppo 2 (22F/16M) era 52,9 ± 17,9 anni. Non vi era una differenza statisticamente significativa tra i due gruppi per sesso o età (p > 0,05). Nei pazienti più gravi del gruppo 1 (n = 7) il livello medio di D-dimero era 1444,9 ± 657,9 μg/L, nei non gravi (n = 34) era 1304,7 ± 350,5 μg/L (p > 0,05). Nel gruppo 2 il livello medio di D-dimero nei gravi (n = 6) era 9,7 ± 2,2 mg/L e nei non-gravi (n = 32) era 5,9 ± 1,3 mg/L (p < 0,05). Il livello medio di D-dimero nei casi gravi in cui è stata utilizzata la metodica con anticorpo monoclonale è risultato significativamente più elevato rispetto ai pazienti meno gravi. Nei pazienti con embolia polmonare il cui D-dimero è più elevato (specie se superiore a 6,6 mg/L con la metodica ad anticorpi monoclonali) dovrebbe essere considerata la possibilità di una embolia massiva. I protocolli diagnostici e di gestione dei pazienti potrebbero perciò essere riformulati sulla base di questi risultati.
Similar content being viewed by others
Introduction
Pulmonary embolism (PE) is diagnosed with increasing frequency nowdays due to advances in the diagnostic methods and the increased awareness of the disease. There is a tendency to use non invasive diagnostic methods for all diseases. Clinical out-come studies have demonstrated that by using algorithms with sequential diagnostic tests, PE can be safely ruled out in patients whose clinical probability indicates PE to be unlikely and whose D-dimer test results are normal.
The blood concentrations of D-dimer, which is a degradation product of cross-linked fibrin, can be elevated in acute venous thromboembolic disorders. Different D-dimer assays have been introduced for rapid and easy emergency testing. These assays have either intermediate sensitivity and specificity (manual whole-blood agglutination {sensitivity 64-96%, specificity 48-84%}, qualitative latex agglutination assays {sensitivity 25-96%, specificity 92-100%} and semi-quantitative latex agglutination assays {sensitivity 66-97%, specificity 43-83%}) or high sensitivity at the cost of low specificity (ELISA {sensitivity 84-99%, specificity 29-71%} and quantitative latex agglutination assays {sensitivity 88-98%, specificity 36-64%}). Higher sensitivity results in a higher negative predictive value and thus less concern about false negative test results, while lower specificity yields less clinical utility as the fraction of patients with normal test results will decrease.
We aimed to detect the relationship between disease severity in PE and the D-dimer levels measured with two different methods.
Materials and Methods
Patients
Consecutive inpatients with PE were evaluated at the pulmonology department of a university teaching hospital. The exclusion criteria were: refusal or inability to consent to the study and being on therapeutic or prophylactic anticoagulant therapy at time of presentation. Other causes of exclusion regarding the samples were insufficient sample volume and poor sample conditions (e.g. hemolyzed or partially clotted). The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. The study protocol, patient information form and informed consent form were approved by the local ethics committee. All patients provided written informed consent before beginning the study.
A total of 89 patients who were diagnosed between 2006 and 2008 were included in the study. The patients who had right ventricular (RV) dysfunction on echocardiography and hemodynamic instability were considered as massive PE. Echocardiographic findings of PE-induced RV pressure overload include the following: RV dilatation/hypokinesis; paradoxical septal motion; PA dilatation; and tricuspid regurgitation. Hemodynamic instability means low systolic blood pressure (< 90 mmHg) and low diastolic blood pressure (< 60 mmHg). PE diagnosis was based on lung scanning and computerised tomography with contrast for all patients. The patients, after PE diagnosis, were randomly assigned to Group 1 or 2. In each group, D-dimer levels were measured by a different method (see below).
D-dimer measurements
We compared the D-dimer levels in cases of massive pulmonary embolism in the two groups. Group 1 included patients whose D-dimer levels were measured with the immunoturbidimetric polyclonal antibody method (D-dimer PLUS®), while Group 2 made use of the immunoturbidimetric monoclonal antibody method (Innovance D-DIMER®).
D-dimer PLUS (Dade Behring, USA) is a latex-enhanced turbidimetric test for the quantitative determination of cross-linked fibrin degradation products (D-dimer) in human plasma. D-dimer measurements were performed in platelet-poor citrate plasma in duplicate. D-dimer PLUS assays were performed and interpreted by independent operators without knowledge of the radiographic results of this study.
Statistics
In each group, D-dimer levels of massive and other cases were compared. The Mann Whitney U test SPSS 13 was used for the statistical analysis.
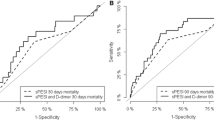
In addition, we accepted significant differences as p < 0.05. To derive the optimal cut-off value, a receiver operator characteristic (ROC) curve was constructed by plotting sensitivity (true positive fraction) versus 100 - specificity (false positive fraction), and area under the curve was calculated (AUC) using MedCalc Software (Belgium).
Results
The mean age of Group 1 (25 F/26 M) was 56.0 ± 17.9 years, and that of Group 2 (22 F/16 M) was 52.9 ± 17.9 years. There was no statistical difference in gender and mean age between the two groups (NS). The patients' hemodynamic parameters are shown in Table 1. There were significant differences between hemodynamic parameters via Mann Whitney U test (mean heart rate, mean systolic blood pressure and mean diastolic blood pressure). In Group 1, the mean D-dimer level of massive PE patients (n = 7) was 1444.9 ± 657.9 µg/L and that of non-massive PE patients (n = 34) was 1304.7 ± 350.5 µg/L (NS). In Group 2, the mean D-dimer level of massive PE patients (n = 6) was 9.7 ± 2.2 mg/L and that of non-massive PE patients (n = 32) was 5.9 ± 1.3 mg/L (p < 0.05). The mean D-dimer levels of massive PE patients, as measured with the monoclonal antibody method, were significantly higher com-pared with those of non-massive patients.
An ROC curve was made displaying sensitivity and specificity for the immunoturbidimetric monoclonal antibody method (Innovance D-DIMER®) at different cut-off levels having an AUC of 0.802, sensitivity 83.3% (95% CI: 36.1- 97.2) and specifity 78.1% (95% CI: 60.0 - 90.7). From this ROC curve, a most appropriate cut-off value of 6.6 mg/L was found for massive PE (ROC curve shown Figure 1). Two patients who had non-massive PE died. The cause of death was not related to PE. The other patients were discharged.
Discussion
Every year, nearly 100,000-200,000 pulmonary embolism cases result in death in the USA [1]. While the mortality rates of undiagnosed patients are around 30%, if pulmonary embolism is properly diagnosed in time, these mortality rates can decrease to below 10%. Suspicion is the first step to diagnosing pulmonary embolism. The clinical and physical findings depend on the degree of vascular obstruction (the number, size and location of the embolus) and the age of the patient and presence of coexisting cardiopulmonary diseases. The clinical and laboratory findings, ECG, chest X-ray and arterial blood gas analysis are not enough to diagnose or to exclude the diagnosis of pulmonary embolism. The use of sequential diagnostic strategies, including D-dimer measurement, has been shown to be useful to rule out acute PE without further imaging testing in a large proportion of patients.
Within the last decade, the measurement of D-dimer, a degradation product formed when the cross-linked fibrin contained in a thrombus is proteolyzed by plasmin, has been introduced for the purpose of PE diagnosis. Various D-dimer assays are available, including latex assays, turbidimetric immunoassays and enzyme-linked immunosorbent as-says (ELISA). However, the reported D-dimer assay sensitivities and specificities vary so widely that its diagnostic utility has been questioned. Moreover, due to the absence of a D-dimer reference standard, the inter-assay correlation is poor [2].
A large variety of D-dimer assays are commercially available. In general, the microplate enzyme-linked immunosorbent assays (ELISA) and the enzyme-linked immunofluorescent immunoassays (ELFA) are accepted as the gold standard of the current D-dimer assays, as they have higher sensitivities for acute PE. Whether the other D-dimer assays (latex quantitative, latex semiquantitative and whole-blood assays) have similar accuracy remains controversial; however, these assays are also widely used. Stein et al. [3] reported the superiority of the ELISA and ELFA over all other D-dimer tests, including latex quantitative assays. In contrast, a recent systematic review by Di Nisio et al. [4] showed that ELISA, ELFA and latex quantitative assays have a comparably higher sensitivity (from 93 to 97%), but lower specificity (from 43 to 53%) than the latex semi-quantitative and whole-blood D-dimer assays, resulting in a more confident exclusion of acute PE at the expense of more additional imaging testing.
D-dimer tests have been reported to be useful predictors of both lower limb DVT and PE [5, 6]. Previous studies have shown different sensitivity and specificity percentages for numerous D-dimer assay methods. We aimed to investigate the relationship between disease severity and D-dimer assay methods. We found that the D-dimer levels as measured with the monoclonal antibody method were related to the disease severity.
The sensitivity, specificity and negative predictive value vary depending on the type of D-dimer assay. With the current rapid tests, both the sensitivity and the negative predictive value are usually high (mostly > 95%) [7–10]. Wermeer et al., by comparing D-dimer PLUS in combination with preclinical scores, have provided a useful tool for clinicians to safely exclude PE or DVT [11].
The combination of pretest clinical probability and the D-dimer test can lead to a reduction of up to 50% in diagnostic imaging tests in a population with a very low disease incidence, as in the case of suspected PE outpatient populations [12]. Because of the poor standardisation of D-dimer assays [13], before inclusion into diagnostic strategies for suspected PE, new D-dimer methods should be appropriately validated in prospective outcome studies or, alternatively, in studies in which plasma samples stored from outcome studies are used.
Breen et al. reported that diagnosis based purely on D-dimer, even in the face of supposed "low risk" is not 100% reliable and clinical suspicion still has a role in clinical medicine [13].
The most sensitive D-dimer tests are the enzyme-linked immunosorbent (ELISA) assays. The initial ELISA membrane plate D-dimer assays had sensitivities of over 95% [14]. ELISA D-dimer tests are much less specific than agglutination assays, with reported specificities varying from 20% to 50% [14, 15]. Thus, more false-positive test results would be expected with ELISA assays, potentially resulting in further unnecessary investigations for many patients with suspected deep vein thrombosis [16]. Our study showed that the sensitivity 83.3% and specificity 78.1% of immunoturbidimetric monoclonal antibody method for D-dimer assays could be of help. Furthermore we can propose a cut-off level of D-dimer to differentiate the severity of pulmonary embolism. Previous studies achieved similar results: Blamoun et al. reported a cut-off level to distinguish mild/moderate from severe/very severe PE at a concentration of 12.35 μg/mL, and found that this threshold selected a higher inpatient mortality and higher 60-week recurrence [17]. We did not follow up our patients after discharge. Only two patients died during hospital stay. We did not find any relationship between D-dimer levels and mortality rate.
According to the RIETE Registry, patients with D-dimer levels in the fourth quartile (≥ 4200 ng/mL-1, latex agglutination test) had an increased risk for overall death, fatal PE or major bleeding. We found a similar relationship between D-dimer levels (immunoturbidimetric monoclonal antibody) and massive PE [18, 20]. Vuilleumier et al. investigated several different biomarkers for diagnosising non-massive PE. They reported that D-dimer level > 2000 ng/mL was a significant cut-off level for non-massive PE diagnosis [19].
Previous reports have found different cut-off levels of D-dimer as a marker of disease severity or mortality. These differences may be related to the use of different D-dimer measurement methods. However, both the sensitivity and specificity of D-dimer measurements depend on the prevalence of disease in the study population, and this explains the variety of results in different reports [21].
In conclusion, we found that if the monoclonal antibody method is used, patients whose D-dimer levels are higher (especially higher than 6.6 mg/L) should be considered as possibly having massive PE. Appropriate diagnostic procedures and management can be planned according to this finding.
Note
This study was presented at the ERS Congress in Vienna, 2009.
Conflict of Interest Statement
None of the authors has any conflict of interest to declare in relation to the subject matter of this manuscript.
References
Horlander KT, Mannino DM, Leeper KV: Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003, 163: 1711-1717. 10.1001/archinte.163.14.1711.
Shitrit D, Heyd J, Raveh D, Rudensky B: Diagnostic value of the D-dimer test in deep vein thrombosis: improved results by a new assay method and by using discriminate levels. Thromb Res. 2001, 102: 125-131. 10.1016/S0049-3848(01)00221-3.
Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Brant R, Biel RK, Bharadia V, Kalra NK: D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004, 140: 589-602.
Di Nisio M, Squizzato A, Rutjes AW, Büller HR, Zwinderman AH, Bossuyt PM: Diagnostic accuracy of Ddimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost. 2007, 5: 296-304. 10.1111/j.1538-7836.2007.02328.x.
Francis CW, Marder VJ, Barlow GH: Plasmic degradation of crosslinked fibrin. Characterization of new macromolecular soluble complexes and a model of their structure. J Clin Invest. 1980, 66: 1033-1043. 10.1172/JCI109931.
Rowbotham BJ, Carroll P, Whitaker AN, Bunce IH, Cobcroft RG, Elms MJ, Masci PP, Bundesen PG, Rylatt DB, Webber AJ: Measurement of crosslinked fibrin derivatives: use in the diagnosis of venous thrombosis. Thromb Haemost. 1987, 57: 59-61.
Gosselin RC, Owings JT, Kehoe J, Anderson JT, Dwyre DM, Jacoby RC, Utter G, Larkin EC: Comparison of six D-dimer methods in patients suspected of deep vein thrombosis. Blood Coagul Fibrinolysis. 2003, 14: 545-550. 10.1097/00001721-200309000-00005.
Sijens PE, Oudkerk M, Berghout A, van Ingen HE, Kemperman H: Comparison of a quantitative latex and a quantitative ELISA plasma D-dimer assay in the exclusion of segmental and subsegmental pulmonary embolism. Thromb Haemost. 2001, 86: 1580-1582.
Anderson FA, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, Forcier A, Dalen JE: A population-based perspective of the hospital incidence and casefatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991, 151: 933-938. 10.1001/archinte.1991.00400050081016.
Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, Conti A, Agnelli G, Berni G: Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000, 101: 2817-2822. 10.1161/01.CIR.101.24.2817.
Vermeer HJ, Ypma P, L van Strijen MJ, Muradin AA, Hudig F, Jansen RW, Wijermans PW, Gerrits WB: Exclusion of venous thromboembolism: evaluation of D-dimer PLUS for the quantitative determination of D-dimer. Thromb Res. 2005, 115: 381-386. 10.1016/j.thromres.2004.09.005.
Ten Cate-Hoek AJ, Prins MH: Management studies using a combination of D-dimer test result and clinical probability to rule out venous thromboembolism: a systematic review. J Thromb Haemost. 2005, 3: 2465-2470. 10.1111/j.1538-7836.2005.01556.x.
Breen M, Dorfman M, Chan SB: Pulmonary embolism despite despite negative ELİSA D-dimer: a case report. J Emerg Med. 2009, 37: 290-292. 10.1016/j.jemermed.2007.11.028.
Anderson DR, Wells PS: Improvements in the diagnostic approach for patients with suspected deep vein thrombosis or pulmonary embolism. Thromb Haemost. 1999, 82: 878-886.
Bounameaux H, de Moerloose P, Perrier A, Reber G: Plasma measurement of D-dimer as diagnostic aid in suspected venous thromboembolism: an overview. Thromb Haemost. 1994, 71: 1-6.
Anderson DR, Wells PS, Stiell I, MacLeod B, Simms M, Gray L, Robinson KS, Bormanis J, Mitchell M, Lewandowski B, Flowerdew G: Management of patients with suspected deep vein thrombosis in the emergency department: combining use of a clinical diagnosis model with D-dimer testing. J Emerg Med. 2000, 19: 225-230. 10.1016/S0736-4679(00)00225-0.
Blamoun J, Alfakir M, Sedfawy AI, Moammar MQ, Maroules M, Khan MA, DeBari VA: The association of D-dimer levels with clinical outcomes in patients presenting with acute pulmonary embolism. Lab Hematol. 2009, 15: 4-9. 10.1532/LH96.08014.
Lobo JL, Zorrilla V, Aizpuru F, Grau E, Jiménez D, Palareti G, Monreal M, RIETE Investigators: D-dimer levels and 15-day outcome in acute pulmonary embolism. Findings from the RIETE Registry. J Thromb Haemost. 2009, 7: 1795-1801. 10.1111/j.1538-7836.2009.03576.x.
Vuilleumier N, Le Gal G, Verschuren F, Perrier A, Bounameaux H, Turck N, Sanchez JC, Mensi N, Perneger T, Hochstrasser D, Righini M: Cardiac biomarkers for risk stratification in non-massive pulmonary embolism: a multicenter prospective study. J Thromb Haemost. 2009, 7: 391-398. 10.1111/j.1538-7836.2008.03260.x.
Grau E, Tenías JM, Soto MJ, Gutierrez MR, Lecumberri R, Pérez JL, Tiberio G, RIETE Investigators: D-dimer levels correlate with mortality in patients with acute pulmonary embolism: findings from the RIETE registry. Crit Care Med. 2007, 35: 1937-1941. 10.1097/01.CCM.0000277044.25556.93.
Lippi G, Favaloro EJ: D-dimer measurement and laboratory feedback. J Emerg Med. 2009, 37 (1): 82-83. 10.1016/j.jemermed.2008.07.033.
Acknowledgements
We acknowledge the support of the Biochemistry Department of Uludag University, Bursa, Turkey. The manuscript was edited by American Journal Experts (AJE).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Coskun, F., Yilmaz, D., Ursavas, A. et al. Relationship between disease severity and D-dimer levels measured with two different methods in pulmonary embolism patients. Multidiscip Respir Med 5, 168 (2010). https://doi.org/10.1186/2049-6958-5-3-168
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2049-6958-5-3-168