Abstract
Live yeast (Saccharomyces cerevisiae) constitutes an effective additive for animal production; its probiotic effect may be related to the concentrate-to-forage ratio (CTFR). The objective of this study was to assess the effects of S. cerevisiae (SC) on fiber degradation and rumen microbial populations in steers fed diets with different levels of dietary concentrate. Ten Simmental × Local crossbred steers (450 ± 50 kg BW) were assigned to a control group or an SC group. Both groups were fed the same basal diet but the SC group received SC supplementation (8 × 109 cfu/h/d through the ruminal fistula) following a two-period crossover design. Each period consisted of four phases, each of which lasted 17 d: 10 d for dietary adaptation, 6 d for degradation study, and 1 d for rumen sample collection. From the 1st to the 4th phase, steers were fed in a stepwise fashion with increasing CTFRs, i.e., 30:70, 50:50, 70:30, and 90:10. The kinetics of dry matter and fiber degradation of alfalfa pellets were evaluated; the rumen microbial populations were detected using real-time PCR. The results revealed no significant (P > 0.05) interactions between dietary CTFR and SC for most parameters. Dietary CTFR had a significant effect (P < 0.01) on degradation characteristics of alfalfa pellets and the copies of rumen microorganism; the increasing concentrate level resulted in linear, quadratic or cubic variation trend for these parameters. SC supplementation significantly (P < 0.05) affected dry matter (DM) and neutral detergent fiber (NDF) degradation rates (cDM, cNDF) and NDF effective degradability (EDNDF). Compared with the control group, there was an increasing trend of rumen fungi and protozoa in SC group (P < 0.1); copies of total bacteria in SC group were significantly higher (P < 0.05). Additionally, percentage of Ruminobacter amylophilus was significantly lower (P < 0.05) but percentage of Selenomonas ruminantium was significantly higher (P < 0.05) in the SC group. In a word, dietary CTFR had a significant effect on degradation characteristics of forage and rumen microbial population. S. cerevisiae had positive effects on DM and NDF degradation rate or effective degradability of forage; S. cerevisiae increased rumen total bacteria, fungi, protozoa, and lactate-utilizing bacteria but reduced starch-degrading and lactate-producing bacteria.
Similar content being viewed by others
Introduction
Compounds isolated from Saccharomyces cerevisiae have been used as antibiotic substitutes to improve cattle production efficiency, especially after antibiotics were banned by the European Union[1]. Studies have shown that the S. cerevisiae CNCM I-1077 strain (Levucell SC, Lallemand, Toulouse, France) has positive effects on milk production and daily feed intake of dairy cows and goats[2–4]. The primary mechanisms by which live yeasts affect animal performance appears to be related to the effect of yeast on rumen bacterial populations[5], consequently, on nutrient degradation. However, the effect of live yeast on nutrient degradation in the rumen is highly variable. Guedes et al.[6] reported that supplementing cattle with live yeast increased ruminal neutral detergent fiber (NDF) degradation of maize silage, while Mir and Mir[7] reported that live yeast supplementation had no effects on ruminal dry matter (DM) or NDF degradation. Part of these differences may be attributed to different basal diets, especially levels of dietary concentrates[8, 9]. According to previous studies[6], probiotic role of live yeast is relating to its effect on stabilizing ruminal pH. However, ruminal pH is function of the level of concentrate. Hence, the effect of live yeast on nutrient degradation characteristics and rumen bacteria population may be related to concentrate to forage ratios.
Although several studies have reported that S. cerevisiae has positive effects on animal production performance, nutrient degradation, and rumen bacterial population, the majority of these studies focused on dairy cattle and small ruminants such as sheep and goats[2–4]. Few studies have focused on the effects of live yeast on nutrient degradation and rumen microbial population of beef cattle fed different levels of dietary concentrates. Therefore, the objective of this study was to investigate the effects of supplementation with S. cerevisiae (SC) on nutrient degradation and rumen microbial population of beef cattle fed diets with different concentrate-to-forage ratios.
Materials and methods
Animals, diets, and experimental design
Ten Simmental × local crossbred steers (450 ± 50 kg body weight) fitted with 10-cm diameter rumen cannulas were used as experimental animals. This study was approved by the Animal Care and Use Committee of the College of Animal Science and Technology of China Agricultural University (ACUC-CAST, #20120806BCRC004). The steers were individually housed in tie-stall barns, and feed, fresh water were available ad libitum. Steers were randomly assigned to one of two groups: a control group, which received the basal diet with no SC supplementation, or an SC group, which received the basal diet with SC supplementation, following a 2-period crossover design. Each period consisted of four phases; each phase lasted 17 d: 16 d for dietary adaptation and 1 d for rumen liquor sampling. From the 1st to the 4th phase, steers were fed with increasing dietary concentrate levels in a stepwise fashion. Between periods, steers were fed low level concentrate diet without any treatment during 65 d as a wash-out period. During the wash-out period, ruminal pH and protozoa number were monitored until which recovered to normal level. The stepwise diets (Table 1) were formulated to meet the nutrient requirements (NRC, 2000), with concentrate-to-forage ratios (CTFR) of 30:70 (Phase 1), 50:50 (Phase 2), 70:30 (Phase 3), and 90:10 (Phase 4). Prior to each phase, the diets were mixed and pressed into high density bales using a specialized wrapping machine (DK-850C, Jintudi Co, Baoding, China). During the experiment, active dry SC (I-1077, Levucell SC, Lallemand, Toulouse, France) at 8 × 109 cfu/h/d was added directly into the rumen through the cannulas just prior to the morning feeding.
In situ rumen incubation
Alfalfa pellets were passed through a 2-mm screen in a Wiley laboratory mill; 5 g DM was transferred to separate number-coded nylon bags (8 cm × 12 cm) of 38 μm pore size. For incubation purposes, each steer had a total of three bags per sample. Rumen incubations were performed according to the method of Ørskov et al.[10] but following the gradual addition/all out schedule[11]. Samples were incubated in the rumen for 168, 96, 48, 24, 12, 6, and 0 h, respectively; for NDF, the samples were incubated for 240 h. All bags were inserted at the same time (0800 h) just prior to the morning feeding and apart from the 12 h bags and 6 h bags, which were inserted at 0700 h and 1300 h. Following incubation, the bags were removed from the rumen and rinsed with cold tap water to remove excess ruminal contents and to remove microbial activity. The bags were washed with cold water without detergent in a washing machine and dried at 60°C for 48 h. The 0 h incubation samples were only washed. The bags were weighed and residues were pooled according to group and incubation times and passed through a 1-mm screen. DM and NDF were analyzed. Data for DM and NDF disappearance at different incubation times were fitted to the following models[10],
where p is the fraction disappearance at time t; a is the soluble or rapidly degradable fraction; b is the insoluble but potentially degradable fraction; c is the rate constant of degradation of potentially degradable insoluble fraction (/h); t is the time of rumen incubation (h); k is the rumen passage rate (/h), and ED is the effective degradability. In this experiment, k = 0.03/h was used[12]. DM in feedstuff and residues was measured by drying the samples at 60°C for 48 h in a forced-air oven. NDF[13] was measured using an ANKOM200 Fiber Analyzer (ANKOM Technology Corp., Fairport, NY, USA). NDF was assayed with heat stable amylase (aNDF) and without sodium sulfite; NDF results included residual ash[14].
Ruminal fluid collection
On the last day of each phase, rumen samples were collected by hand from four locations in the rumen and reticulum through rumen cannulas at 3 h after the morning feeding. Aliquots were filtered through two layers of cheese cloth; 40 mL of the filtered rumen fluid was transferred to 50-mL centrifuge tubes, which were flash frozen in liquid nitrogen, transferred to the laboratory, and stored at −80°C prior to DNA extraction; these steps lasted 10 min.
DNA extraction and real-time PCR
Total genomic DNA from 200 μL of frozen rumen samples was extracted using TIANGEN® TIANamp Stool DNA Kit (Tiangen Biotech Co., Ltd., Beijing, China). DNA concentration and purity (OD260/280 and OD260/230, respectively) were determined using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies).
Quantitative real-time PCR was performed with the 7300 Real-Time PCR System (Applied Biosystems) using SYBR green chemistry (SuperReal PreMix Plus, Tiangen Biotech Co., Ltd.). The primers of target microorganism are shown in Table 2. DNA extract (1 μL) was added to the amplification reaction (20 μL), containing 0.3 μL of each primer, 7.9 μL of 2× SuperReal PreMix Plus (with SYBR Green), 8 μL of ddH2O, and 2.5 μL of 50 × ROX Reference Dye. The thermal cycling conditions consisted of an initial Taq activation step at 95°C for 15 min, followed by 40 cycles of 95°C for 15 s and of 60°C for 30 s, followed by an amplicon dissociation stage (95°C for 15 s and 60°C for 1 min, increasing 0.5°C/cycle until 95°C was reached), which confirmed specificity via dissociation curve analysis of PCR end products. Fluorescence detection was performed at the end of each denaturation and extension step. For robustness, 3 replicates of each DNA sample were analyzed in the same plate.
For the absolute quantification of total bacteria 16S rDNA, rumen fungi and protozoa 18S rDNA gene copies, total bacteria, fungi and protozoa rDNA extracts from mixed rumen samples were loaded on a 1% agarose gel and visualized by ethidium bromide staining respectively. The resulting products were purified using the TIANGEN® TIANgel Midi Purification Kit (Tiangen Biotech Co., Ltd.). The purified PCR product was used as a standard whose concentration was measured in the ND-1000 spectrophotometer and converted to concentration using the following equation, DNA (number of molecules) = (NL × A × 10−9)/ (660 × n), where NL is the Avogadro constant (6.02 × 1023 molecules per mol); A is the molecular weight of the molecule in the standard; and n is the length of the amplicon in base pairs[21]. Standard curves were constructed with purified total bacteria, rumen fungi, and protozoa rDNA when the copies of total bacteria, rumen fungi, and protozoa were measured.
For quantification of individual species, Relative Quantification ΔCT method[22] was used and the total bacteria used as reference. Amplification efficiencies were calculated using serial dilutions and only efficiencies between 90%-110% were taken into consideration.
Statistical analyses
SAS (1990) software was used for statistical analyses. Nutrient degradation data and rumen microbial population data were analyzed using the MIXED procedure with the following model,
where μ represented the overall mean, Period represented the period (1 or 2), Treatment accounted for the fixed effect of yeast supplementation, Diet represented the fixed effect of different dietary CTFR, Steer accounted for the random effect of each individual animal, ϵ account for the unexplained random error. Linear, quadratic and cubic responses for dietary CTFR were assessed using orthogonal polynomial contrast statements.
Results
The results revealed that there were no significant difference between the 2 periods and there were no interactions between dietary CTFR and SC (P > 0.1; Tables 3,4 and5) for most parameters except aDM, cNDF, and copies of R. ablus. Therefore, the main effects of diet and SC are discussed independently.
Effect of diet on alfalfa pellet degradation
The degradation characteristics of alfalfa pellet DM are shown in Table 3. There were statistically significant differences (P < 0.01) in aDM, bDM, cDM, and EDDM among the different diets. Increasing concentrate level resulted in a cubic variation trend in aDM and bDM (P < 0.05), and a linear and quadratic decrease in cDM and EDDM respectively (P < 0.01).
The degradation characteristics of alfalfa pellet NDF are shown in Table 4. Dietary CTFR significantly (P < 0.01) affected NDF degradation characteristics and the variation trends were similar to those obtained for DM.
Effect of SC on alfalfa pellet degradation
There were no significant differences (P > 0.05) in aDM, bDM, or EDDM of alfalfa pellet between the SC and control groups (Table 3). However, SC supplementation increased (P < 0.01) cDM of alfalfa pellet compared to the control group.
There were no differences in aNDF, bNDF, (P > 0.05) between the SC and control groups (Table 4); however, cNDF and EDNDF of SC were significantly different from that of the control group (P < 0.05).
The relative DM effective degradability had an increasing trend with increasing dietary CTFR and it reached 5.93% (calculated from 45.03%–42.51%)/42.51% × 100%) when dietary CTFR was 90:10.
In addition, the relative NDF effective degradability presented an increasing trend with increasing dietary CTFR and it reached 9.49% (calculated from 23.38%–21. 35%)/21.35% × 100%) when dietary CTFR was 90:10.
Effect of diet on rumen microbial population
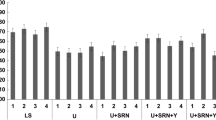
There were significant differences (P < 0.01) in rumen microbial population among the four phases (Table 5). With increasing dietary concentrate levels, percentage of Butyrivibrio fibrisolvens and Lactobacillus species linearly decreased and increased respectively (P < 0.01); percentage of Fibrobacter succinogenes and Ruminobacter amylophilus quadratically decreased respectively (P < 0.01); total bacteria, fungi, protozoa and other target bacteria presented cubic variation trend (P < 0.05).
Effect of SC on rumen microbial population
The total bacteria copies in the SC group were significantly higher (P < 0.05) than in the control group (Table 5). The copies of rumen fungi and protozoa with SC supplementation increased (P < 0.1) compared to the control group. When SC was supplemented, the percentage of R. amylophilus and S. ruminantium significantly (P < 0.05) decreased and increased, respectively. However, there were no significant differences (P > 0.05) in other bacterial species.
Discussion
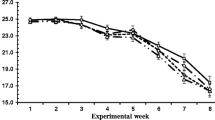
There was an interaction of dietary CTFR and SC supplementation on aDM, cNDF, and copies of R. ablus, which can be illustrated by polynomial contrast results and visually shown in Figures 1,2,3 and4. Without yeast, aDM showed quadratic variation trend (Quadratic, P < 0.01), with yeast aDM presented cubic variation trend (Cubic, P < 0.05). Similarly, SC supplementation changed the variation trend of cNDF, and copies of R. ablus. The interaction suggested that SC possess biological effect on fiber degradation, which was related to dietary concentrate to forage ratio.
Roughage is degraded by fiber-degrading bacteria; however, the major fiber-degrading bacteria such as F. succinogenes, R. ablus, and R. flavfaciens are sensitive to low pH values. With increasing dietary concentrate levels, more sugars, starch, and other non-structural carbohydrates are consumed, which contribute to a higher concentration of volatile fatty acids (VFAs). Higher VFA concentrations reduce ruminal pH values and, consequently, the number and activities of fiber-degrading bacteria decreased. Therefore, the effect of dietary CTFR on aDM/aNDF, bDM/bNDF, cDM/cNDF, and EDDM/EDNDF can easily be understood. On the other hand, with increasing dietary concentrate levels, the percentage of lactate-producing bacteria such as S. bovis and Lactobacillus increased; however, lactate-utilizing species such as S. ruminantium decreased (Table 5), therefore, lactate may accumulate in the rumen. Consequently, rumen pH declined and total bacteria, fungi, and protozoa levels rapidly declined (Table 5).
When SC was supplemented, total bacteria, fungi, protozoa, fiber-degrading, and lactate-utilizing bacteria increased while starch-degrading and lactate-producing bacteria decreased. Meanwhile, degradation rate or effective degradability of alfalfa DM and NDF improved (Tables 3,4 and5). According to Chaucheyras-Durand et al.[5], the probiotic role of SC is attributed to several factors. Firstly, SC possesses the capacity to scavenge unnecessary oxygen in the rumen, reducing the redox potential[23, 24]. Therefore, SC contributes to an ecological condition that favors the growth and activities of anaerobic microorganisms and of fiber-degrading bacteria, which improve the DM and NDF degradation rate or effective degradability[6]. Secondly, SC provides thiamin, which is required by fungi for zoosporogenesis[25]. Rumen fungi play important roles in fiber degradation. Thirdly, SC outcompetes starch-degrading bacteria such as S. bovis and Ruminobacter amylophilus for carbohydrate utilization[5]. As a result, rumen VFA concentration was lower in SC than in the control group[26]. Moreover, SC provides growth factors such as amino acids, peptides, and organic acids, which are essential for lactate-utilizing bacteria[27, 28]; consequently, the number of lactate-utilizing bacteria such as S. ruminantium improved significantly (Table 5) and the concentration of lactate was reduced[29]. In addition, SC can stimulate rumen protozoa especially ciliate Entodiniomorphid protozoa, which rapidly engulf starch granules and stabilize ruminal pH[3]; the same effect of SC on protozoa was obtained in this study (Table 5). The conclusions of Chaucheyras-Durand et al. (2008) were obtained by in vitro experiments, and some experts doubted whether the SC can play the same important role in vivo as in vitro. This experiment proved that in vivo SC really has a positive effect on nutrient degradation and rumen microorganism balance[5].
One the other hand, the effect of SC on rumen nutrient degradation characteristics was inconsistent. For example, results from this and previous studies revealed that SC supplementation improved the degradation rate or effective degradability of forage feedstuff[6, 30]. However, other studies revealed that SC supplementation had no effects on DM or NDF degradation[31]. The inconsistent results of SC on forage nutrient degradation characteristics may be related to the dietary concentrate-to-forage ratios.
Conclusions
Dietary CTFR had a significant effect on degradation characteristics of forage and rumen microbial population. S. cerevisiae had positive effects on DM and NDF degradation rate or effective degradability of forage; S. cerevisiae increased rumen total bacteria, fungi, protozoa, and lactate-utilizing bacteria but reduced starch-degrading and lactate-producing bacteria.
References
Anadón A: The EU ban of antibiotics as feed additives: alternatives and consumer safety. J Vet Pharmacol Therap. 2006, 29 (Suppl 1): 41-46.
de Ondarza MB, Sniffen CJ, Graham H, Wilcock P: Case study: Effect of Supplemental Live Yeast on Yield of Milk and Milk Components in High-Producing Multiparous Holstein Cows. The Professional Animal Scientist. 2010, 26: 443-449.
Brossard L, Chaucheyras-Durand F, Michalet-Doreauand B, Martin C: Dose effect of live yeasts on rumen microbial communities and fermentations during butyric latent acidosis in sheep: new type of interaction. Anim Sci. 2006, 82 (6): 829-836. 10.1017/ASC200693.
Moallem U, Lehrer H, Livshitz L, Zachut M, Yakoby S: The effects of live yeast supplementation to dairy cows during the hot season on production, feed efficiency, and digestibility. J Dairy Sci. 2009, 92 (1): 343-351. 10.3168/jds.2007-0839.
Chaucheyras-Durand F, Walker ND, Bach A: Effects of active dry yeasts on the rumen microbial ecosystem: Past, present and future. Anim Feed Sci Tech. 2008, 145 (1–4): 5-26.
Guedes CM, Gongalves D, Rodrigues MAM, Dias-da-Silva A: Effects of a Saccharomyces cerevisiae yeast on ruminal fermentation and fibre degradation of maize silages in cows. Anim Feed Sci Tech. 2008, 145 (1–4): 27-40.
Mir Z, Mir PS: Effect of the Addition of Live Yeast (Saccharomyces cerevisiae) on Growth and Carcass Quality of Steers Fed High-Forage or High Grain Diets and on Feed Digestibility and In Situ Degradability. J Anim Sci. 1994, 72 (3): 537-545.
Stella AV, Paratte R, Valnegri L, Cigalino G, Soncini G, Chevaux E, Dell’Orto Savoini G: Effect of administration of live Saccharomyces cerevisiae on milk production, milk composition, blood metabolites, and faecal flora in early lactating dairy goats. Small Rumin Res. 2007, 67 (1): 7-13. 10.1016/j.smallrumres.2005.08.024.
Desnoyers M, Giger-Reverdin S, Bertin G, Duvaux-Ponter C, Sauvant D: Meta-analysis of the influence of Saccharomyces cerevisiae supplementation on ruminal parameters and milk production of ruminants. J Dairy Sci. 2009, 92 (4): 1620-1632. 10.3168/jds.2008-1414.
Ørskov ER, DeB Hovell FD, Mould F: The use of the nylon bag technique for the evaluation of feedstuffs. Trop Anim Prod. 1980, 5 (3): 195-213.
Yu P, Niu Z: Using a complex non-TDN based model (the DVE/OEB system) to predict microbial protein synthesis, endogenous protein, degradation balance, and total truly absorbed protein supply of different varieties of cereal oats for ruminants. Anim Sci J. 2009, 80 (3): 273-279. 10.1111/j.1740-0929.2009.00641.x.
Alexandrov AN: Effect of ruminal exposure and subsequent microbial contamination of dry matter and protein degradability of various feedstuffs. Anim Feed Sci Tech. 1998, 71 (1–2): 99-107.
Van Soest PJ, Robertson JB, Lewis BA: Methods for dietary fiber neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991, 74 (10): 3583-3597. 10.3168/jds.S0022-0302(91)78551-2.
Hristov AN, Varga G, Cassidy T, Long M, Heyler K, Karnati SKR, Corl B, Hovde CJ, Yoon I: Effect of Saccharomyces cerevisiae fermentation product on ruminal fermentation and nutrient utilization in dairy cows. J Dairy Sci. 2010, 93 (2): 682-692. 10.3168/jds.2009-2379.
Denman SE, McSweeney CS: Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol. 2006, 58 (3): 572-582. 10.1111/j.1574-6941.2006.00190.x.
Carberry CA, Kenny DA, Han S, McCabe MS, Waters SM: Effect of phenotypic residual feed intake and dietary forage content on the rumen microbial community of beef cattle. Appl Environ Microbiol. 2012, 78 (14): 4949-4958. 10.1128/AEM.07759-11.
Stevenson DM, Weimer PJ: Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl Microbiol Biotechnol. 2007, 75 (1): 165-174. 10.1007/s00253-006-0802-y.
Li M, Penner GB, Hernandez-Sanabria E, Oba M, Guan LL: Effects of sampling location and time, and host animal on assessment of bacterial diversity and fermentation parameters in the bovine rumen. J Appl Microbiol. 2009, 107 (6): 1924-1934. 10.1111/j.1365-2672.2009.04376.x.
Tajima K, Aminov RI, Nagamine T, Matsui H, Nakamura M, Benno Y: Diet-dependent shifts in the bacterial population of the rumen revealed with real-time PCR. Appl Environ Microbiol. 2001, 67 (6): 2766-2774. 10.1128/AEM.67.6.2766-2774.2001.
Lettat A, Nozière P, Silberberg M, Morgavi DP, Berger C, Martin C: Rumen microbial and fermentation characteristics are affected differently by bacterial probiotic supplementation during induced lactic and subacute acidosis in sheep. BMC Microbiol. 2012, 12: 142-154. 10.1186/1471-2180-12-142.
Hernandez-Sanabria E, Goonewardene LA, Wang Z, Durunna ON, Moore SS, Guan LL: Impact of feed efficiency and diet on adaptive variations in the bacterial community in the rumen fluid of cattle. Appl Environ Microbial. 2012, 78 (4): 1203-1214. 10.1128/AEM.05114-11.
Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time 401 quantitative PCR and the 2-ΔΔCT Method. Methods. 2001, 25: 402-408. 10.1006/meth.2001.1262.
Chaucheyras-Durand F, Fonty G: Influence of a probiotic yeast (Saccharomyces cerevisiae CNCM I-1077) on microbial colonization and fermentation in the rumen of newborn lambs. Microb Ecol Health Dis. 2002, 14 (1): 30-36. 10.1080/089106002760002739.
Křížová L, Richter M, Třináctý J, Říha J, Kumprechtová D: The effect of feeding live yeast cultures on ruminal pH and redox potential in dry cows as continuously measured by a new wireless device. Czech J Anim Sci. 2011, 56 (1): 37-45.
Chaucheyras F, Fonty G, Bertin G, Gouet P: Effects of live Saccharomyces cerevisiae cells on zoospore germination, growth, and cellulolytic activity of the rumen anaerobic fungus, Neocallimastix frontalisMCH3. Curr Microbiol. 1995, 31: 201-205. 10.1007/BF00298373.
Bach A, Iglesias C, Devant M: Daily rumen pH pattern of loose-housed dairy cattle as affected by feeding pattern and live yeast supplementation. Anim Feed Sci Tech. 2007, 136 (1–2): 146-153.
Newbold CJ, McIntosh FM, Wallace RJ: Changes in the microbial population of a rumen-simulating fermenter in response to yeast culture. Can J Anim Sci. 1998, 78: 241-244.
Rossi F, Luccia AD, Vincenti D, Cocconcelli PS: Effects of peptidic fractions from Saccharomyces cerevisiae culture on growth and metabolism of the ruminal bacteria Megasphaera elsdenii. Anim Res. 2004, 53: 177-186. 10.1051/animres:2004009.
Monnerat JP, Paulino PV, Detmann E, Valadares Filho SC, Valadares RD, Duarte MS: Effects of Saccharomyces cerevisiae and monensin on digestion, ruminal parameters, and balance of nitrogenous compounds of beef cattle fed diets with different starch concentrations. Trop Anim Health Prod. 2013, 45 (5): 1251-1257. 10.1007/s11250-013-0356-9.
Chaucheyras-Durand F, Fonty G: Establishment of cellulolytic bacteria and development of fermentative activities in the rumen of gnotobiotically-reared lambs receiving the microbial additive Saccharomyces cerevisiae CNCM I-1077. Reprod Nutr Dev. 2001, 41: 57-68. 10.1051/rnd:2001112.
Carro MD, Lebzien P, Rohr K: Influence of yeast culture on the in vitro fermentation (Rusitec) of diets containing variable portions of concentrates. Anim Feed Sci Tech. 1992, 37 (3–4): 209-220.
Acknowledgements
This study was financially supported by the Earmarked Fund for Modern Agro-Industry Technology Research System (Beef Cattle and Yaks, CARS-38), and the Chinese Universities Scientific Fund (No. 2013QT034)”. The authors acknowledge the Lallemand (China) for supplying the yeast products.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
QXM and YC conceived the study and designed the experiment. GZD carried out the statistical analysis and drafted the manuscript. GZD, LPZ, ZMZ and LPR performed the animal experiments and rumen microbial population studies. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ding, G., Chang, Y., Zhao, L. et al. Effect of Saccharomyces cerevisiae on alfalfa nutrient degradation characteristics and rumen microbial populations of steers fed diets with different concentrate-to-forage ratios. J Animal Sci Biotechnol 5, 24 (2014). https://doi.org/10.1186/2049-1891-5-24
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2049-1891-5-24