Abstract
Background
The aim of this study is to investigate the effects of polyphenol extract from Phyllanthus emblica (PEEP) on cervical cancer cells and to explore the underlying mechanism.
Methods
MTT assay was used to measure inhibition of proliferation of cervical cancer (HeLa) cells after treatment with PEEP at concentrations of 0, 50, 100, 150, and 200 mg/ml for 48 hours. HeLa cells were treated with PEEP (150 mg/ml) for 48 hours in the following analysis. Karyomorphism was assessed by immunofluorescence using DAPI staining, and cell apoptosis and cell cycle were assessed using flow cytometry. Three apoptotic marker proteins, namely, Fas, FasL, and cleaved caspase-8, were assessed by western blotting.
Results
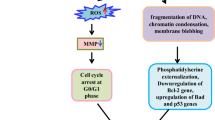
PEEP inhibited the growth of HeLa cells, and the optimum concentration of PEEP was 150 mg/ml. In addition, the karyomorphism of HeLa cells after treatment with PEEP was abnormal. Furthermore, PEEP induced arrest of the HeLa cell cycle at G2/M phase, and triggered apoptosis. PEEP also induced significant Fas and FasL activation, and cleavage of caspase-8.
Conclusions
Our study indicates that PEEP is effective in inhibiting HeLa cell proliferation by inducing cell cycle arrest at G2/M phase and inducing apoptosis.
Similar content being viewed by others
Background
Cervical cancer is ranked as the second leading cause of female cancer mortality worldwide, with an annual incidence of approximately 200,000 deaths and more than 500,000 new cases diagnosed [1–3]. The incidence of cervical cancer is high in developing countries, and more than 28.8% of the world’s cases occur in China [4].
Human papillomavirus (HPV) infection is considered the greatest risk factor in the development of cervical cancer [5]. Although HPV vaccines have been licensed in several areas, such as the USA, Europe, Canada, and Australia, the incidence of HPV infection-related cervical cancer has not been eliminated [6]. This is because the vaccines are effective only against some types of HPV, and they are not yet widely used in developing countries [7]. Curative surgery is the first option for patients with early-stage cervical cancer, while radiotherapy and chemotherapy have proven to be effective treatments for patients in the advanced stages. However, the curative effect of traditional chemotherapeutic drugs is limitedm and their side effects, such as neurological and/or renal [8] and cardiac [9] toxicity, are serious. Therefore, research into novel chemotherapeutic drugs is essential for effective treatment of cervical cancer.
Phyllanthus emblica (PE; syn. Emblica officinalis, also known as the Indian or Nepalese gooseberry or emblic leaf-flower) is a species belonging to the family Euphorbiaceae, which is used as a traditional medicine, especially in Asia [10]. A previous study showed that PE could induce apoptosis in mouse and human carcinoma cell lines, including Dalton’s lymphoma ascites and CeHa cell lines [11]. In addition, Ngamkitidechakul et al. reported that PE was able to inhibit proliferation of a series of cancer cell lines, including A549, HepG2, HeLa, MDA-MB-231, SK-OV3, and SW620, suggesting potential for PE in oncotherapy [12].
The ingredients of PE are complex, and include tannin and phenolic glycosides, flavonoids, terpenes, sterols, and several human essential trace elements such as vitamins and amino acids [10]. In the present study, we isolated polyphenol extract from PE (PEEP), and measured its effect on the proliferation, cell cycle and apoptosis of cervical cancer (HeLa) cells. We also assessed karyomorphism of the cells after incubation with PEEP for 48 hours, and assessed expression of three apoptotic marker proteins: Fas, FasL, and cleaved caspase-8, using western blotting.
Methods
Preparation of PEEP
Polyphenols were extracted from the leaves of PE plants as described previously [13]. Briefly, the leaves were homogenized for 5 minutes with chilled 70% acetone, followed by homogenization at high speed for 5 minutes, then the homogenate was centrifuged for 10 minutes. This process was performed in triplicate. Finally, the extract was dissolved in dimethyl sulfoxide (DMSO, Sigma, St Louis, MO,USA) and stored at −20°C until used.
Cell culture
HeLa cells were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences, (Shanghai, China), and were maintained in RPMI1640 (Gibco, Uxbridge, UK) with 10% fetal bovine serum (Hyclone, UT, USA) at 37°C in an atmosphere of 5% CO2. HeLa cells in the logarithmic phase were seeded into 96-well tissue culture plates at a density of 1 × 105 cells per well, and allowed to grow for 24 hours before being treated with PEEP. Cells were exposed to different concentrations (50, 100, 150, and 200 mg/ml) of PEEP, with phosphate-buffered saline (PBS) used as a negative control.
Proliferation assay
Cells were incubated for 48 hours at 37°C, then 20 μl MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, 5 mg/ml; Sigma, St Louis, MO, USA) was added to each well, and cells were maintained at 37°C for a further 4 hours. After this, 150 μl DMSO was added to each well, and the optical density (OD) of each well at 570 nm was measured by a microplate reader (Thermo Fisher Scientific, Waltham, MA). All experiments performed in triplicate. Inhibition rate was calculated by the following formula:
Immunofluorescence assay
HeLa cells in the logarithmic phase that had been treated with PEEP (150 mg/ml) for 48 hours were seeded at a density of 2 × 105 cells onto coverslips, and maintained at 37°C in an atmosphere of 5% CO2 for another 48 hours. After that, cells were washed three times with PBS, and fixed in 3.7% paraformaldehyde for 20 minutes. The nuclei were stained with DAPI (4′, 6-diamidino-2-phenylindole; Roche, Mannheim, Germany) for 15 minutes. Images were captured using a confocal microscope.
Cell cycle analysis
HeLa cells in the logarithmic phase were seeded on a 96-well plate at a density of 1 × 105 cells per well and treated with PEEP (150 mg/ml), with PBS used as a control. At 48 hours after treatment, the cells were harvested and washed twice with PBS. The cells were then stained with 0.1% Triton X-100 containing propidium iodide (Amersham, Buckinghamshire, UK) and RNase (NEB, Ipswich, USA). Fluorescence from the propidium iodide-DNA complex was measured by flow cytometry (Millipore, Billerica, USA).
Analysis of apoptosis
For analysis of cell apoptosis, HeLa cells in the logarithmic phase were seeded at a density of 1 × 105 cells per well and treated with PEEP (150 mg/ml) for 48 hours before harvesting. Cells were washed twice with PBS and resuspended in PBS at a density of 1 × 105 cells/ml. Before being analyzed by fluorescence-activated cell sorting (FACS) (FACSCaliburl Milipore, Billerica, USA), 100 μl of cells were mixed with 100 μl Guava Nexin reagent (Milipore, Billerica, USA) for 20 minutes. All experiments were carried out in triplicate.
Western blotting
Cells in the logarithmic phase were harvested and lysed on ice for 30 minutes. After centrifugation at 2,000 rpm (626 g) for 10 minutes, the supernatant was collected, and protein concentrations were determined by bicinchoninic acid assay (BCA). Proteins were separated by 10% SDS-PAGE (20 μg protein per well). Then proteins were transferred to PVDF membrane (Bio-Rad, Hercules, USA). The membrane was blocked in Tris-buffered saline containing 5% non-fat milk (Wyeth) for 2 hours, and subsequently incubated with primary caspase-8 (1:200 dilution), Fas (1:200 dilution), FasL(1:200 dilution), and GAPDH antibodies (Abcam, Cambridge, USA) overnight at 4°C, followed by incubation with secondary antibody (Abcam, Cambridge, USA) at room temperature for 1 hour. The proteins of interest were visualized using an enhanced chemiluminescence (ECL) system (Millipore). GAPDH expression was used as internal control.
Results
PEEP inhibits HeLa cell proliferation
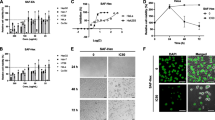
To investigate the effect of PEEP on HeLa cell proliferation, we assessed the inhibition rate of HeLa cells after PEEP treatment. PEEP inhibited proliferation in a dose-dependent fasion up to 200 mg/ml, after which it decreased. The maximum inhibition rate was 39% when the PEEP concentration was 150 mg/ml (Figure 1).
Polyphenol extract of Phyllanthus emblica (PEEP) inhibits HeLa cell proliferation. HeLa cells were treated with different concentrations of PEEP (50, 100, 150, 200 mg/ml) for 48 hours. Quantification of the number of viable cells was determined by MTT assay. The data are expressed as the means ± SD obtained from three independent experiments.
PEEP induces G2/M arrest of HeLa cells
To explore the role of PEEP in cell cycle regulation, we treated HeLa cells with 150 mg/ml PEEP for 48 hours, and found that cell cycle arrest was induced at the G2/M phase after PEEP treatment. The number of HeLa cells in G2/M was 10% with PBS, and this increased to 31% after PEEP treatment. However, the number of cells in G1 phase decreased from 65% to 25%, and those in the S phase decreased from 51% to 18% (Figure 2). These results suggest that PEEP is able to induce arrest of HeLa cells in G2/M phase.
Polyphenol extract of Phyllanthus emblica (PEEP) induces cell cycle arrest of HeLa cells at G2/M phase. HeLa cells were treated with 0 mg/ml PEEP (A), 150 mg/ml PEEP (B) for 48 hours, and the cell cycle stage was determined by flow cytometry. The panels of M1, M2, and M3inside the image are the marks of stage borders of G0/G1, S, and G2/M, respectively.
PEEP changes the karyomorphism of HeLa cells
The significant effects of PEEP on HeLa cell proliferation and cell cycle arrest led us to consider its effect on the karyomorphism of HeLa cells. Therefore, we performed immunofluorescence experiments of HeLa cells treated with PEEP. In normal cells, the chromatin was well distributed and the karyotheca were integrated. By contrast, after treatment with PEEP for 48 hours, the chromatin was condensed and the karyotheca were ruptured (Figure 3).
Polyphenol extract of Phyllanthus emblica (PEEP) changes the karyomorphism of HeLa cells. HeLa cells were seeded onto coverslips, and treated with 0 mg/ml PEEP (A), 150 mg/ml PEEP (B) for 48 hours before harvesting. DAPI staining was used to highlight the nucleus. The panels inside the image represent the size of 10 μm.
PEEP induce apoptosis of HeLa cells
To identify the functional role of PEEP in mediating apoptosis of HeLa cells, the apoptosis rate of HeLa cells after PEEP treatment was determined by FACS. The rate of apoptosis increased from 4.8 ± 2.1% to 45 ± 2.4% after PEEP treatment (Figure 4).
Polyphenol extract of Phyllanthus emblica (PEEP) induces HeLa cell apoptosis. HeLa cells were treated with 0 mg/ml PEEP (A), 150 mg/ml PEEP (B) for 48 hours. The cells were harvested and resuspended in Guava Nexin. Cell cycle stage was determined by flow cytometry. The panels inside the image are marks of the quadrants, cells in the left lower quadrant are living cells, in the right upper quadrant are dead cells, and in the right lower quadrant are apoptotic cells.
In order to understand the mechanism underlying this effect of PEEP on apoptosis of HeLa cells, expression of three apoptotic marker proteins (Fas, Fas L and clevaved caspase-8 were analyzed by western blotting. PEEP treatment induced significant Fas and FasL activation, and cleavage of caspase-8 (Figure 5). Based on these results, we concluded that PEEP is able to activate the cell apoptosis pathway in HeLa cells.
Discussion
PE is a traditional medicine that has been investgated fo antiviral [14, 15] and anti-cancer [12] propertied, with satisfactory results. Although PE seems to have an anti-cancer effect, the active ingredients of PE are not very clear. The structure of PEEP contains an aromatic nucleus, one or more phenolic hydroxyl groups, and other elements. In the present study, we isolated polyphenols from PE and determined that in HeLa cells, this extract is capable of inhibiting cell proliferation, inducing cell cycle arrest at G2/M phase, and triggering apoptosis. We suggest that these effects were mainly due to the unique characteristics of polyphenols.
There are two common mechanisms identified in drug-induced inhibition of cancer cell proliferation [16–18]. First, chemotherapeutic drugs induce cell cycle arrest, especially in S or G2/M phase, with consequent inhibition of cell growth. Second, such drugs trigger the apoptotic pathway in cells. Fas, FasL, and caspase-8 are all molecular markers in the apoptotic pathway [19]. Zhang et al. indicates that polyphenols extracted from fruit juice or from PE leaves had a strong inhibitory effect on melanoma cells [20]. In addition, PEEP is efficient in facilitating the cytotoxicity of other drugs, such as doxorubicin and cis-platinum, when co-administered [21]. In the current study, we determined that the inhibition of HeLa cell proliferation increased as PEEP concentration increased, reaching a maximum inhibition level of 39% at 150 mg/ml PEEP. Further experiments showed that PEEP inhibited HeLa proliferation by inducing cell cycle arrest at G2/M phase and triggering apoptosis.
It has been reported previously that PEEP induced G2/M phase arrest of lung cancer cells and triggered cell apoptosis [22]. Our data suggest that PEEP similarly induces HeLa cell cycle arrest at G2/M phase. Further experiment showed that cell apoptosis was also triggered. Consistent with this, three apoptotic markers, Fas, FasL and cleaved caspase-8, were increased, suggesting that PEEP inhibits cell proliferation by inducing cell cycle arrest at G2/M phase and triggering apoptosis in cervical cancer cells.
Conclusions
PEEP was able to inhibit proliferation and promote apoptosis of cervical cancer HeLa cells by inducing cell cycle arrest at G2/M phase and triggering apoptosis. PEEP may be a potential future chemotherapy drug with definite functional components.
References
Chaturvedi AK, Engels EA, Gilbert ES, Chen BE, Storm H, Lynch CF, Hall P, Langmark F, Pukkala E, Kaijser M, et al.: Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst 2007, 99: 1634–1643. 10.1093/jnci/djm201
Hu X, Schwarz JK, Lewis JS Jr, Huettner PC, Rader JS, Deasy JO, Grigsby PW, Wang X: A microRNA expression signature for cervical cancer prognosis. Cancer Res 2010, 70: 1441–1448. 10.1158/0008-5472.CAN-09-3289
Kleinerman RA, Boice JD Jr, Storm HH, Sparen P, Andersen A, Pukkala E, Lynch CF, Hankey BF, Flannery JT: Second primary cancer after treatment for cervical cancer. An international cancer registries study. Cancer 1995, 76: 442–452. 10.1002/1097-0142(19950801)76:3<442::AID-CNCR2820760315>3.0.CO;2-L
Yumin W, Jie C, Wenhui Z, Wangdong H, Fangyou Y: Study of the prevalence of human Papillomavirus infection in Chinese women with cervical cancer. Afr J Microbiol Res 2012, 6: 1048–1053.
Kumar V, Robbins SL: Robbins Basic Pathology. 8th edition. Philadelphia, PA: Saunders/Elsevier; 2007.
Lowy DR, Schiller JT: Prophylactic human papillomavirus vaccines. J Clin Invest 2006, 116: 1167–1173. 10.1172/JCI28607
NCI factsheet: human papillomavirus (HPV) vaccines: questions and answers [Internet]. [http://www.cancer.gov/cancertopics/factsheet/risk/HPV-vaccine]
Florea A-M, Büsselberg D: Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancer 2011, 3: 1351–1371. 10.3390/cancers3011351
Monsuez J-J, Charniot J-C, Vignat N, Artigou J-Y: Cardiac side-effects of cancer chemotherapy. Int J Cardiol 2010, 144: 3–15. 10.1016/j.ijcard.2010.03.003
Xia Q, Xiao P, Wan L, Kong J: Ethnopharmacology of Phyllanthus emblica L. Zhongguo Zhong Yao Za Zhi 1997, 22: 515–518. 525, 574
Rajeshkumar NV, Pillai MR, Kuttan R: Induction of apoptosis in mouse and human carcinoma cell lines by Emblica officinalis polyphenols and its effect on chemical carcinogenesis. J Exp Clin Cancer Res 2003, 22: 201–212.
Ngamkitidechakul C, Jaijoy K, Hansakul P, Soonthornchareonnon N, Sireeratawong S: Antitumour effects of Phyllanthus emblica L.: induction of cancer cell apoptosis and inhibition of in vivo tumour promotion and in vitro invasion of human cancer cells. Phytother Res 2010, 24: 1405–1413. 10.1002/ptr.3127
He X, Liu RH: Phytochemicals of apple peels: isolation, structure elucidation, and their antiproliferative and antioxidant activities. J Agric Food Chem 2008, 56: 9905–9910. 10.1021/jf8015255
Liu Q, Wang YF, Chen RJ, Zhang MY, Yang CR, Zhang YJ: Anti-coxsackie virus B3 norsesquiterpenoids from the roots of Phyllanthus emblica. J Nat Prod 2009, 72: 969–972. 10.1021/np800792d
Xiang Y, Pei Y, Qu C, Lai Z, Ren Z, Yang K, Xiong S, Zhang Y, Yang C, Wang D, et al.: In vitro anti-herpes simplex virus activity of 1,2,4,6-tetra-O-galloyl-beta-D-glucose from Phyllanthus emblica L. (Euphorbiaceae). Phytother Res 2011, 25: 975–982. 10.1002/ptr.3368
Shi M, Cai Q, Yao L, Mao Y, Ming Y, Ouyang G: Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Cell Biol Int 2006, 30: 221–226. 10.1016/j.cellbi.2005.10.024
Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N, Neungton N: Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J Ethnopharmacol 2004, 90: 161–166. 10.1016/j.jep.2003.09.048
Liang Y, Hou M, Kallab AM, Barrett JT, El Etreby F, Schoenlein PV: Induction of antiproliferation and apoptosis in estrogen receptor negative MDA-231 human breast cancer cells by mifepristone and 4-hydroxytamoxifen combination therapy: a role for TGFbeta1. Int J Oncol 2003, 23: 369–380.
Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, et al.: Inhibition of death receptor signals by cellular FLIP. Nature 1997, 388: 190–195. 10.1038/40657
Zhang YJ, Nagao T, Tanaka T, Yang CR, Okabe H, Kouno I: Antiproliferative activity of the main constituents from Phyllanthus emblica. Biol Pharm Bull 2004, 27: 251–255. 10.1248/bpb.27.251
Pinmai K, Chunlaratthanabhorn S, Ngamkitidechakul C, Soonthornchareon N, Hahnvajanawong C: Synergistic growth inhibitory effects of Phyllanthus emblica and Terminalia bellerica extracts with conventional cytotoxic agents: doxorubicin and cisplatin against human hepatocellular carcinoma and lung cancer cells. World J Gastroenterol 2008, 14: 1491–1497. 10.3748/wjg.14.1491
Khan MTH, Lampronti I, Martello D, Bianchi N, Jabbar S, Choudhuri MSK, DATTA BK, Gambari R: Identification of pyrogallol as an antiproliferative compound present in extracts from the medicinal plant Emblica officinalis: effects on in vitro cell growth of human tumor cell lines. Int J Oncol 2002, 21: 187–192.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XXZ and HFL designed the experiments: XXZ, YO, and WWH performed and interpreted the experiments: and JJW wrote the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zhu, X., Wang, J., Ou, Y. et al. Polyphenol extract of Phyllanthus emblica (PEEP) induces inhibition of cell proliferation and triggers apoptosis in cervical cancer cells. Eur J Med Res 18, 46 (2013). https://doi.org/10.1186/2047-783X-18-46
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2047-783X-18-46