Abstract
Background
Squamous odontogenic tumor (SOT) is a rare benign odontogenic epithelial neoplasm. A slow-growing painless expansive swelling is the common presenting symptom. Histopathologically, SOT can be easily misdiagnosed as an acanthomatous ameloblastoma. Although Notch receptors and ligands have been shown to play a role in cell fate decisions in ameloblastomas, the role of these cell signaling molecules in SOT is unknown.
Case report
This paper describes a case of SOT affecting the anterior mandible of a 10-year-old Indian female. The patient was treated by local surgical excision and there has been no follow-up clinical record of recurrence 5 years after primary treatment. Histopathological examination revealed a solid, locally-infiltrative neoplasm composed of bland-looking squamatoid islands scattered in a mature fibrous connective tissue stroma and the diagnosis was SOT. Immunohistochemical evaluation showed positive reactivity of varying intensity in the neoplastic epithelial cells for Notch1, Notch3, Notch4, and their ligands Jagged1 and Delta1. Expression patterns showed considerable overlap. No immunoreactivity was detected for Notch2 and Jagged2.
Conclusions
Present findings suggest that Notch receptors and their ligands play differential roles in the cytodifferentiation of SOT.
Similar content being viewed by others
Introduction
Squamous odontogenic tumor (SOT) is a rare tumor with less than 50 cases reported [1]. It was first described as a distinct entity by Pullon et al. in 1975 [2]. The aetiopathogenesis of this benign locally-invasive odontogenic epithelial neoplasm is unclear. Clinicopathologically, three main types are identified: intraosseous [1], mural (mural SOT-like proliferations in cyst) [3] and extraosseous forms [4]. SOT affects a wide age range, shows a slight male preponderance and occurs more frequently in the mandible [1]. Aggressive [5] and multifocal [6] variants have been reported. Histopathologically it is composed of islands of well-differentiated non-keratinizing squamous epithelium surrounded by a mature fibrous connective tissue [1]. There is no cellular atypia. In the epithelial islands, cystic degeneration as well as calcification may occur. Invasion into cancellous bone may be present [7].
Mammalian Notch is a four-member family of receptors (Notch1-4) that mediates short-range events [8, 9]. The Notch receptor is a single transmembrane protein containing distinct structural extracellular and intra-cellular domains. The structure of the four Notch receptors is highly homologous with only some differences in these domains. Notch signaling pathway is activated when cell surface-anchored ligands (Jagged1, Jagged2, Delta1, Delta3 and Delta4) from neighboring cells bind the receptors and trigger the proteolytic cleavage of Notch receptors. The activation of Notch signaling pathway leads to different outcomes ranging from control of proliferation to apoptosis, differentiation, maintenance of stemness and cell fate decision [9]. Deregulation of Notch signaling has been implicated in some genetic diseases and tumorigenesis [10]. Notch signaling in a variety of tumors can be either oncogenic or tumor suppressive, depending on the specific cellular context, also in odontogenic neoplasms [11–13].
The potential role for Notch signaling pathway in the development and cytodifferentiation of odontogenic neoplasms has gained attention only recently. In others [14] and our studies [15–17], Notch expression was observed in plexiform and follicular ameloblastoma [4, 15], ameloblastic carcinoma [16] and ameloblastic fibroma [17] but not in the odontogenic myxoma [17]. A search of the English language literature disclosed that Notch signaling activity in SOT is not known. In this report, the expression patterns of Notch1-4 and their ligands, Jagged1, Jagged2 and Delta1 in a case of SOT are presented and the significance of these findings speculated.
Case report
Clinical summary
A 10-year-old Indian female patient was seen for a complaint of a slowly-enlarging, non-tender swelling of unknown duration in her anterior mandible. No further clinical or radiographic information was available as to the presentation of this lesion in the jaw. A pre-operative diagnosis of ossifying fibroma was made. The lesion was surgically excised under general anesthesia, and submitted for histopathological examination. No follow-up information was available as to the outcome of the patient five years after primary treatment.
Histopathology
Microscopic examination of the lesional area disclosed a solid, locally-infiltrative, benign odontogenic epithelial neoplasm. It was composed of bland-looking islands of well-differentiated squamous epithelium set against a mature fibrous connective tissue stroma (Figure 1). These tumor islands did not show any evidence of peripheral columnar cells, reversal nuclear polarity or central stellate reticulum-like cells. Cellular atypia was absent. There was central keratinization and cystic degeneration. Foci of dystrophic calcifications and occasional clear cell clusters were noted. These histopathological findings led us to daiagnose the lesion to be SOT.
Immunohistochemistry
The immunohistochemical detection of Notch receptors and ligands was performed using the Envision technique as previously described [14]. Primary antibodies used are detailed in Table 1. Stromal endothelium and fibroblasts served as internal positive controls while negative controls were performed by substituting the primary antibody with phosphate-buffered saline.
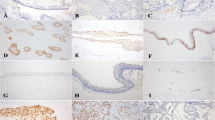
SOT showed positive expression for Notch1, Notch3 and Notch4 in the well-differentiated squamous epithelial islands, central keratinization and cystic degeneration (Figure 2). Notch2 was not detected. Expression for Jagged1 was moderate while that for Delta1 was weak within the neoplastic epithelium. Jagged2 was consistently absent. Clear cell nests and dystrophic calcific foci showed similar expression patterns (Figure 3). Stromal endothelium and fibroblasts were variably positive for notch receptors and ligands. furthermore, there were some notch1,3,4 positive cells scattered in the stromal connective tissue.
Immunohistochemical staining for Notch receptors: A, Notch1 shows moderate expression in a squamous island with central keratinization; B, Notch3 weak expression in squamous tumor islands but moderate to strong in vascular endothelium and stromal fibroblasts; and C, Notch4 overexpression in epithelial keratinizing cysts. (original magnification, A, ×400; B, ×200; C, ×100).
Staining intensity of Sot was analyzed according to the criteria of published studies [14, 16, 17] (table 2) where expression results of immunohistochemistry (IHC) and in situ hybridization (ISH) in the neoplastic epithelium were quantified as negative (-) when no cells were stained, mild (+) when less than 25% cells stained positive, moderate (++) for 25 -50% positive cells, and strong (+++) for greater than 50% positive.
Discussion
SOT is classified as a benign, locally-infiltrative odontogenic epithelial neoplasm with three possible sources of origin depending on its location. Intraosseous SOT putatively originates from the cell rests of Malassez whereas its peripheral counterpart arises from the dental lamina remnants (glands of Serres) or the gingival surface epithelium [18]. SOT must be distinguished from other intraosseous neoplasms notably ameloblastoma and primary intraosseous squamous cell carcinoma. Histopathologically, tumor islands in SOT consisted of well-differentiated squamous epithelium without peripheral palisading unlike those in the classic ameloblastoma which characteristically exhibits a peripheral layer of pre-ameloblasts and central stellate reticulum-like cells [3, 18]. Primary intraosseous squamous cell carcinoma can be ruled out on grounds of absence of cytological features of malignancy in the tumor islands of SOT [3, 7]. In the present case all these aspects were considered before arriving at a diagnosis of SOT.
The Notch family of receptors has been characterized as critical determinants of cell fate in a variety of organisms [8–10]. Notch receptors participate in cell fate decisions by the process of lateral inhibition or inductive signaling. In the developing tooth, Notch receptors and ligands have been found to be expressed in dental epithelium and/or ectomesenchyme, suggesting that Notch signaling may regulate odontogenesis [19]. It is known that the morphological characteristics and inductive relationship between various parts of the developing tooth germ are reproduced to a greater or lesser extent in many of the tumors and tumor-like lesions of the odontogenic apparatus [1]. In the light of this, the potential role of the Notch signaling pathway in the proliferation and cellular differentiation of some of these odontogenic neoplasms was examined. Notch1, 2 and 3, Jagged1 and Delta1 were detected in the central and peripheral cells of the follicular and plexiform ameloblastoma but were absent in areas exhibiting keratinization and granular cell differentiation, and in stromal cells (Table 2) [14]. These findings led the authors to suggest that Notch signaling does not play an oncogenic role in the tumorigenesis of odontogenic epithelium but might contribute to the control of neoplastic cell differentiation and suppress neoplastic cell proliferation in odontogenic epithelium [14]. In the ameloblastic carcinoma, Notch1 was expressed by most neoplastic epithelial cells including small numbers of mitoses, and these observations led to the suggestion that Notch1 plays some roles in the cytological differentiation or acquisition of tissue specific characteristics and may contribute to cell cycle arrest in these neoplastic cells [11, 16]. In the case of ameloblastic fibroma, Notch1 activity was detected in the peripheral and central cells of tumor nests and strands as well as in the dental papilla-like stromal cells, and these findings suggest that Notch signaling might be involved in the specific cell differentiation of the epithelial nests of this neoplasm [17]. During odontogenesis, Notch1 transcriptions were seen in the dental mesenchyme after the cap stage [19]. For the odontogenic myxoma, Notch signaling activity was absent and this was attributed to the fact that the mesenchymal tissue in this neoplasm is in a less advanced stage of differentiation than the dental papilla-like tissue in the ameloblastic fibroma [17].
In the current study, Notch1, 3 and 4, Jagged1 and Delta1 were expressed by the peripheral and central cells of the tumor islands in the SOT as well as in areas of keratinization, cystic degeneration, clear cell differentiation and dystrophic calcification foci. Notch2 and Jagged2 were consistently absent. These preliminary findings suggest that Notch signaling may be involved in the cellular differentiation of SOT. It is further observed that ameloblastoma and SOT are both benign neoplasms of odontogenic epithelium without odontogenic ectomesenchymal participation but their Notch expression patterns differ. We believed that these differences may be due to their different stages of cellular differentiation. In the ameloblastoma tumor epithelium, the peripheral cells resembled the pre-ameloblasts while the central cells resembled the stellate reticulum of the developing tooth germ after cap stage [14]. In the SOT, the tumor epithelium has a squamous characteristic. These differences in Notch expression patterns may be related to the different functional activities of these cell signaling molecules during the cellular differentiation of the ameloblastoma and SOT.
In summary, a case of SOT is reported here and its Notch immunoexpression profile defined. As a wide range of neoplasms including odontogenic neoplasms occurs in the oral and craniofacial regions research on cell differentiation phenomenon and cell signaling factors is currently ongoing [11–17, 20–25]. Studies on larger series of SOT are also recommended to help refine the role of these signaling molecules in the development of this neoplasm.
References
Reichart PA: Squamous odontogenic tumour. In World Health Organization Classification of Tumours: Pathology and genetics of tumours of the head and neck. 301. Edited by: Bames L, Eveson JW, Reichart P, Sidransky D. IARC Press, Lyon, France; 2005.
Pullon PA, Shafer WG, Elzay RP, Kerr DA, Corio RL: Squamous odontogenic tumor: Report of six cases of a previously undescribed lesion. Oral Surg Oral Med Oral Pathol 1975, 40: 616–630. 10.1016/0030-4220(75)90372-2
Oliveira JA, Costa IM: Squamous odontogenic tumor-like proliferations (SOT-LP) versus intraosseous squamous cell carcinoma in residual cyst. J Oral Maxillofac Surg 2006, 64: 1325.
Baden E, Doyle J, Mesa M, Fabie M, Lederman D, Eichen M: Squamous odontogenic tumor: Report of three cases including the first extraosseous case. Oral Surg Oral Med Oral Pathol 1993, 75: 733–738. 10.1016/0030-4220(93)90432-4
Ruhin B, Raoul G, Kolb F, Casiraghi O, Lecomte-Houcke M, Ghoul S, Auriol M, Ferri J: Aggressive maxillary squamous odontogenic tumour in a child: histological dilemma and adaptive surgical behaviour. Int J Oral Maxillofac Surg 2007, 36: 864–866. 10.1016/j.ijom.2007.03.002
Leider AS, Jonker LA, Cook HE: Multicentric familial squamous odontogenic tumor. Oral Surg Oral Med Oral Pathol 1989, 68: 175–181. 10.1016/0030-4220(89)90189-8
Kim K, Mintz SM, Stevens J: Squamous odontogenic tumor causing erosion of the lingual cortical plate of the mandible: a report of 2 cases. J Oral Maxillofac Surg 2007, 65: 1227–1231. 10.1016/j.joms.2006.05.051
Miele L: Notch signaling. Clin Cancer Res 2006, 12: 1074–1079. 10.1158/1078-0432.CCR-05-2570
Artavanis-Tsakonas S, Rand MD, Lake RJ: Notch signaling: Cell fate control and signal integration in development. Science 1999, 284: 770–776. 10.1126/science.284.5415.770
Leong KG, Karsan A: Recent insights into the role of Notch signaling in tumorigenesis. Blood 2006, 107: 2223–2233. 10.1182/blood-2005-08-3329
Nakano K, Nagatsuka H, Tsujigiwa H, Gunduz M, Katase N, Siar CH, Kawakami T: Immunohistochemical characteristics of odontogenic neoplasms and their physiological counterparts. J Hard Tissue Biol 2008, 17: 79–90. 10.2485/jhtb.17.79
Kawakami T, Nagatsuka H: Cell differentiation of neoplastic cells originating in the oral and craniofacial regions. Nova Science Publishers, Inc. New York 2009, 1–56.
Kawakami T, Nagatsuka H, Nakano K, Shimizu T, Tsujigiwa H, Hasegawa H, Nagai N: Chapter 1: Cell differentiation of neoplastic cells originating in the oral and craniofacial regions. In Cell Differentiation Research Developments. Edited by: Ivanova LB. Nova Science Publishers, Inc. New York; 2008:1–30.
Kumamoto H, Ohki K, Ooya K: Expression of notch signaling molecules in ameloblastomas. J Oral Pathol Med 2008, 37: 228–234. 10.1111/j.1600-0714.2007.00629.x
Siar CH, Ng KH, Ariff Z, Muraki E, Shimizu T, Tsujigiwa H, Nagatsuka H, Nagai N, Kawakami T: A case report of ameloblastoma of the mandible with examination of Notch signaling. Oral Med Pathol 2006, 11: 35–39. 10.3353/omp.11.35
Nakano K, Siar CH, Tsujigiwa H, Nagatsuka H, Nagai N, Kawakami T: Notch signaling in benign and malignant ameloblastic neoplasms. Eur J Med Res 2008, 13: 476–480.
Nakano K, Chelvanayagam P, Born K, Siar CH, Ng KH, Nagatsuka H, Kawakami T: A study of recurrent giant odontogenic myxoma of the mandible with immunohistochemical examination of Notch. Oral Med Pathol 2008, 12: 53–56. 10.3353/omp.12.53
Tatemoto Y, Okada Y, Mori M: Squamous odontogenic tumor: immunohistochemical identification of cytokeratins. Oral Surg Oral Med Oral Pathol 1989, 67: 63–67. 10.1016/0030-4220(89)90303-4
Mitsiadis TA, Regaudiat L, Gridley T: Role of Notch signaling pathway in tooth morphogenesis. Arch Oral Biol 2005, 50: 137–140. 10.1016/j.archoralbio.2004.10.006
Chuah KS, Siar CH, Nakano K, Nagatsuka H, Khoo SP, Ng KH, Kawakami T: Wingless-type protein-1 (Wnt-1) expression in primary conventional and unicystic ameloblastomas and their recurrent tumors. J Hard Tissue Biol 2009, 18: 63–70. 10.2485/jhtb.18.63
Nagatsuka H, Katase N, Pwint HP, Tsujigiwa H, Siar CH, Nakajima M, Naomoto Y, Tamamura R, Kawakami T, Gunduz M: Heparanase and its related molecules in odontogenic tumors. Oral Med Pathol 2009, 13: 81–89. 10.3353/omp.13.81
Han PP, Nagatsuka H, Tamamura R, Katase N, Bernard M, Hu H, Takagi S, Ishida N, Nakano K, Kawakami T, Gunduz M: Role of Heparanase in the release of heparin sulphate binding growth factors in of odontogenic tumors. J Hard Tissue Biol 2007, 16: 36–41. 10.2485/jhtb.16.36
Inoue M, Nagatsuka H, Tamamura R, Siar CH, Tsujigiwa H, Borkosky S, Fujii M, Setsu K: Localization of oxytalan fiber, type III collagen and BMP family in conventional and desmoplastic ameloblastoma. J Hard Tissue Biol 2008, 17: 23–30. 10.2485/jhtb.17.23
Han PP, Tamamura R, Katase N, Fujii E, Okauchi M, Jin T, Siar CH, Nagatsuka H: Differential distribution of type IV collagen α1 to α6 chains suggests distinct molecular interaction between the epithelial and mesenchymal components of benign odontogenic tumors. J Hard Tissue Biol 2006, 15: 46–53. 10.2485/jhtb.15.46
Heikinheimo K, Mori K, Nagatsuka H, Happonen RP: Transforming growth factor beta (TGF-β) gene family members in developing and neoplastic odontogenic tissues. J Hard Tissue Biol 2006, 15: 1–5. 10.2485/jhtb.15.1
Acknowledgements
This research was supported jointly by the University of Malaya Research Grant FS170/2008C and Grant-in Aid for Scientific Research (C) (20592349) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siar, C., Nakano, K., Ng, K. et al. Squamous odontogenic tumor of the mandible: a case report demonstrating immunoexpression of Notch1, 3, 4, Jagged1 and delta1. Eur J Med Res 15, 180 (2010). https://doi.org/10.1186/2047-783X-15-4-180
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2047-783X-15-4-180