Abstract
Background
Alteration in the immune system is one of the most profound aspects of aging. Progressive changes in the number of B lymphocyte progenitors during aging have been reported but the underlying mechanisms are still elusive. A heterozygous G608G mutation in the LMNA gene leads to a deletion of 50 amino acids in lamin A protein, termed progerin, and is the predominant cause of Hutchinson-Gilford progeria syndrome (HGPS). Lack of Zmpste24, a metalloproteinase responsible for prelamin A processing, leads to progeroid features resembling HGPS. Therefore Zmpste24-deficient mice provide an ideal mouse model to study the impact of lamin A and (premature) aging on the aging-related decline of B lymphopoiesis.
Results
Analysis of bone marrow (BM) nucleated cells revealed a decline of early B cell progenitors in Zmpste24−/− mice. BM transplantation in a congenic strain completely rescued the defects in B lymphopoiesis, indicating that the decline in B cell progenitors in Zmpste24−/− mice is attributable to defective BM microenvironments rather than to cell-intrinsic defects. Further investigation revealed downregulation of a set of important early B lymphopoiesis factors in Zmpste24−/− bone marrow stromal cells (BMSCs), such as Vcam-1, SDF-1α, Flt3L and TSLP, and most of them are under transcriptional control of NF-κB signaling. Though TNFα stimulates IκBα degradation and NF-κB nuclear translocation in Zmpste24−/− BMSCs, NF-κB fails to stimulate IκBα re-expression, which mediates a negative feedback loop of NF-κB signaling in wild-type BMSCs.
Conclusions
Our data demonstrate a cell-extrinsic defect of B cell development in a progeroid mouse model and a critical role for lamin A in the regulation of NF-κB signaling and cytokines that are essential for lymphopoiesis.
Similar content being viewed by others
Background
Aging is a progressive deterioration of physiological functions that are necessary for survival and fertility [1]. Alteration in the immune system is one of the most profound aspects of aging, including shrinkage of the diverse repertoire of immunoglobins in both B and T lymphocytes [2, 3], compromised immune responsiveness to pathogens, which is marked by greater proportion of low-affinity antibodies and involves both humoral and cell-mediated immune response [4, 5], and increased auto-reactive cells leading to higher risk of autoimmune diseases [6].
B lymphocytes are one of the main components of the adaptive immune system and are responsible for the generation of B cell receptors (BCRs, also known as immunoglobulins), which recognize a large repertoire of antigens [7]. The B cell development is a highly ordered process orchestrated by differentiation from hematopoietic stem cells (HSCs). The initial commitment to the B cell lineage is characterized by the expression of CD45R/B220, leading to the earliest fraction of B cell progenitors, precursor of B cell progenitor (pre-pro-B) [8, 9]. Pre-pro-B cells give rise to progenitor B (pro-B) cells [10, 11]. The following stage is B cell precursors (pre-B), comprising mainly small resting cells. The subsequent expression of surface immunoglobulin M (sIgM) is the hallmark of the progression from pre-B cells to immature B cells when they start to leave bone marrow (BM) niches and enter the peripheral blood for further maturation. Contrasting to myeloid compartments, which are relatively intact during aging, B lymphopoiesis declines significantly with age [12]. However, the underlying mechanisms remain elusive.
Hutchinson-Gilford progeria syndrome (HGPS) is an extremely rare genetic disorder of early onset premature aging. Patients with HGPS can only live for 12 to 16 years and are clinically characterized with early growth retardation, small body size, lipodystrophy, loss of hair, stiff joints, reduced bone density, dilated cardiomyopathy and atherosclerosis [13, 14]. HGPS is predominantly caused by a de novo p.G608G lamin A mutation. Lamin A is first synthesized as prelamin A with an additional 18 amino acids on the C-terminus, which dictates a series of processing events involving farnesylation, proteolysis and methylation [15–17]. ZMPSTE24, a metalloprotease, is required for the proteolytic cleavages during lamin A maturation [18]. The G608G mutation activates a cryptic splicing donor signal in exon 11, leading to a 150-nucleotide deletion in the LMNA transcript and a 50-residue truncation in the prelamin A protein, referred to as progerin. Progerin lacks the second proteolytic cleavage site of ZMPSTE24 but retains the CAAX motif [19, 20]. Mice lacking Lmna surfer from growth retardation and muscle dystrophy, resembling Emery-Dreifuss muscular dystrophy (EDMD) [21]; depleting Zmpste24 in mice recapitulates many progeroid features found in HGPS patients [16]. Lmna−/−Zmpste24−/− double knockout mice phenotypically resemble Lmna single knockouts, while depleting only one allele of Lmna ameliorates progeroid phenotypes and extends lifespan in Zmpste24−/− mice [22, 23]. This suggests that prelamin A is most likely the only substrate of Zmpste24, and unprocessed prelamin A is the direct cause of premature aging imposed by Zmpste24 deficiency.
Alternate splicing also happens at the wild type LMNA locus, leading to expression of low levels of progerin [24] and indicating that HGPS might share, at least partially, mechanism(s) with normal aging process. This idea is supported because the expression of ectopic progerin results in defective proliferation and premature senescence in human cells [25, 26]; the number of progerin positive cells gradually increase during aging in normal individuals [27]; and telomere shortening or dysfunction activates progerin production [28]. Therefore Zmpste24-deficient mice provide an ideal mouse model to study the impact of lamin A and (premature) aging on the decline of B lymphopoiesis. It has been recently reported that loss of Lmna in mice causes non-cell autonomous defects of B cell development, possibly attributable to compromised bone marrow stromal cells (BMSCs) and/or the overall unhealthiness of the mutants [29]. In the current study, we asked whether the accumulation of prelamin A affects B lymphopoiesis in Zmpste24−/− mice. We found an extrinsic decline of early B lymphocyte progenitors in Zmpste24−/− mice. Defects in early B lymphopoiesis are most likely attributable to defective BM niches as in vitro cultured Zmpste24−/− BMSCs are compromised in NF-κB signaling and the secretion of cytokines necessary for early B lymphopoiesis, including Vcam-1, SDF-1α, Flt3L, TSLP, etcetera. Our data reveal a critical role for lamin A in regulating NF-κB signaling that is essential for lymphopoiesis.
Results
Defective B lymphopoiesis in Zmpste24−/−mice
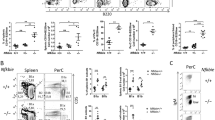
We first examined whether there is any difference in the B cell development in Zmpste24−/− mice. As shown in Figure 1A, the number of nucleated BM cells from femurs and tibias decreased in Zmpste24−/− mice when comparing with Zmpste24+/− mice, which are phenotypically indistinguishable from their Zmpste24+/+ littermates. Of note, while the number of nucleated BM cells increased along with age in Zmpste24+/− mice, no further increase was observed after 3 months of age in Zmpste24−/− mice. We then performed FACS analysis on Zmpste24−/− mice at 1 month, 3 months and 5 months of age using their sex-matched heterozygous littermates as controls. While 1-month-old Zmpste24−/− mice had a similar profile of early B cells to that of their heterozygous littermates, Zmpste24−/− mice of 3 months and 5 months of age showed a significant decrease in the number of B lymphocytes as well as the percentage of pro-B (identified as B220loCD43+) and pre-B (identified as B220loCD43−) populations (Figure 1B-D), suggesting a defect in the generation of B lineage lymphocyte progenitors, which is consistent with the previous reports on physiologically senescent mice [30–32].
Defective B cell development in Zmpste24−/−mice. (A) Decreased bone marrow nucleated cells in Zmpste24−/− mice compared to their wild-type littermates. Data represent mean ± SEM, n ≥5. *P <0.05. (B) Decreased B lymphocytes in Zmpste24−/− mice started from 3 months of age, compared to their wild-type littermates. Data represent mean ± SEM, n = 5. *P <0.05. (C) Representative B cell profile in 5-month-old Zmpste24−/− mice and control, showing decreased pro-B (B220loCD43+) and pre-B (B220loCD43−) cells. (D) Decreased pro-B and pre-B cells in 5-month-old Zmpste24−/− mice. Data represent mean ± SEM, n = 5. *P <0.05. circ. B, circulating B cells; pre-B cells, B cell precursors; pro-B cells, progenitor B cells.
Defective B lymphopoiesis in Zmpste24−/−mice is not cell-intrinsic
The defective B lymphopoiesis observed in Zmpste24−/− mice could be either cell-intrinsic or cell-extrinsic. To identify this, the BM transplantation test was performed with congenic strain B6 as recipients. A comparable number of nucleated BM cells from either 6-month-old Zmpste24 null mice or their wild-type littermates was transplanted into lethally irradiated B6 mice and kept for 2 months before fluorescence-activated cell sorting (FACS) analysis. The BM cells of donor origin (CD45.2+) are identified with FITC-CD45.2 antibody. Two months after the transplantation, FACS analysis showed that the engraftment efficiency is between 90% and 96% (Figure 2A). However, the donor-derived cells of Zmpste24 null mice showed no significant difference in the profiling of early B cell development, including the proportions of B lineage progenitors and circulating B cells when compared with wild-type origin (Figure 2). These results indicate that the defects observed in early B cell development in Zmpste24-deficient mice are not cell-intrinsic, but rather the consequence of a defective BM microenvironment.
Defective early B lymphopoiesis in Zmpste24−/−mice is cell-extrinsic. (A) Representative plots showing B cell profile (pro- and pre-B) analyses in recipients with Zmpste24−/− or Zmpste24+/+ donor cells. (B) Detailed B cell profile analyses, including the number of pro-B cells, pre-B cells and circ. B cells, showed a complete rescue of defective early B cell development in Zmpste24−/− mice. Data represent mean ± SEM, n = 4. circ. B, circulating B cells; pre-B cells, B cell precursors; pro-B cells, progenitor B cells.
Compromised bone marrow niches in Zmpste24−/−mice
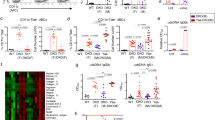
The bone marrow hematopoietic niches provide cytokines and cell adhesion molecules essential for B cell development and survival [10, 33]. However the cellular components in bone marrow niches are highly complex and still poorly understood. Recent studies revealed that a subset of bone marrow stromal cells (BMSCs) expressing vascular cell adhesion molecule-1 (Vcam-1) is essential for B-lymphopoiesis [34]. Of these reticular Vcam-1+ stromal cells, roughly 46% express interleukin 7 (IL-7) and around 17% express stromal cell-derived factor-1α (SDF-1α), also known as chemokine (C-X-C motif) ligand 12 (CXCL12) or pre-B-cell growth-stimulating factor (PBSF), both of which are critical for B lymphopoiesis [35]. To investigate the potential niche defects responsible for the impaired B lymphopoiesis in Zmpste24−/− mice, BMSCs were cultured and subjected to cytokine array from 6-month-old Zmpste24−/− mice in comparison with their heterozygous littermates. With quantification by Image J, 20 out of 96 cytokines showed significant alteration in their expression level (Figure 3 and [see Figure S1 in Additional file 1) and most of them are transcriptional targets of NF-κB ([See Figure S2 in Additional file 2 and online source at http://www.bu.edu/nf-kb/gene-resources/target-genes/), indicating a defective NF-κB pathway in Zmpste24−/− mice. Among those affected, Vcam-1, SDF-1α, tyrosine kinase 3 ligand (Flt3L) and thymic stromal lymphopoietin (TSLP) are most important for the early B cell development; Vcam-1, SDF-1α and TSLP are under transcriptional control of NF-κB signaling [36–38].
Reduced essential factors required for B lymphopoiesis owing to defective NF-κB signaling in Zmpste24−/−bone marrow stromal cells. (A) Representative blots showing cytokine expression in wild-type and Zmpste24−/− bone marrow stromal cells (BMSCs). Corresponding cytokines showed significant difference in (B) were labeled with number. (B) Downregulated cytokines in Zmpste24−/− BMSCs and most of them (#) are under transcriptional control of NF-κB, especially those required for early B cell development, including Vcam-1, SDF-1α, Flt3L and TSLP. Data represent mean ± SEM, n = 3. P <0.05, Zmpste24−/− BMSCs versus wild-type. POS, positive control (GAPDH); NEG, negative control (Blank).
Defective NF-κB pathway in Zmpste24−/−mice
In canonical NF-κB pathway, the p65/p50 complex is sequestered in cytoplasm by IκBα/β/ε; upon cytokine stimulation (for instance, TNFα), IκBα/β/ε is phosphorylated by IκB kinase β (IKKβ) and subjected to ubiquitination and proteasomal degradation, and then the p65/p50 complex is released and enters the nucleus to activate the relative gene expression [39]. NF-κB pathway activation is tightly regulated by an IκB-NF-κB negative feedback loop [40]. In Hoffmann’s model, the nuclear p65/p50 complex activates transcription of IκBα/β/ε, and newly synthesized IκBα/β/ε inhibited amplification NF-κB signaling. Analyses of the affected chemokines and TNFα family members by STRING 8.2 (an online tool for analysis of protein-protein interaction) suggested a defect in NF-κB pathway in Zmpste24−/− BMSCs [See Figure S2 in Additional file 2. As determined by nuclear fractionation and western blotting, compared with wild-type, a downregulation of the nuclear level of p65 in Zmpste24−/− BMSCs was found (Figure 4, untreated). We further investigated the dynamic changes of NF-κB signaling in Zmpste24−/− and wild-type BMSCs upon stress. We transiently stimulated BMSCs with TNFα and examined the nuclear translocation of p65 protein and cytoplasmic level of IκBα/β. As shown in Figure 4, in Zmpste24+/+ BMSCs, the nuclear level of p65 peaked at 10 minutes, then gradually decreased to the lowest level until 110 minutes after the stimulation and finally stabilized to normal level (before treatment) around 160 minutes after the stimulation. Tightly correlated with the dynamic changes of p65 level, the lowest level of IκBα/β was observed around 10 minutes after stimulation and went to the highest level at 110 minutes and stabilized around 160 minutes. These results show a beautiful negative feedback loop of IκB-NF-κB pathway. However, in Zmpste24−/− BMSCs, the highest level of nuclear p65 was slightly delayed until 20 minutes and then remained at the highest level until 160 minutes. In consistent with the changes of p65, IκBα/β went to its lowest level around 20 minutes after stimulation and remained unchanged until 160 minutes. Since IκBα/β is transcriptionally activated by NF-κB, these data suggested that although the nuclear accumulation of p65 was not much affect, the transcriptional activity of NF-κB was significantly compromised in Zmpste24−/− BMSCs.
Defective NF-κB signaling in Zmpste24−/−bone marrow stromal cells. (A) Representative western blotting results showing that upon transient TNFα stimulation, NF-κB was defective in activating IκBα/β expression and the following negative feedback in Zmpste24−/− bone marrow stromal cells (BMSCs). (B) Quantification of p65 and IκBα/β after TNFα treatment in (A) by Image J (see Materials and Methods)
Discussion
Bone marrow (BM) hematopoietic niches are essential for B cell development; they provide cytokines and cell adhesion molecules that are necessary for the survival of the B cells [10]. About two decades ago, Whitlock and Witte showed that adherent non-hematopoietic cells have great potential in supporting B-lymphopoiesis [41]. However the cellular basis that regulates B-lymphopoiesis is still poorly understood. Recent studies found that a subset of bone marrow mesenchymal cells expressing Vcam-1 is critical for B-lymphopoiesis [34, 35]. Schaumann et al. found that FACS-purified adherent Vcam-1+ stromal cells share similar surface markers with BMSCs, such as CD13, CD31, CD90, CD105, etcetera [34], suggesting that BMSCs, at least the Vcam-1+ subpopulation, might recapitulate part of the in vivo B cell niches. In the current study, we did cytokine array analysis for all BMSCs regardless of the expression of Vcam-1 because all BM-derived adherent non-hematopoietic cells have potentials to support B-lymphopoiesis [41]. We found that a series of important early B cell factors, including Vcam-1, SDF-1α and Flt3L, are primarily and significantly affected in Zmpste24−/− BMSCs. Vcam-1 interacts with very late antigen integrins (VLA-4), thus mediating the adhesion of B cell precursors to stromal cells [42, 43]. Depleting Vcam-1 in mice results in defective B cell homing to the bone marrow [44, 45]. SDF-1α belongs to chemokine family and is mainly recognized by CXC-chemokine receptor 4 (Cxcr4). Sdf1−/− embryos showed a significantly decreased number of the earliest stage of B cell precursors in fetal liver [46]. Adoptive transfer experiments showed that Cxcr4−/− fetal liver cells failed to generate pro-B cells [47]. In chimeric mice reconstituted with Cxcr4−/− fetal liver cells, the number of donor-derived pro-B and pre-B cells was significantly decreased in BM but increased in peripheral blood [48, 49]. It has been shown that Vcam-1 is expressed in almost all of the SDF-1α-expressing cells, whereas only 17% of all the Vcam-1+ stromal cells are SDF-1α positive and the rest comprises of a high proportion of IL-7+ cells [34, 35]. IL-7 is the first defined cytokine essential for B cell development [50]. Research based on both gene targeting and in vitro culture have shown that IL-7 is necessary for the development from pre-pro-B and pro-B stages [10, 51]. Although no significant change of IL-7 expression was observed in Zmpste24−/− BMSCs (data not shown), we found that Flt3L, which synergizes with IL-7 to support early B cell development [52], is significantly decreased. Therefore defects in both Vcam-1+SDF-1α+IL-7− and Vcam-1+SDF-1α−IL-7+ bone marrow stromal cells might be responsible for the defective B-lymphopoiesis in Zmpste24−/− mice. In addition to Vcam-1, SDF-1α and Flt3L, 17 other cytokines also show significant changes in Zmpste24−/− BMSCs (see Figure 3). Some of these cytokines, for example, TSLP, are important for early murine B cell development [53]. However it is still unclear whether changes in these cytokines represent defects in Vcam-1− BMSCs that might also be responsible for the defective B-lymphopoiesis in Zmpste24−/− mice. Our data are consistent with a previous report showing extrinsic defects in B and T cell development in Lmna null mice [29]. However, Hale and colleagues showed that engrafted Lmna null thymus is comparable in the ability to support T lymphopoiesis and concluded that the defective T cell development is attributable to the overall unhealthiness of the host animal instead of impaired stromal cells. In the current study, we employed in vitro expanded BMSCs; therefore, the defects in various cytokines are independent of the overall healthiness of examined animals. In this regard, the effects of lacking lamin A in Lmna null mice and accumulation of prelamin A in Zmpste24 null mice are likely different in the regulation of lymphopoietic niches.
Our data are also consistent with a report showing defective NF-κB pathway in Lmna−/− mice [54], where loss of Lmna compromised IL-1β-stimulated NF-κB-regulated luciferase activity, although the binding of NF-κB to target sequences was increased. It is worthwhile to elucidate the underlying molecular mechanism of defective NF-κB signaling in Zmpste24−/− mice in future studies. The transcriptional activity of NF-κB can also be regulated by co-activators, including p300/CBP, PCAF, p160 proteins (SRC-1/2/3), etcetera, and co-repressors, such as HDAC1/2/3, SMRT, NcoR, etcetera, which modulate local chromatin structure and NF-κB signaling. Given that the chromatin structure is disorganized in HGPS and normal aging cells [24, 55, 56], one possibility is that accumulation of unprocessed prelamin A may affect local chromatin remodeling and thus impede NF-κB-mediated transcription activation in Zmpste24−/− BMSCs. This may also explain the finding that only a set of targets are affected by defective NF-κB signaling in Zmpste24−/− BMSCs.
Conclusions
In this study, we found a significant decline in the number of B cell progenitors in Zmpste24−/− mice. Further investigation revealed that the defective B-lymphopoiesis is most likely attributable to decreased NF-κB signaling in BMSCs, which likely resembles in vivo B cell niches. Of those 20 affected cytokines in Zmpste24−/− BMSCs, Vcam-1, SDF-1α, Flt3L and TSLP are among the most well-studied and are essential for early B lymphopoiesis. Collectively, our data demonstrate a cell-extrinsic defect of B cell development in a progeroid mouse model and a critical role for lamin A in the regulation of NF-κB signaling and essential cytokines in B-lymphopoiesis. As progerin accumulates in and contributes to healthy aging, our data also suggest a mechanistic explanation for aging-related decline in B cell populations in aged individuals.
Methods
Antibodies
PE/Cy5 anti CD45R/B220 (RA3-6B2), R-PE anti CD43 and FITC anti-mouse CD45.2 were sourced from BioLegend (San Diego, CA, USA). Mouse lineage panel (anti-CD3ε, anti-CD11b, anti-B220, anti-Ly-6G, anti-Ly-6C, and anti-TER-119) and anti Biotin microbeads were purchased from BD Biosciences (San Jose, CA, USA). Rabbit anti p65, IκB-α, IκB-β and lamin A/C antibodies were from Santa Cruz (Santa Cruz, CA, USA). Mouse anti β-actin antibody was from Sigma (St. Louis, MO, USA).
Animals
Mouse experiments were performed under the regulations and guidelines of the Committee on the Use of Live Animals in Teaching and Research (CULATR) at the University of Hong Kong. Zmpste24−/− mice were described previously [16].
B cell profile analysis and bone marrow transplantation
Bone marrow cells were flushed out into HBSS supplemented with 2% FBS, stained with B cell markers (PE/Cy5-anti-CD45R/B220 and R-PE-anti-CD43) and subjected to FACS analysis. For total bone marrow transplantation, 6-month-old B6.SJL/BoyJ mice were irradiated with 9 Gy and served as recipients. A total of 1×107 bone marrow nucleated cells from either Zmpste24−/− mice or wild-type littermates were suspended in 100 μl of HBSS supplemented with 2% FBS and injected into recipients via tail vain. FITC-anti-CD45.2 antibody was used to identify donor-derived lymphocytes.
Bone marrow stromal cell culture and RayBio™ Mouse Cytokine Antibody Array
BMSCs were cultured as described [57]. Briefly, total bone marrow cells were flushed out with HBSS buffer (containing 2% FBS) and taken into culture with 3 ml α-MEM medium (containing 20% FBS and penicillin-streptomycin) directly in a 60-mm petri dish. Culture medium was replaced with fresh α-MEM (containing 20% FBS and no antibiotics) after 24 h, which cleared most of the unattached cells (including red blood cells) and cell debris. After 6 days, the P0 culture was trypsinized (with 0.025% trypsin and 0.01% EDTA) for 10 minutes and the detached cells were transferred into a 10-cm petri dish for culture. The cells were then subcultured every 3 to 4 days. After passage four, the BMSC cultures were subjected to magnetic sorting with BD mouse lineage panel (anti-CD3ε, anti-CD11b, anti-B220, anti-Ly-6G, anti-Ly-6C, and anti-TER-119) and anti Biotin microbeads according to the manufacture’s instruction. Cells from three different mice were pooled together for the sake of decrease the background noise and increasing the robustness of the difference between mutant and heterozygous mice in the following cytokine array and protein expression analysis. RayBio™ Mouse Cytokine Antibody Array III and IV (RayBiotech, USA) were used to compare the cytokine expression pattern in Zmpste24−/− and Zmpste24+/+ BMSCs. The experiment was performed according to the instruction of the manufacturer.
Protein extraction and western blotting
Total cell lysate was prepared by suspending the cells in five volumes of suspension buffer, and then adding five volumes of Laemmli buffer and boiling for 5 minutes. For protein fractionation, cells were suspended in 100 μl ice-cold buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, 1 mM DTT, protease inhibitors). After the addition of 0.1% Triton X-100, the cell suspension was mixed gently, incubated on ice for 5 minutes and centrifuged at 1300×g at 4°C for 4 minutes. The supernatant (S1) was transferred to a new tube and clarified by high-speed centrifugation (12000×g, 10 minutes, 4°C). The remaining nuclei pellet (P1) was washed once with 100 μl buffer A and then resuspended in 100 μl Laemmli buffer and boiled for 5 minutes. Western blotting was performed as described previously [58].
Image quantification and statistics analysis
Photos were processed with Photoshop CS® (Adobe Systems Incorporated, San Jose, CA, USA) when necessary. The pixel intensity of western blotting and dot blotting was measured by Image J gel analysis function [59] and normalized to housekeeping controls. Student’s t-test was performed for statistical comparison.
Abbreviations
- BCRs:
-
B cell receptors
- BM:
-
Bone marrow
- BMSCs:
-
Bone marrow stromal cells
- FACS:
-
Fluorescence-activated cell sorting
- HGPS:
-
Hutchinson-Gilford progeria syndrome
- HSCs:
-
Hematopoietic stem cells
- PBSF:
-
Pre-B-cell growth-stimulating factor
- pre-B:
-
B cell precursors
- pre-pro-B:
-
Precursor of B cell progenitor
- pro-B:
-
Progenitor B
- sIgM:
-
Surface immunoglobulin
- TSLP:
-
Thymic stromal lymphopoietin
- Vcam-1:
-
Vascular cell adhesion molecule-1.
References
Gilbert SF: Developmental Biology. 2000, Sunderland: SINAUER ASSOCIATES, INC, 6th
Ghia P, Melchers F, Rolink AG: Age-dependent changes in B lymphocyte development in man and mouse. Exp Gerontol. 2000, 35: 159-165. 10.1016/S0531-5565(99)00095-9.
Goronzy JJ, Weyand CM: T cell development and receptor diversity during aging. Curr Opin Immunol. 2005, 17: 468-475. 10.1016/j.coi.2005.07.020.
Solana R, Pawelec G, Tarazona R: Aging and innate immunity. Immunity. 2006, 24: 491-494. 10.1016/j.immuni.2006.05.003.
Weng NP: Aging of the immune system: how much can the adaptive immune system adapt?. Immunity. 2006, 24: 495-499. 10.1016/j.immuni.2006.05.001.
Pistoresi-Palencia MC, Romero-Piffiguer M, Moron G, Ferro ME: Effect of aging on autoimmune response to rat male accessory glands: young, but not aged, antigen-presenting cells efficiently induce suppression in aged rats. Mech Ageing Dev. 1994, 76: 33-41. 10.1016/0047-6374(94)90005-1.
Bassing CH, Alt FW: The cellular response to general and programmed DNA double strand breaks. DNA Repair (Amst). 2004, 3: 781-796. 10.1016/j.dnarep.2004.06.001.
Ogawa M, ten Boekel E, Melchers F: Identification of CD19(−)B220(+)c-Kit(+)Flt3/Flk-2(+)cells as early B lymphoid precursors before pre-B-I cells in juvenile mouse bone marrow. Int Immunol. 2000, 12: 313-324. 10.1093/intimm/12.3.313.
Li YS, Wasserman R, Hayakawa K, Hardy RR: Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996, 5: 527-535. 10.1016/S1074-7613(00)80268-X.
Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K: Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991, 173: 1213-1225. 10.1084/jem.173.5.1213.
Allman D, Li J, Hardy RR: Commitment to the B lymphoid lineage occurs before DH-JH recombination. J Exp Med. 1999, 189: 735-740. 10.1084/jem.189.4.735.
Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL: Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci USA. 2005, 102: 9194-9199. 10.1073/pnas.0503280102.
Hennekam RC: Hutchinson-Gilford progeria syndrome: review of the phenotype. Am J Med Genet A. 2006, 140: 2603-2624.
Pollex RL, Hegele RA: Hutchinson-Gilford progeria syndrome. Clin Genet. 2004, 66: 375-381. 10.1111/j.1399-0004.2004.00315.x.
Beck LA, Hosick TJ, Sinensky M: Isoprenylation is required for the processing of the lamin A precursor. J Cell Biol. 1990, 110: 1489-1499. 10.1083/jcb.110.5.1489.
Pendas AM, Zhou Z, Cadinanos J, Freije JM, Wang J, Hultenby K, Astudillo A, Wernerson A, Rodriguez F, Tryggvason K, Lopez-Otin C: Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet. 2002, 31: 94-99.
Bergo MO, Gavino B, Ross J, Schmidt WK, Hong C, Kendall LV, Mohr A, Meta M, Genant H, Jiang Y, Wisner ER, Van Bruggen N, Carano RA, Michaelis S, Griffey SM, Young SG: Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc Natl Acad Sci USA. 2002, 99: 13049-13054. 10.1073/pnas.192460799.
Rusinol AE, Sinensky MS: Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors. J Cell Sci. 2006, 119: 3265-3272. 10.1242/jcs.03156.
Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS: Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003, 423: 293-298. 10.1038/nature01629.
Reddel CJ, Weiss AS: Lamin A expression levels are unperturbed at the normal and mutant alleles but display partial splice site selection in Hutchinson-Gilford progeria syndrome. J Med Genet. 2004, 41: 715-717. 10.1136/jmg.2004.019323.
Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B: Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999, 147: 913-920. 10.1083/jcb.147.5.913.
Varela I, Cadinanos J, Pendas AM, Gutierrez-Fernandez A, Folgueras AR, Sanchez LM, Zhou Z, Rodriguez FJ, Stewart CL, Vega JA, Tryggvason K, Freije JM, López-Otín C: Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 2005, 437: 564-568. 10.1038/nature04019.
Fong LG, Ng JK, Meta M, Cote N, Yang SH, Stewart CL, Sullivan T, Burghardt A, Majumdar S, Reue K, Bergo MO, Young SG: Heterozygosity for Lmna deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice. Proc Natl Acad Sci USA. 2004, 101: 18111-18116. 10.1073/pnas.0408558102.
Scaffidi P, Misteli T: Lamin A-dependent nuclear defects in human aging. Science. 2006, 312: 1059-1063. 10.1126/science.1127168.
Candelario J, Sudhakar S, Navarro S, Reddy S, Comai L: Perturbation of wild-type lamin A metabolism results in a progeroid phenotype. Aging Cell. 2008, 7: 355-367. 10.1111/j.1474-9726.2008.00393.x.
Kudlow BA, Stanfel MN, Burtner CR, Johnston ED, Kennedy BK: Suppression of proliferative defects associated with processing-defective lamin A mutants by hTERT or inactivation of p53. Mol Biol Cell. 2008, 19: 5238-5248. 10.1091/mbc.E08-05-0492.
McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, Collins FS, Djabali K: The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One. 2007, 2: e1269-10.1371/journal.pone.0001269.
Cao K, Blair CD, Faddah DA, Kieckhaefer JE, Olive M, Erdos MR, Nabel EG, Collins FS: Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J Clin Invest. 2011, 121: 2833-2844. 10.1172/JCI43578.
Hale JS, Frock RL, Mamman SA, Fink PJ, Kennedy BK: Cell-extrinsic defective lymphocyte development in Lmna(−/−) mice. PLoS One. 2010, 5: e10127-10.1371/journal.pone.0010127.
Riley RL, Kruger MG, Elia J: B cell precursors are decreased in senescent BALB/c mice, but retain normal mitotic activity in vivo and in vitro. Clin Immunol Immunopathol. 1991, 59: 301-313. 10.1016/0090-1229(91)90026-7.
Stephan RP, Sanders VM, Witte PL: Stage-specific alterations in murine B lymphopoiesis with age. Int Immunol. 1996, 8: 509-518. 10.1093/intimm/8.4.509.
Johnson KM, Owen K, Witte PL: Aging and developmental transitions in the B cell lineage. Int Immunol. 2002, 14: 1313-1323. 10.1093/intimm/dxf092.
Wilson A, Trumpp A: Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006, 6: 93-106. 10.1038/nri1779.
Schaumann DH, Tuischer J, Ebell W, Manz RA, Lauster R: VCAM-1-positive stromal cells from human bone marrow producing cytokines for B lineage progenitors and for plasma cells: SDF-1, flt3L, and BAFF. Mol Immunol. 2007, 44: 1606-1612. 10.1016/j.molimm.2006.08.021.
Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T: Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004, 20: 707-718. 10.1016/j.immuni.2004.05.001.
Iademarco MF, McQuillan JJ, Rosen GD, Dean DC: Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1). J Biol Chem. 1992, 267: 16323-16329.
Lee HC, Ziegler SF: Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci USA. 2007, 104: 914-919. 10.1073/pnas.0607305104.
Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR: The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002, 17: 525-535. 10.1016/S1074-7613(02)00423-5.
Vallabhapurapu S, Karin M: Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009, 27: 693-733. 10.1146/annurev.immunol.021908.132641.
Hoffmann A, Levchenko A, Scott ML, Baltimore D: The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002, 298: 1241-1245. 10.1126/science.1071914.
Whitlock CA, Witte ON: Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci USA. 1982, 79: 3608-3612. 10.1073/pnas.79.11.3608.
Ryan DH, Nuccie BL, Abboud CN, Winslow JM: Vascular cell adhesion molecule-1 and the integrin VLA-4 mediate adhesion of human B cell precursors to cultured bone marrow adherent cells. J Clin Invest. 1991, 88: 995-1004. 10.1172/JCI115403.
Dittel BN, McCarthy JB, Wayner EA, LeBien TW: Regulation of human B-cell precursor adhesion to bone marrow stromal cells by cytokines that exert opposing effects on the expression of vascular cell adhesion molecule-1 (VCAM-1). Blood. 1993, 81: 2272-2282.
Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA: Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001, 193: 741-754. 10.1084/jem.193.6.741.
Leuker CE, Labow M, Muller W, Wagner N: Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs B cell localization and T cell-dependent humoral immune response. J Exp Med. 2001, 193: 755-768. 10.1084/jem.193.6.755.
Egawa T, Kawabata K, Kawamoto H, Amada K, Okamoto R, Fujii N, Kishimoto T, Katsura Y, Nagasawa T: The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001, 15: 323-334. 10.1016/S1074-7613(01)00185-6.
Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA: Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998, 95: 9448-9453. 10.1073/pnas.95.16.9448.
Kawabata K, Ujikawa M, Egawa T, Kawamoto H, Tachibana K, Iizasa H, Katsura Y, Kishimoto T, Nagasawa T: A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc Natl Acad Sci USA. 1999, 96: 5663-5667. 10.1073/pnas.96.10.5663.
Ma Q, Jones D, Springer TA: The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999, 10: 463-471. 10.1016/S1074-7613(00)80046-1.
Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D, Gillis S: Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988, 333: 571-573. 10.1038/333571a0.
von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R: Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995, 181: 1519-1526. 10.1084/jem.181.4.1519.
Namikawa R, Muench MO, de Vries JE, Roncarolo MG: The FLK2/FLT3 ligand synergizes with interleukin-7 in promoting stromal-cell-independent expansion and differentiation of human fetal pro-B cells in vitro. Blood. 1996, 87: 1881-1890.
Ray RJ, Furlonger C, Williams DE, Paige CJ: Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur J Immunol. 1996, 26: 10-16. 10.1002/eji.1830260103.
Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT: Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004, 113: 370-378.
Columbaro M, Capanni C, Mattioli E, Novelli G, Parnaik VK, Squarzoni S, Maraldi NM, Lattanzi G: Rescue of heterochromatin organization in Hutchinson-Gilford progeria by drug treatment. Cell Mol Life Sci. 2005, 62: 2669-2678. 10.1007/s00018-005-5318-6.
Shumaker DK, Dechat T, Kohlmaier A, Adam SA, Bozovsky MR, Erdos MR, Eriksson M, Goldman AE, Khuon S, Collins FS, Jenuwein T, Goldman RD: Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci USA. 2006, 103: 8703-8708. 10.1073/pnas.0602569103.
Meirelles Lda S, Nardi NB: Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003, 123: 702-711. 10.1046/j.1365-2141.2003.04669.x.
Liu B, Wang J, Chan KM, Tjia WM, Deng W, Guan X, Huang JD, Li KM, Chau PY, Chen DJ, Cao Y, Cheah KS, Tryggvason K, Zhou Z: Genomic instability in laminopathy-based premature aging. Nat Med. 2005, 11: 780-785. 10.1038/nm1266.
Schneider CA, Rasband WS, Eliceiri KW: NIH Image to ImageJ: 25 years of image analysis. Nature methods. 2012, 9: 671-675. 10.1038/nmeth.2089.
Acknowledgements
We thank Ms. Alice Lui for technical assistance. This project is supported by research grants (HKU7655/06M, CRF/HKU3/07C) from Research Grant Council of Hong Kong, the 973 Project (2007CB507400) from the Ministry of Science and Technology of China, a NSFC grant (30871440) and a Guangdong NSF grant (8452402301001450). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BL and ZZ designed experiments. BL and SZ conducted experiments. BL, XL, KZ, FZ and ZZ analyzed the data. BL, SZ and ZZ wrote the manuscript. All authors discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
13685_2012_10_MOESM2_ESM.jpeg
Additional file 2: Figure S2. Downregulated cytokines in Zmpste24−/− BMSCs are correlated with NF-κB pathway. (A) Interacting network among NF-κB pathway and downregulated factors in Zmpste24−/− BMSCs was predicted by online tool STRING (http://string.embl.de/). (B) Interacting network among those downregulated factors that are essential for B cell development and NF-κB signaling, predicted by STRING. *Significantly downregulated cytokines in Zmpste24−/− BMSCs. (JPEG 836 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Liu, B., Zhou, S., Liu, X. et al. Accumulation of prelamin A compromises NF-κB-regulated B-lymphopoiesis in a progeria mouse model. Longev Healthspan 2, 1 (2013). https://doi.org/10.1186/2046-2395-2-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2046-2395-2-1