Abstract
To develop and characterize a novel cell culture method for the generation of undifferentiated and differentiated human mesenchymal stem cell 3D structures, we utilized the RWV system with a gelatin-based scaffold. 3 × 106 cells generated homogeneous spheroids and maximum spheroid loading was accomplished after 3 days of culture. Spheroids cultured in undifferentiated spheroids of 3 and 10 days retained expression of CD44, without expression of differentiation markers. Spheroids cultured in adipogenic and osteogenic differentiation media exhibited oil red O staining and von Kossa staining, respectively. Further characterization of osteogenic lineage, showed that 10 day spheroids exhibited stronger calcification than any other experimental group corresponding with significant expression of vitamin D receptor, alkaline phosphatase, and ERp60 . In conclusion this study describes a novel RWV culture method that allowed efficacious engineering of undifferentiated human mesenchymal stem cell spheroids and rapid osteogenic differentiation. The use of gelatin scaffolds holds promise to design implantable stem cell tissue of various sizes and shapes for future regenerative treatment.
Similar content being viewed by others
Introduction
Stem cell-based therapies offer tremendous advantages for the treatment of orthopedic defects. One of the most widely studied stem cells are human mesenchymal stem cells (hMSC). HMSCs display a very high degree of plasticity and are found in virtually all organs, however, the bone marrow contains the highest density [1]. HMSCs serve as renewable source for mesenchymal [2] and potentially epithelial cells and have pluripotent ability of differentiating into several cell lineages, including osteoblasts, chondrocytes, adipocytes, skeletal and cardiac myocytes, endothelial cells, and neurons in vitro upon appropriate stimulation, and in vivo after transplantation [3]. Although the pathophysiologic functions of hMSCs are critically under investigation, the in vitro pluripotency of hMSC suggests a role in tissue regeneration, wound healing, or tissue repair after transplantation [4]. These characteristics make hMSCs good vehicles for autologous transplantation with the genuine benefits for tissue regeneration or cell-based gene therapies [5].
HMSCs isolated from the bone marrow have several limitations, however, the most paramount is the limited number of cells easily obtainable. Limited cell number presents further constraints, particularly for autologous transplantation, as the number of cells per area on tissue culture plates requires multiple passaging and potential loss of pluripotency [6]. An additional hurdle is the length of time required to promote lineage-specific differentiation. For example, it is well established that 3 to 4 weeks of in vitro incubation of hMSC monolayers with osteogenic differentiation media is required for calcium accumulation and positive von Kossa staining [7]. Various matrices or scaffolds have been employed to promote differentiation such as porous gelatin, polyethylene terephthalate, or thermo-reversible gelatin polymer [8–10]. The scaffold is a very important component for promoting tissue differentiation because it represents a structure, which the cells attach to and colonize in order to produce three-dimensional (3D) tissues. One such scaffold is gelatin sponge. Gelatin sponge is a porous denatured gelatin scaffold and has been previously reported to promote hMSC osteogenic, chondrogenic and adipogenic differentiation under appropriate conditions [11]. In addition gelatin sponge is an ideal scaffold as its porous gelatin properties favor cell adhesion proteins, which is the first basic step toward cell growth [12]. Furthermore, the use of gelatin-based scaffolds avoids the need for chemical surfactants that could be detrimental to the biocompatibility in certain systems.
To circumvent the challenges of in vitro and in vivo expansion of hMSCs, several ex vivo systems have been proposed. Ex vivo approach has several advantages, particularly for tissue-specific repair, delivery of targeted anticancer agents, or for allogenic transplantation [4]. While all these methods were able to induce hMSC differentiation, efficacy (i.e. cell load) has been poor and the time to differentiation has still remained at the 3 to 4 week period. The system we used in this study, the Rotary Wall Vessel (RWV) system, has been previously used to simulate the effects of a microgravity or RWV environment on numerous cell culture systems [13, 14].
Herein we present the use of RWV microgravity (MG) system as a novel culture methodology that facilitates the generation of multicellular undifferentiated mesenchymal stem cell spheroids that retain differentiation potential into multiple independent cell lineages. Our work has established defined ex vivo culture conditions for enhanced hMSC multicelluar aggregation and accelerated differentiation with the potential to deliver either undifferentiated or pre-differentiated cells to the site of injury to aid repair. Therefore, culture conditions that reduce hMSC differentiation predictably would provide standardization that is critical to establish a broad clinical adoption and potential allogenic transplantation.
Materials and methods
Cell culture
Human mesenchymal stem cells were purchased from Lonza (Walkersville, MD, #PT-2501). As specified by the company, hMSC are harvested and cultured from normal human bone marrow. Cells are tested for purity by flow cytometry and for their ability to differentiate into osteogenic, chondrogenic, and adipogenic lineages. Cells are positive for CD29, CD44, CD105, CD133 and CD166. Cells are negative for CD14, CD34, and CD45. HMSCs were cultured in mesenchymal stem cell media (Lonza, Walkersville, MD, #PT-3001) and cell propagation was limited to passage 7.
Three-dimensional cell culture
1 × 1 × 1 mm porous gelatin (Ferrosan, Soeborg, DK, #H206197412), was soaked in undifferentiated stem cell media until all air was expelled; it remained positively buoyant. 10 ml of cell suspension including porous gelatin (scaffold) were transferred to a RWV vessel and air was removed. The RWV system was set at a rotational speed of 4 rounds per minute (rpm) and cells were cultured at 37°C and 5% CO2. A spheroid adhering to the scaffold was generated, and media was exchanged every 3 to 4 days. Differentiation was induced utilizing chondrogenic, osteogenic, and adipogenic differentiation media, obtained from Lonza (#PT-3003, #PT-3002, #PT-3004), after 3 days of RWV culture. Spheroids in undifferentiated control growth media or differentiation media were cultured for additional 7 days.
DNA quantification
The number of hMSCs attached to the porous gelatin core was determined by quantification of DNA content per spheroid. DNA quantification was performed using the DNeasy tissue kit (Qiagen, Valencia, CA, #69504) and cell number extrapolated from an hMSC standard curve.
Histology
All specimens were fixed in 10% formalin, embedded in paraffin, and stained for H&E. In addition, frozen sections were assessed for adipogenic differentiation with oil red O stain and for osteogenic differentiation with von Kossa stain. Chondrogenic differentiation was evaluated by immunohistochemistry (IHC) using antibodies against collagen type II (Santa Cruz, CA, sc-52658) at a dilution of 1: 10, and against aggrecan (Santa Cruz, CA, sc-73693) at a dilution of 1:100. CD44 (Abcam, Cambridge, MA, ab24504) at 1:50, CD133 (Abcam, Cambridge, MA, ab19898) at 1:250, and CD166 (Abcam, Cambridge, MA, ab53442) at 1:10 antibodies were used to evaluate stem cell surface markers. Cell proliferation was assessed by staining Ki-67 (Santa Cruz, CA, sc-7844) with a 1:100 antibody dilution. Spheroid calcification was further studied by IHC against alkaline phosphatase (AP) (Abcam, Cambridge, MA, ab75699) at 1:100, nuclear vitamin D receptor (Santa Cruz, CA, sc-1008) at 1:500, membrane vitamin D receptor (ERp60) (Alpha Diagnostics International, San Antonio, TX, Ab100) at 1:1000. Intra- and intercellular architecture was studied by transmission electron microscopy (TEM), performed at University of Alabama, Birmingham.
Results
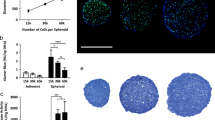
Gelatin sponge is a scaffold, which has pores separated by thin (few μm in thickness) walls [15]. We employed a cell loading procedure utilizing empirically determined 1 × 1 × 1 mm sponge scaffold to maximize hMSC loading and future engrafting (Figure 1).
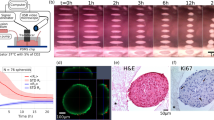
Schematic of generation of multicellular spheroids . 2D cultured hMSC were suspended in growth media in the presence of scaffold. Cell suspensions containing scaffolds were transferred to RWV rotary cell culture system and cultured at 4 rounds per minute for designated time intervals. After designated time intervals multicellular spheroids were sectioned and examined for cellularity and differentiation status.
As hMSC loading onto gelatin sponges in MG conditions has not previously been characterized, we first determined efficacy of hMSC loading onto the gelatin scaffold under MG conditions. 2 × 106 to 4 × 106 hMSC were introduced to the RWV system along with 1 mm3 gelatin scaffold. 2 × 106 hMSC/10 ml resulted in incomplete loading of the scaffold and partial spheroid formation, as determined by histological examination (data not shown). However, 3 × 106 cells/10 ml produced a homogeneous multi-cellular tissue-like spheroid around the porous gelatin core
We further characterized the length of time required to maximum spheroid size and cell loading. 3 x106 cells/10 ml hMSC spheroids were harvested at 1, 2, 3, 4, and 8 days of MG culture. Total DNA of spheroids was extracted and normalized to 2D cultured cells to extrapolate the exact number of cells within spheroids. Our results show that maximum spheroid loading was achievable by 3 days with a mean of 3.67 × 106cells per spheroid, representing 12% efficacy in cell loading (Figure 2A). Spheroid loading progressively declined after day 3, as determined by number of remaining cells in suspension; however spheroid size was maintained throughout (Figure 2B). Upon subsequent removal of hMSC coated gelatin scaffolds, we noticed that the rigid structure was maintained, suggesting an implantable construct (Figure 1). Hematoxylin and eosin (H&E) staining of sectioned spheroids confirmed these finding and highlighted a concentric rim of cells in the periphery with cell-cell connections and multi-cellular aggregation, with cells sparsely adherent near the porous space adjacent to the center of the spheroid (Figure 2C). Throughout the spheroid hMSC appear to maintain their mesenchymal phenotype and form cell-cell connections, which is further evident at increased magnification by TEM (Figure 2D). Since we determined the most significant cellularity and adherence to the gelatin scaffold at 3 days, we continued further characterization from this time point on.
Quantification of hMSC loading onto gelatin scaffolds under MG conditions. (A) Number of cells loaded onto spheroids was determined by quantification of DNA content per spheroid over an 8 day period. (B) Number of cells that remained in suspension over an 8 day period. All data presented are the mean of three independent experiments ± SE. (C) H&E staining of day 3 spheroid at 10x and 40x magnification shows multi-cellularity and absence of necrosis. (D) TEM images of day 3 spheroid show cell structure was maintained in MG. Images shown are representative of 4 individual experiments.
Numerous reports have provided compelling evidence that hMSC enhance organ repair. However, a major obstacle is the delivery of a sufficient number of undifferentiated cells to the site of injury. Therefore, it is essential that MG conditions do not alter hMSC multi-lineage differentiation potential. To determine whether 3 day spheroids, cultured under maintenance conditions alone, retained stem cell markers, we evaluated expression of stem cell markers (CD44, CD133, CD166) and common markers for osteogenic, adipogenic, and chondrogenic differentiation by staining for von Kossa, oil red O, collagen II, and aggrecan, respectively over an additional 10 day period. Spheroids harvested after three days showed robust expression of CD44, CD133, and CD166 (Figure 3A), which is similar to hMSC cells cultured on 2D surfaces [16]; however, spheroids harvested after 10 days demonstrated a loss of CD133 and CD166 expression. Additionally, 10 day spheroids did not exhibit von Kossa staining or collagen II/aggrecan expression, however, we did observe low levels of oil red O staining (Figure 3B).
Differentiation of hMSC spheroids cultured under MG conditions . (A) Immunohistochemistry analysis of spheroids for stem cells makers CD44, CD133, CD166, and differentiation markers von Kossa, oil red O, collagen-II showed that day 3 spheroids retained CD44, CD133, and CD166 expression and lacked differentiation markers. (B) Day 10 spheroids retain CD44 expression and were positive for oil red O staining. Images shown are representative of 4 individual experiments.
Since 3 day spheroids appeared to retain stem cell markers, we sought to determine if lineage-specific differentiation could be induced subsequent to 3 days of culture under MG conditions. Therefore, 3 day spheroids, previously cultured under maintenance conditions, were cultured in pre-defined media for chondrogenic, osteogenic and adipogenic differentiation for an additional 7 days, and compared to cells that remained in growth media only.
Spheroids cultured in chondrogenic media demonstrated histological changes on H&E stain (data not shown) such as clear cell appearance and cell aggregates indicating imminent differentiation. While CD133 and CD166 expression was undetectable, CD44 expression was detected throughout (Figure 4A). Expression of chondrogenic marker collagen type II and aggrecan was also absent after 3 and 10 days of culture in both control and chondrogenic media (Additional file 1: Figure S1), however, there were significant levels of adipogenic marker oil red O staining (Figure 4A).
Induced differentiation of hMSC spheroids cultured under MG conditions. (A) Immunohistochemistry analysis of hMSC cultured for 10 days in A, chrondrogenic media shows negative staining for collagen. (B) hMSC spheroids cultured under adipogenic conditions show positive oil read O staining. (C) hMSC spheroids cultured under osteogenic conditions were positive with von Kossa staining. Images shown are representative of 4 individual experiments.
In spheroids cultured under adipogenic conditions, we observed a similar pattern with decreased expression of CD44 and CD133 expression compared to controls (Figure 4A). As expected we observed significant increases in oil red O staining, demonstrating increased amounts of lipids in all specimens of all experimental groups. We did not observe positive von Kossa or collagen II staining, suggesting a commitment to adipogenic lineage (Figure 4B).
Lastly, we determined whether osteogenic differentiation conditions would override the seemly adipogenic default lineage observed with 3 day and 10 day cultures. 10 day spheroids in osteogenic differentiation conditions resulted in significant von Kossa staining, suggesting mineralization and calcification in 10 day spheroids (Figure 4C). As with the chondrogenic and adipogenic spheroids, we did not observe expression of CD133 or CD166, however, CD44 expression remained (Figure 4C). We did observe low level of oil red staining O but collagen II and aggrecan staining was completely absent.
Since mineralization appeared to occur on day 10, we further sought to determine if this mineralization was associated with additional markers of osteogenesis, alkaline phosphatase (ALP), nuclear vitamin D receptor (VDR) and membrane vitamin D receptor (ERp60) over the 10 day culture period. ALP staining was completely absent in 3 day spheroids, however, we did detect expression at 10 days under control and osteogenic conditions (Figure 5A). We also observed significant levels of nuclear VDR and ERp60 expression in the 3 day and 10 day control and osteogenic spheroids (Figure 5A). Robust Ki-67 expression was observed in undifferentiated and differentiated cultured conditions. (Figure 5B). Thus, it appears that these studies provide proof-of-principle that hMSC can be induced toward the osteogenic lineage under MG conditions.
Osteogenic differentiation of hMSC spheroids cultured under MG conditions. (A) Immunohistochemical analysis of alkaline phosphatase (AP), vitamin D receptor (VDR), and ERp60 expression in hMSC spheroids after day 3 and day 10 with and without osteogenic conditions. (B) Immunohistochemical analysis of proliferation marker Ki-67 at day 3 and day 10. Images shown are representative of 4 individual experiments.
Discussion
Conventional reconstructive surgery has employed autologous, biological, and artificial materials in an effort to reestablish normal human anatomy and function, however, often trigger immunological or organ-specific complications. Tissue engineering seeks to overcome the inherent problems of introduced materials by generating tissue that conforms to site-specific anatomy and function. To facilitate tissue regeneration, several studies have been performed using various biomaterials in association with different cell types (i.e. osteoblasts, osteoclasts and endothelial cells) under 2D and 3D conditions for bone repair, however, there have been few reports investigating the osteogenic differentiation of hMSC under MG conditions.
The Rotary Wall Vessel (RWV) is a device designed to simulate microgravity conditions, however, we and others have utilized this system for the RWV culture of cells [17, 18]. This system encompasses the features of low-shear stress, high mass transfer environment, comparable to the weightless state that is characteristic of blood, lymphatic fluid, or even in the bone marrow [19, 20]. Additionally, this system has been extremely useful in determining cellular interactions between cancer and host bone marrow stromal cells [21], and in studying therapeutic options for many pathological situations, which include radiation resistance, phenotypic differentiation, and response to targeted drug delivery. In this study we utilized the RWV system, which models microgravity (MG) conditions, with a gelatin sponge scaffold, to explore the possibility that hMSC, with an appropriate scaffold, will facilitate cell growth and organization, as well as directed differentiation, and that it can be utilized for regeneration of bone cells.
Previous studies have shown that gelatin scaffold supports the differentiation of hMSC or other stem cells into multiple lineages including osteogenic, chondrogenic, and adipogenic on 2D surfaces [11, 22, 23]. However, differentiation of hMSC cultured under modeled microgravity has not been determined. Our initial results indicate that hMSC cultured in the RWV system with gelatin scaffold support randomized adherence to the gelatin scaffold with near complete cell uptake within the gelatin-based spheroid as measured by DNA content after 3 days of culture. These findings are supported by previous reports that numerous scaffolds including microcarrier beads or collagen-coated sponges support cell attachment in vitro[24]. Although collagen is a major component of the bone matrix, we did not observe significant cell attachment with collagen-coated scaffolds (data not shown). Thus, we utilized uncoated gelatin sponges for the remainder of our studies.
Upon histological examination, 3 day control spheroids showed increased cell organization with a homogeneous multi-cellular tissue-like structure that completely coated the 1x1x1 mm scaffold. A high proliferative index, determined by Ki-67 staining, was seen as well. Interestingly, we did not observe any necrosis, which is typical of RWV engineered tissue-like structures greater than 200 μm in size in bioreactor systems [25, 26]. Thus, it is possible that the gelatin sponge facilitates diffusion of nutrients and oxygen, not typically observed with more rigid scaffold designs. Surprisingly, these findings are in contrast to a study [27] that reported a decrease in the viability and proliferation of cells cultured in the RWV bioreactor under modeled MG conditions. Although we did find a decrease in attachment of free floating cells, viability and proliferation remained through all experimental time points.
In order to determine the effects of MG on hMSC differentiation, we further characterized the spheroids over a 10 day period. Early differentiation (3 days) was not observed since undifferentiated stem cell markers CD44, CD133, and CD166 were robustly expressed and typical markers associated with lineage-specific differentiation (von Kossa, oil red O, collagen II, aggrecan) were not detected. Indeed, we did detect isolated lipid deposits, (oil red O staining) within the spheroids, suggesting the spheroids were pre-adipogenic. However, given the relatively low oil red O staining and presence of stem cells markers, it appears that after 3 days of MG, hMSCs largely remain undifferentiated.
The pluripotent potential of spheroids was further demonstrated after 10 day culture under defined differentiation conditions (shown to promote adipogenic, chondrogenic, and osteogenic differentiation, respectively). As expected, we observed early lipid deposits (3 days) and strong lipid deposits in 10 day control and adipogenic cultured cells. These findings are similar to Zayzafoon’s study [24] that concluded that hMSC, cultured on microcarrier beads in MG, undergo adipogenic differentiation opposed to other lineages including cells cultured in divergent differentiation-inducing conditions. The authors further concluded that the adipogenic lineage represents the default pathway of hMSC cultured under MG conditions. However, our findings do not completely reflect this hypothesis. hMSC cultured under osteogenic conditions, after 10 days exhibited robust von Kossa staining, suggesting calcification and mineralization, and VDR staining which is responsible for mineral stability [28–30]. Additionally, we observed ALP expression, which is produced by osteoblastic cells and represents a definitive marker for early osteogenesis [31, 32]. Loss of stem cell markers CD133 and CD166 expression in all differentiation conditions further support the pluripotency of 3 day spheroids and the commitment to cellular differentiation after 10 days.
Osteogenic differentiation of mouse bone marrow stromal cells in RWV system has been previously reported [27]. Conditioned media from mature osteoblasts were able to induce increased osteoclastogenesis and bone resorption in mouse bone marrow cultures via indirect stimulation of osteoclast formation and activity by regulating osteoblast secretion of regulatory factors such as RANKL and OPG. VDR regulates the expression of bone matrix proteins and promotes osteoclast differentiation by inducing the expression of RANKL. We observed an increase in VDR expression and constant expression of ERp60, a membrane-associated receptor implicated in the rapid actions of 1,25(OH)2D [33], in 3 day and 10 day spheroids (control or osteogenic condition), along with increased ALP expression and mineralization. These findings suggest that exogenous or endogenous soluble ligands (i.e. RANKL/OPG) are required to override the adipogenic default lineage and promote osteoblast formation of hMSC in MG conditions. Our observation of a low level of ALP expression after 10 days, even without osteogenic conditions, further supports a directive role of gelatin scaffold. Indeed, since the biocompatibility of gelatin likely facilitates cell attachment, which is required to promote differentiation and proliferation, it likely plays a role in promoting osteogenic properties of hMSC under MG conditions
In conclusion, this study provides greater insight into the effects of MG on hMSC osteogenic differentiation. Furthermore, this model establishes a rapid culture method that overcomes the bone diminishing effects of MG on hMSC osteogenesis and provides a system to determine the cellular and molecular mechanisms that are associated with this decline. Although the pluripotency of MG-cultured hMSC within the gelatin scaffold has yet to be verified in vivo in regard to bone regeneration, our system provides undifferentiated, cell-coated, and implantable constructs for tissue engineering and ex vivo modeled organogenesis.
References
da Silva Meirelles L, Chagastelles PC, Nardi NB: Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 2006, 119: 2204–2213. 10.1242/jcs.02932
Aggarwal S, Pittenger MF: Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105: 1815–1822. 10.1182/blood-2004-04-1559
Caplan AI: Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007, 213: 341–347. 10.1002/jcp.21200
Atala A: Tissue engineering and regenerative medicine: concepts for clinical application. Rejuvenation Res 2004, 7: 15–31. 10.1089/154916804323105053
van der Laan LJ, Lockey C, Griffeth BC, Frasier FS, Wilson CA, Onions DE, Hering BJ, Long Z, Otto E, Torbett BE, Salomon DR: Infection by porcine endogenous retrovirus after islet xenotransplantation in SCID mice. Nature 2000, 407: 90–94. 10.1038/35024089
Chamberlain G, Fox J, Ashton B, Middleton J: Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25: 2739–2749. 10.1634/stemcells.2007-0197
Lund AW, Bush JA, Plopper GE, Stegemann JP: Osteogenic differentiation of mesenchymal stem cells in defined protein beads. J Biomed Mater Res B Appl Biomater 2008, 87: 213–221.
Grayson WL, Ma T, Bunnell B: Human mesenchymal stem cells tissue development in 3D PET matrices. Biotechnol Prog 2004, 20: 905–912. 10.1021/bp034296z
Ponticiello MS, Schinagl RM, Kadiyala S, Barry FP: Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res 2000, 52: 246–255. 10.1002/1097-4636(200011)52:2<246::AID-JBM2>3.0.CO;2-W
Hishikawa K, Miura S, Marumo T, Yoshioka H, Mori Y, Takato T, Fujita T: Gene expression profile of human mesenchymal stem cells during osteogenesis in three-dimensional thermoreversible gelation polymer. Biochem Biophys Res Commun 2004, 317: 1103–1107. 10.1016/j.bbrc.2004.03.165
Hong L, Peptan IA, Colpan A, Daw JL: Adipose tissue engineering by human adipose-derived stromal cells. Cells Tissues Organs 2006, 183: 133–140. 10.1159/000095987
Dawson JI, Wahl DA, Lanham SA, Kanczler JM, Czernuszka JT, Oreffo RO: Development of specific collagen scaffolds to support the osteogenic and chondrogenic differentiation of human bone marrow stromal cells. Biomaterials 2008, 29: 3105–3116. 10.1016/j.biomaterials.2008.03.040
Begley CM, Kleis SJ: The fluid dynamic and shear environment in the NASA/JSC rotating-wall perfused-vessel bioreactor. Biotechnol Bioeng 2000, 70: 32–40. 10.1002/1097-0290(20001005)70:1<32::AID-BIT5>3.0.CO;2-V
Hammond TG, Hammond JM: Optimized suspension culture: the rotating-wall vessel. Am J Physiol Renal Physiol 2001, 281: F12-F25.
Rohanizadeh R, Swain MV, Mason RS: Gelatin sponges (Gelfoam) as a scaffold for osteoblasts. J Mater Sci Mater Med 2008, 19: 1173–1182. 10.1007/s10856-007-3154-y
Turnovcova K, Ruzickova K, Vanecek V, Sykova E, Jendelova P: Properties and growth of human bone marrow mesenchymal stromal cells cultivated in different media. Cytotherapy 2009,:1–12.
Sung SY, Hsieh CL, Law A, Zhau HE, Pathak S, Multani AS, Lim S, Coleman IM, Wu LC, Figg WD, et al.: Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer Res 2008, 68: 9996–10003. 10.1158/0008-5472.CAN-08-2492
Wang R, Xu J, Juliette L, Castilleja A, Love J, Sung SY, Zhau HE, Goodwin TJ, Chung LW: Three-dimensional co-culture models to study prostate cancer growth, progression, and metastasis to bone. Semin Cancer Biol 2005, 15: 353–364. 10.1016/j.semcancer.2005.05.005
Goodwin TJ, Prewett TL, Spaulding GF, Becker JL: Three-dimensional culture of a mixed mullerian tumor of the ovary: expression of in vivo characteristics. In Vitro Cell Dev Biol Anim 1997, 33: 366–374. 10.1007/s11626-997-0007-4
Schwarz RP, Goodwin TJ, Wolf DA: Cell culture for three-dimensional modeling in rotating-wall vessels: an application of simulated microgravity. J Tissue Cult Methods 1992, 14: 51–57. 10.1007/BF01404744
Josson S, Sharp S, Sung SY, Johnstone PA, Aneja R, Wang R, Gururajan M, Turner T, Chung LW, Yates C: Tumor-stromal interactions influence radiation sensitivity in epithelial- versus mesenchymal-like prostate cancer cells. J Oncol 2010, Article ID 232831: 10.
Mattii L, Battolla B, D’Alessandro D, Trombi L, Pacini S, Cascone MG, Lazzeri L, Bernardini N, Dolfi A, Galimberti S, Petrini M: Gelatin/PLLA sponge-like scaffolds allow proliferation and osteogenic differentiation of human mesenchymal stromal cells. Macromol Biosci 2008, 8: 819–826. 10.1002/mabi.200700331
Angele P, Kujat R, Nerlich M, Yoo J, Goldberg V, Johnstone B: Engineering of osteochondral tissue with bone marrow mesenchymal progenitor cells in a derivatized hyaluronan-gelatin composite sponge. Tissue Eng 1999, 5: 545–554. 10.1089/ten.1999.5.545
Zayzafoon M, Gathings WE, McDonald JM: Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology 2004, 145: 2421–2432. 10.1210/en.2003-1156
Kunz-Schughart LA: Multicellular tumor spheroids: intermediates between monolayer culture and in vivo tumor. Cell Biol Int 1999, 23: 157–161. 10.1006/cbir.1999.0384
Santini MT, Rainaldi G: Three-dimensional spheroid model in tumor biology. Pathobiology 1999, 67: 148–157. 10.1159/000028065
Rucci N, Rufo A, Alamanou M, Teti A: Modeled microgravity stimulates osteoclastogenesis and bone resorption by increasing osteoblast RANKL/OPG ratio. J Cell Biochem 2007, 100: 464–473. 10.1002/jcb.21059
Marcellini S, Bruna C, Henriquez JP, Albistur M, Reyes AE, Barriga EH, Henriquez B, Montecino M: Evolution of the interaction between Runx2 and VDR, two transcription factors involved in osteoblastogenesis. BMC Evol Biol 2010, 10: 78. 10.1186/1471-2148-10-78
Takeyama K, Yamamoto Y, Kato S: VDR knockout mice and bone mineralization disorders. Clin Calcium 2007, 17: 1560–1566.
Zhang X, Rahemtulla F, Zhang P, Beck P, Thomas HF: Different enamel and dentin mineralization observed in VDR deficient mouse model. Arch Oral Biol 2009, 54: 299–305. 10.1016/j.archoralbio.2009.01.002
Chevallier N, Anagnostou F, Zilber S, Bodivit G, Maurin S, Barrault A, Bierling P, Hernigou P, Layrolle P, Rouard H: Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials 2010, 31: 270–278. 10.1016/j.biomaterials.2009.09.043
Tohma Y, Ohgushi H, Morishita T, Dohi Y, Tadokoro M, Tanaka Y, Takakura Y: Bone marrow-derived mesenchymal cells can rescue osteogenic capacity of devitalized autologous bone. J Tissue Eng Regen Med 2008, 2: 61–68. 10.1002/term.67
Boyan BD, Wang L, Wong KL, Jo H, Schwartz Z: Plasma membrane requirements for 1alpha,25(OH)2D3 dependent PKC signaling in chondrocytes and osteoblasts. Steroids 2006, 71: 286–290. 10.1016/j.steroids.2005.09.018
Acknowledgments
We thank Dr. Carlos Abramowsky and Carla Shoffeit from the Department of Pediatric Pathology at Children’s Healthcare of Atlanta for their help in tissue preparation and staining. This grant was supported by PO-1 CA-098912, NIH/RCMI G12 RR03059-21A1NIH/NCHD, NIH/NCI 1 U54 CA118623.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interest
The authors declare that they have no competing interests.
Author’s contribution
WC and CY conceived, designed, and performed the experiments and analyzed the data. SS, BB HZ performed experiments and data analysis. LWKC, WC, and CY wrote the manuscript. All authors have read and approve the final manuscript.
Electronic supplementary material
13619_2011_2_MOESM1_ESM.tiff
Additional file 1: hMSC spheroids do not display chrondrogenic markers cultured under MG conditions. Immunohistochemistry analysis of day 3 or day 10 period spheroids cultured in control or chrondrogenic media conditions were negative for chrondrogenic markers aggrecan or collagen-II. Images shown are representative of 4 individual experiments. (TIFF 2 MB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cerwinka, W.H., Sharp, S.M., Boyan, B.D. et al. Differentiation of human mesenchymal stem cell spheroids under microgravity conditions. Cell Regen 1, 2 (2012). https://doi.org/10.1186/2045-9769-1-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2045-9769-1-2