Abstract
The objective of this review is to focus on putative modified epithelial functions related to allergy. The dysregulation of the epithelial barrier might result in the allergen uptake, which could be the primary defect in the pathogenesis of allergic reaction. We review the literature of the role of respiratory epithelium as an active barrier, how allergens are transported through it and how it senses the hostile environmental allergens and other dangerous stimuli.
Similar content being viewed by others
Introduction
Acute allergic reactions are known to be symptomatic type-I hypersensitivity responses caused by an allergen cross-linking specifically with anti-IgE molecules on the surface of pre-sensitized mast cells [1–4]. In Europe the incidence of allergies rapidly increasing and thus we are facing an epidemic outburst of these diseases [5]. Even though costs per a patient are usually small allergies cause a major economical burden to the society, since up to 20% of the population now suffers from these disorders for instance in Finland [6]. Because of this the Finnish authorities have launched an "Allergy project 2008-2018", where a new direction has been taken. Here the patients will now be tolerated towards the antigens instead of avoiding them [6].
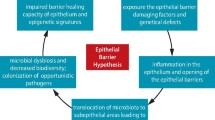
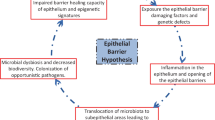
Currently there are two, partially different basic theories on the pathogenesis of allergy. For decades the primary assumption has been that allergy is caused by unbalanced and overactive immunological responses against allergens, mostly driven by activated Th2 cells and due to aberrant T- regulatory cells. The second more recent hypothesis relies on the dysregulation of the epithelial barrier, which might result in the allergen uptake as a primary defect in the pathogenesis of allergic reactions. There is a wealth of literature on T cell responses in allergic diseases [7, 8], and thus the objective of this review is to focus on putative modified epithelial functions related to allergy [9–11].
Epithelium as an active barrier
Respiratory epithelium is the first barrier to the external environment and thus crucially important in the protection of the internal environment. Normally three independent and complex networks connect epithelial cells to each other; desmosomes, adherens junctions, and tight junctions [12]. The respiratory epithelium senses changes in its local environment such as the allergen exposure and also actively responds to these changes [13, 14].
Interleukin (IL)-25, IL-33, and thymic stromal lymphopoietin (TSLP) are cytokines, which have a role in Th2-type allergic responses [15]. Previously the epithelium was considered only as a barrier, but now it is considered as a central player in controlling the immune function release of innate cytokines-promoting Th2 responses and the activation of local dendritic cells [16–18]. The exposure of airways to aeroallergens induces a rapid release of these three cytokines from the epithelial cells into the airway lumen and initiates an allergic immune response [19, 20].
Recently, disruption in the epithelial barrier has been included into the primary defects in allergic diseases. Atopic dermatitis (i.e. atopic eczema) is an allergic disease characterized by the defective epidermal barrier function, immunological dysregulation, and IgE-mediated sensitization to pollen allergens and food [21, 22]. Loss-of-function mutations in the filaggrin gene are linked to atopic dermatitis and other related diseases including peanut allergy [22]. Filaggrin dysfunction has been suspected to cause the impaired barrier function in the skin allowing the allergen penetration and followed by a local inflammation. To more directly verify the link between mutated filaggrin and the barrier dysfunction an elegant survey with wasp allergies was conducted. Wasp stings bypass the skin surface and antigen is injected deep into the dermis [21]. Patients with atopic dermatitis have an increased frequency of the most common filaggrin null mutations (R501X and 2282del4) compared to those with wasp allergy and healthy subjects. This data supports the role of mutated filaggrin in the epithelial dysfunction resulting in the allergic sensitization [21].
The respiratory epithelium is constantly facing hostile injurious stimuli causing damage. Proliferation and differentiation of resident progenitor and stem cells repair the damages and try to maintain the protective barrier [23]. The concept of unified airways suggests that in addition to the anatomic continuity, also inflammation influences the homeostasis of other parts of the airway [24–26]. In vivo challenge with the birch pollen allergen, Bet v 1, leads to instant leukocyte extravasation into conjunctival and nasal mucosa only in patients allergic to birch pollen, but not in healthy subjects.
Allergen transport through the epithelium
Several of the known allergens are proteases [27, 28]. Impaired epithelial barrier function can be a direct consequence of proteolytic activity of inhaled allergens, including house dust mite (HDM) [29]. After causing injury to the epithelium Der p 1 m, the major allergen in HDM, can enter the tissue and induce mediate inflammation via Toll-like receptor 4 (TLR4) [30, 31]. Der p 2, another allergen from HDM activates respiratory epithelial cells, indicating that this non-proteolytic allergen, in addition to its immunogenic properties, can aggravate a respiratory airway disease by an adjuvant [32].
Also toxic perturbations such as exposure to cigarette smoke can induce epithelial cell gaps and a decrease in trans-epithelial resistance [33]. Cigarette smoke exposure induces also a significant increase in the allergen uptake to epithelium. Thus smoking reduced the barrier function of the respiratory epithelium for allergens and contributed to exacerbation of an allergic disease [33].
A new concept is to focus on the aberrant innate responses of the respiratory epithelium to viral or bacterial infections and how these responses may increase the risk of allergic diseases [34]. As novel sequencing techniques are emerging the role of the resident respiratory microbiome and its influence on the local immune response can now be studied more efficiently [35, 36].
The very rapid increase in the incidence of allergic diseases over the last 30 years and the differences in the prevalence of an allergic disease between industrialized and developing countries both suggest that something else than variations in human genetics is the cause of this allergic epidemic [5, 6, 37]. These observations led to the generation of the "hygiene hypothesis", i. e, the lack of an early microbial stimulation or an exposure to soil and dirt results in dysregulated immune responses to allergens later in life [6, 38]. The respiratory epithelium is the home to microbiome, which is estimated to be composed of 10 × 1012 microbes, with a diversity of greater than 1,000 species within each individual [39]. The international Human Microbiome Project aims to define the human microbiome from various locations within the body both in health and in various diseases [40, 41]. Several different combinations of local microbes can contribute to the local environment and thus alter the barrier functions of the respiratory epithelium.
Allergen-containing subpollen particles from timothy grass were shown to first bind to and then be internalized by primary epithelial cells in a dose dependent manner. The binding was enhanced with the surfactant protein -D and coincided with the secretion of Interleukin (IL)-8 [42]. Timothy grass pollen allergen Phl p 1 activated respiratory epithelial cells by a non-protease mechanism [43]. Enhancement of TGF-beta, IL-6 and IL-8 induced by Phl p 1 might provide an indirect mechanism through which the allergen may cross the epithelial barrier. Furthermore, recent observations also indicated that timothy grass allergen Phl p 1 is actively transported though the epithelium [44].
Already one minute in vivo challenge with birch pollen caused Bet v 1 binding to conjunctival epithelial surfaces in the allergic, but not in the healthy individuals (Figure 1a) [45]. Bet v 1 entry into the epithelium was evident also in immuno transmission electron microscopy (TEM) in the allergic, but not in the healthy individuals. One minute after the birch pollen perturbation, before the fulminant clinical symptoms, Bet v 1 was found both on the cell surfaces of villae as well as within the villae, in the cytoplasm, in intracellular vesicles, and also in the nuclei of epithelial cells in allergic patients [45]. At the same time there was no Bet v 1 in the conjunctival epithelial cells of the healthy subjects (Cell level, Table 1). In conjunctival biopsies anti-Bet v 1 staining was seen dispersed into the more basal epithelial cell layers (Tissue level, Table 1). These observations support a very rapid traffic through the epithelium in allergic patients, but not in healthy subjects. Currently the putative receptor(s) mediating this allergen binding and facilitating its transport through the epithelium is not known. A striking specificity is observed when birch pollen allergic subjects were also challenged with timothy grass pollen and no entry of this pollen allergen Phl p 1 into epithelial cells was detected (R. Renkonen unpublished data). While the specific transport mechanism for birch pollen remains unsolved the first hints of the role of caveolae in this have been obtained. In the double immunoTEM stainings caveolin 2, but not caveolin 1 or 3, was present on the conjunctival epithelial surface in the same clusters as Bet v 1 (Figure 1). A great majority of anti-Bet v 1 staining in the epithelial cells was colocalized also with another caveolar marker, flotillin 2. Thus the observed Bet v 1 traffic could involve an active lipid raft and caveolar-dependent transport [46].
Bet v 1 binds to and is transported through the conjunctival epithelium only in allergic, but not in healthy subjects. Panel a; A conjunctival epithelial biopsy taken from an allergic patient 1 min after the in vivo birch pollen challenge indicated a clear Bet v 1 location detected with anti- Bet v 1 antibodies (green staining for Bet v 1). Original magnification × 200. Panel b; The strong clustered staining of anti-Bet v 1 detected with gold label particles at the epithelial villus. Bet v 1 was seen to co-localise with caveolin 2 as shown with double immunoTEM using an anti- Bet v 1 10 nm gold-labelled antibody and anti-caveolin 2 5 nm gold-labelled antibody, magnification × 27 500. Bet v 1 was almost solely (75%) co-localised with a caveolar marker protein caveolin 2.
While the above data suggests a caveolar-dependent traffic, the primary contact with the epithelium is still unknown. Small proteins like cholera toxin or much larger cargo like full Pseudomonas bacteria can be transported to and through epithelial cells via lipid raft and caveolar mechanisms [47]. In the gastrointestinal tract food proteins are the targets for lysosomal degradation while selective food allergens are protected from this when they are transported across the intestinal epithelium by their binding to cell surface IgE/CD23 (FcεRII) complexes. The presence of IgE/CD23 on the intestinal epithelium provides means for intact transcytosis for food allergens across the epithelium, and this can lead to classical mast cell-dependent type I anaphylactic allergic reactions [48]. So far this direct interaction with IgE and its low affinity receptor CD23 has been detected only in the intestinal but not in the respiratory epithelium. However, as the transport of pollen allergens through the respiratory epithelium seems to be specific, e.g., our patients allergic to birch pollen do not transport timothy grass allergen and vice versa, it is most likely antibody-dependent. Some other allergens are actively and specifically transported through the respiratory epithelium. Ovalbumin allergen (OVA) is taken up rapidly via the respiratory epithelium both in the nose and lower airways in a rodent model [49]. Also, human respiratory epithelial cells internalize timothy grass pollen allergen, Phl p 1 [44].
Bet v 1 associated epithelial caveolar proteins
Microscopy provided evidence for the novel hypothesis of active allergen transport. This led us to clarify whether the Bet v 1-associated epithelial proteins act as putative receptors in this intraepithelial traffic. With the help the covalently matrix-bound Bet v 1 antigen and nasal epithelial protein lysates collected from both healthy and allergic subjects we began to identify these interacting proteins. By extensive analyses applying both database-related and de novo identifications we generated a shortlist of Bet v 1-binding epithelial proteins found only in the allergic patients. Six out of these sixteen proteins (40%) had previously been identified as caveolar proteins: ACTG, ANXA2, CALM, KCNA5, PLEC1, STML2 (Table 2, [46]). While the entire human proteome consists of less than 1% of caveolar proteins, the Bet v 1 associated list of proteins was highly enriched with the caveolar proteins. This observation provided independent support for the observation that Bet v 1 could be transported actively through the epithelium within caveolae at the onset of an allergic reaction.
Bet v 1 associated epithelial caveolar lipids
The primary contact between the allergen and the epithelium can be mediated by lipids. Bet v 1 has been shown to bind cholesterol and enter the epithelium of allergic patients in cholesterol- and glycolipid-rich caveolae [50–52]. We performed 3-D modeling of Bet v 1 and its possible lipid ligands. We modeled a large group of putative lipid ligands for Bet v 1 on the atomic level with a computational ligand docking using the CDOCKER algorithm, which is based on the CHARMm force field engine [53].
We conducted 3-D docking with both experimentally verified Bet v 1 ligands as well as with larger lipid molecules for which experimental affinity studies were not available. In silico data suggested that Bet v 1 can bind also to more complex amphiphilic molecules like ceramides, sphingomyelins and even glycolipids, all of which are present on lipid rafts and caveolar surfaces [53]. Thus a binding model was drafted, where the hydrophobic tail groups of lipids are located in the central Y-shaped cavity of the Bet v 1 molecule, while the polar head group points out from the molecule. Taken together, these data provide a hypothesis on how Bet v 1 could directly interact with epithelial surface lipids [53]. It remains to be verified experimentally whether any of these lipids actually participate in the birch pollen uptake on the epithelial surfaces during the early phases of allergic reactions.
Allergen induced responses on the epithelium
A hypothesis-free approach in order to analyze the differences between respiratory epithelial swabs from allergic and healthy subjects during the asymptomatic winter period [45, 46, 54], 182 significantly differentially expressed transcripts (and thus proteins after converting them to corresponding proteins) in between these two groups could be detected. Twenty-two epithelial cell surface receptors displayed enhanced expression levels in allergic compared to healthy subjects and eight out of them were found in lipid rafts/caveolae according to manual PubMed literature search (Table 3, [45]). The kinetics of the Bet v 1 caveolar traffic resembles in many ways other previously known caveolar-dependent phenomena, such as the entry of bacterial toxins, whole viruses, bacteria or even parasites [55–61].
In another approach the response of epithelium to the birch pollen challenge was analyzed. Nasal epithelial cell swabs were collected from both allergic and healthy subjects in winter (no symptoms) and during the birch pollen season in May when allergic patients suffer from clinical symptoms. The allergic subjects displayed 331 transcripts between these two time points [46]. Surprisingly the healthy subjects displayed a much stronger response, while they remained without any clinical symptoms through the experiment. Out of the 605 transcripts recorded as altered in healthy controls, the Gene Ontology (GO) category Immune response was the most significantly upregulated. This immune response was not present in allergic subjects giving us the first hint to that allergy might be, at least partially, caused by lack of protective immune defense at the epithelial surfaces [46]. These results suggest that to truly learn about the pathogenesis of diseases one must analyze also how the healthy subjects respond.
A precise time-series analysis was performed with allergic patients and healthy control subjects. The first nasal epithelial specimens were obtained in February when all subjects were symptom-free and the following four nasal epithelial cell swabs were collected from each subject at weekly intervals starting in mid-April when the birch pollen season in Finland begins. While the healthy subjects remained totally symptomless throughout this whole period of time the allergic subjects began to have symptoms already in April, which worsened along with the birch pollen season [54].
Patients allergic to birch pollen showed 105 uniquely regulated nasal epithelial transcripts (converted to proteins for further analyses) with increasing clinical symptoms. Dyneins were among the most enriched Gene Ontology (GO) categories in the list of proteins. Dyneins are molecular motors for the epithelial intracellular caveolar cargo traffic along the microtubuli and ciliary movements [54].
The epithelial cells collected from healthy subjects displayed 22 unique and significantly altered transcripts during the birch pollen season when compared to the winter season. Forty percent of these uniquely regulated genes, i.e., CXCL6, CXCL9, CXCR2, CXL10, FPR1, IL1 and IL8, NKG7, and PGSG belonged to the Gene Ontology category Immune response (GO:0006955). This Immune response category contains altogether only few percent of all annotated proteins of the human proteome (amigo.geneontology.org). Thus the birch pollen allergen induced a strong epithelial immune response in healthy subjects, while this response was lacking from the epithelium of allergic patients [54]. It is remarkable that a similar immunoresponse has been documented on primary human nasal epithelial cells isolated form healthy subjects after HMD challenge and that this response is lacking from the epithelium of allergic subjects. Here also the number of modified transcripts was significantly larger in healthy epithelium that in the allergic (555 compared to 301 probe sets). Many transcripts remain unaffected by the HDM-exposure in the allergic epithelium while these same transcripts were modified by HDM in the healthy epithelium. Genes involved in immunoresponse and with this expression pattern are: IL-1b, IL-8, CXCL2, CXCL3, CCL20, CTGF, HBEGF, and AREG [14, 62, 63].
How does the epithelium detect danger and respond to it? There are extensive recent reviews on the topic [14, 64]. In short the respiratory epithelium continuously senses the changes in its environment. There are several families of receptors to carry out this including eleven human Toll-like receptors (TLR1- 11). They are all membrane bound receptors which mediate effects through dimerization and complex downstream signaling [65]. Other receptors involved in the initiation of epithelial innate immunity include the families protease-activated receptors (PAR1-4), NOD and leucine-rich repeats containing receptors (NLRs) including NOD1 and NOD2 [14, 64]. Although a great number of research data has been published on this field with a special reference to the anti microbial defense the true role of this arm on immunology in the development of allergies and asthma remains to be solved [66, 67].
Conclusions
Could allergy be either a epithelial disease or a disease of the immune system? So far it seems that both answers are correct. There is a wealth of immunological studies indicating an altered regulation of IgE production, Th2-response, and other hyperimmune responses, which we do not discuss further in this Review. The role of the epithelial barrier function in allergies has recently also been identified. Filaggrin mutations have been identified constantly in atopic dermatitis and this provoked increasing interest in studies trying to identify the epithelial barrier dysfunction in allergic diseases [68, 69]. It is also interesting that mutations in the filaggrin gene not only cause atopic dermatitis, but also lead to a significantly increased risk for other allergic diseases and asthma in the context of eczema [70]. The defective airway epithelial barrier functions together with disrupted tight junctions and innate immunity are also encountered in asthma patients. This could explain the significant impact of viruses and bacteria in acute asthma exacerbations [71, 72]. The expensive genome-wide association (GWA) studies have yielded several hypothesis-free candidate genes with known or suspected links to epithelial barrier functions for further analyses in allergic diseases [73, 74]. Finally novel approaches pursuing to measure epithelial proteomics [75] and lipidomics [76] will become possible in near future thus allowing us to broaden the DNA and mRNA based GWA analyses.
Taken together, new data has been generated on the role of modified epithelial barrier functions in the early phases of allergic diseases. Active transport of allergens through the epithelium might be incorporated to the pathogenesis of allergy. It is possible that the healthy epithelium displays a strong immune response against pollen allergens and thus escapes from becoming allergic. This challenges the concept of hypersensitivity as the only driving force behind allergic reactions. In fact, if allergy turns out to be, at least in part, a result of epithelial hyposensitivity, it could have major consequences in the strategies of prevention and treatment of these diseases. Towards this end, a national allergy program was launched in Finland in 2008, which changes the basic idea of trying to avoid allergens to the concept of natural exposure and tolerance [6].
References
Bohle B: The impact of pollen-related food allergens on pollen allergy. Allergy. 2007, 62: 3-10.
D'Amato G, Cecchi L, Bonini S, Nunes C, Annesi-Maesano I, Behrendt H, Liccardi G, Popov T, van Cauwenberge P: Allergenic pollen and pollen allergy in Europe. Allergy. 2007, 62: 976-990. 10.1111/j.1398-9995.2007.01393.x.
Larche M, Akdis CA, Valenta R: Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006, 6: 761-771. 10.1038/nri1934.
Ring J, Grosber M, Mohrenschlager M, Brockow K: Anaphylaxis: acute treatment and management. Chem Immunol Allergy. 2010, 95: 201-210.
Bousquet J, Burney PG, Zuberbier T, Cauwenberge PV, Akdis CA, Bindslev-Jensen C, Bonini S, Fokkens WJ, Kauffmann F, Kowalski ML, Lodrup-Carlsen K, Mullol J, Nizankowska-Mogilnicka E, Papadopoulos N, Toskala E, Wickman M, Anto J, Auvergne N, Bachert C, Bousquet PJ, Brunekreef B, Canonica GW, Carlsen KH, Gjomarkaj M, Haahtela T, Howarth P, Lenzen G, Lotvall J, Radon K, Ring J: GA2LEN (Global Allergy and Asthma European Network) addresses the allergy and asthma 'epidemic'. Allergy. 2009, 64: 969-977. 10.1111/j.1398-9995.2009.02059.x.
Haahtela T, von Hertzen L, Makela M, Hannuksela M: Finnish Allergy Programme 2008-2018--time to act and change the course. Allergy. 2008, 63: 634-645. 10.1111/j.1398-9995.2008.01712.x.
Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA: Role of Treg in immune regulation of allergic diseases. Eur J Immunol. 2010, 40: 1232-1240. 10.1002/eji.200940045.
Ray A, Khare A, Krishnamoorthy N, Qi Z, Ray P: Regulatory T cells in many flavors control asthma. Mucosal Immunol. 2010, 3: 216-229. 10.1038/mi.2010.4.
Davies DE: The role of the epithelium in airway remodeling in asthma. Proc Am Thorac Soc. 2009, 6: 678-682. 10.1513/pats.200907-067DP.
Holgate ST: Has the time come to rethink the pathogenesis of asthma?. Curr Opin Allergy Clin Immunol. 2010, 10: 48-53. 10.1097/ACI.0b013e3283347be5.
Schleimer RP, Kato A, Peters A, Conley D, Kim J, Liu MC, Harris KE, Kuperman DA, Chandra R, Favoreto S, Avila PC, Grammer LC, Kern RC: Epithelium, inflammation, and immunity in the upper airways of humans: studies in chronic rhinosinusitis. Proc Am Thorac Soc. 2009, 6: 288-294. 10.1513/pats.200808-088RM.
Groschwitz KR, Hogan SP: Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009, 124: 3-20. 10.1016/j.jaci.2009.05.038. quiz 21-22
Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H: The Danger Signal, Extracellular ATP, Is a Sensor for an Airborne Allergen and Triggers IL-33 Release and Innate Th2-Type Responses. J Immunol. 2011, 186: 4375-4387. 10.4049/jimmunol.1003020.
Vroling AB, Fokkens WJ, van Drunen CM: How epithelial cells detect danger: aiding the immune response. Allergy. 2008, 63: 1110-1123. 10.1111/j.1398-9995.2008.01785.x.
Comeau MR, Ziegler SF: The influence of TSLP on the allergic response. Mucosal Immunol. 2010, 3: 138-147. 10.1038/mi.2009.134.
Bulek K, Swaidani S, Aronica M, Li X: Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010, 88: 257-268. 10.1038/icb.2009.113.
Godoy L: Allergic inflammation: where epithelial function interacts with immune response in atopic diseases. Drug News Perspect. 2009, 22: 233-236. 10.1358/dnp.2009.22.4.1368087.
Hammad H, Lambrecht BN: Dendritic cells and airway epithelial cells at the interface between innate and adaptive immune responses. Allergy. 2011, 66: 579-587. 10.1111/j.1398-9995.2010.02528.x.
Broide DH: Allergic rhinitis: Pathophysiology. Allergy Asthma Proc. 2010, 31: 370-374. 10.2500/aap.2010.31.3388.
Turvey SE, Broide DH: Innate immunity. J Allergy Clin Immunol. 2010, 125: S24-32. 10.1016/j.jaci.2009.07.016.
Aslam A, Lloyd-Lavery A, Warrell DA, Misbah S, Ogg GS: Common filaggrin null alleles are not associated with hymenoptera venom allergy in europeans. Int Arch Allergy Immunol. 2011, 154: 353-355. 10.1159/000321829.
Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, Northstone K, Henderson J, Alizadehfar R, Ben-Shoshan M, Morgan K, Roberts G, Masthoff LJ, Pasmans SG, van den Akker PC, Wijmenga C, Hourihane JO, Palmer CN, Lack G, Clarke A, Hull PR, Irvine AD, McLean WH: Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011, 127: 661-667. 10.1016/j.jaci.2011.01.031.
Zheng T, Yu J, Oh MH, Zhu Z: The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011, 3: 67-73. 10.4168/aair.2011.3.2.67.
Compalati E, Ridolo E, Passalacqua G, Braido F, Villa E, Canonica GW: The link between allergic rhinitis and asthma: the united airways disease. Expert Rev Clin Immunol. 2010, 6: 413-423.
Guilemany JM, Angrill J, Alobid I, Centellas S, Pujols L, Bartra J, Bernal-Sprekelsen M, Valero A, Picado C, Mullol J: United airways again: high prevalence of rhinosinusitis and nasal polyps in bronchiectasis. Allergy. 2009, 64: 790-797. 10.1111/j.1398-9995.2008.01892.x.
Hellings PW, Prokopakis EP: Global airway disease beyond allergy. Curr Allergy Asthma Rep. 2010, 10: 143-149. 10.1007/s11882-010-0107-1.
Jacquet A: Interactions of airway epithelium with protease allergens in the allergic response. Clin Exp Allergy. 2011, 41: 305-311. 10.1111/j.1365-2222.2010.03661.x.
Mari A, Rasi C, Palazzo P, Scala E: Allergen databases: current status and perspectives. Curr Allergy Asthma Rep. 2009, 9: 376-383. 10.1007/s11882-009-0055-9.
Turi GJ, Ellis R, Wattie JN, Labiris NR, Inman MD: The effects of inhaled house dust mite on airway barrier function and sensitivity to inhaled methacholine in mice. Am J Physiol Lung Cell Mol Physiol. 2011, 300: L185-190. 10.1152/ajplung.00271.2010.
Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN: House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009, 15: 410-416. 10.1038/nm.1946.
Hongjia L, Qingling G, Meiying L, Weixuan W, Lihong Z, Yongsheng G, Yanli L, Jinxiang W, Liang D: House dust mite regulate the lung inflammation of asthmatic mice through TLR4 pathway in airway epithelial cells. Cell Biochem Funct. 2010, 28: 597-603. 10.1002/cbf.1697.
Osterlund C, Gronlund H, Polovic N, Sundstrom S, Gafvelin G, Bucht A: The non-proteolytic house dust mite allergen Der p 2 induce NF-kappaB and MAPK dependent activation of bronchial epithelial cells. Clin Exp Allergy. 2009, 39: 1199-1208. 10.1111/j.1365-2222.2009.03284.x.
Gangl K, Reininger R, Bernhard D, Campana R, Pree I, Reisinger J, Kneidinger M, Kundi M, Dolznig H, Thurnher D, Valent P, Chen KW, Vrtala S, Spitzauer S, Valenta R, Niederberger V: Cigarette smoke facilitates allergen penetration across respiratory epithelium. Allergy. 2009, 64: 398-405. 10.1111/j.1398-9995.2008.01861.x.
Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, Woyke T, Allgaier M, Bristow J, Wiener-Kronish JP, Sutherland ER, King TS, Icitovic N, Martin RJ, Calhoun WJ, Castro M, Denlinger LC, Dimango E, Kraft M, Peters SP, Wasserman SI, Wechsler ME, Boushey HA, Lynch SV: Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011, 127: 372-381. 10.1016/j.jaci.2010.10.048. e371-373
Lee YK, Mazmanian SK: Has the microbiota played a critical role in the evolution of the adaptive immune system?. Science. 2010, 330: 1768-1773. 10.1126/science.1195568.
Round JL, O'Connell RM, Mazmanian SK: Coordination of tolerogenic immune responses by the commensal microbiota. J Autoimmun. 2010, 34: J220-225. 10.1016/j.jaut.2009.11.007.
Bousquet J, Bieber T, Fokkens W, Kowalski M, Humbert M, Niggemann B, Simon HU, Cruz AA, Haahtela T: In Allergy, 'A new day has begun'. Allergy. 2008, 63: 631-633. 10.1111/j.1398-9995.2008.01730.x.
Okada H, Kuhn C, Feillet H, Bach JF: The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010, 160: 1-9.
Huffnagle GB: The microbiota and allergies/asthma. PLoS Pathog. 2010, 6: e1000549-10.1371/journal.ppat.1000549.
Hsiao WW, Fraser-Liggett CM: Human Microbiome Project--paving the way to a better understanding of ourselves and our microbes. Drug Discov Today. 2009, 14: 331-333. 10.1016/j.drudis.2009.03.001.
Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C, Baker CC, Di Francesco V, Howcroft TK, Karp RW, Lunsford RD, Wellington CR, Belachew T, Wright M, Giblin C, David H, Mills M, Salomon R, Mullins C, Akolkar B, Begg L, Davis C, Grandison L, Humble M, Khalsa J, Little AR: The NIH Human Microbiome Project. Genome Res. 2009, 19: 2317-2323.
Schleh C, Erpenbeck VJ, Winkler C, Lauenstein HD, Nassimi M, Braun A, Krug N, Hohlfeld JM: Allergen particle binding by human primary bronchial epithelial cells is modulated by surfactant protein D. Respir Res. 2010, 11: 83-10.1186/1465-9921-11-83.
Röschmann K, Farhat K, König P, Suck R, Ulmer A, Petersen A: Timothy grass pollen major allergen Phl p 1 activates respiratory epithelial cells by a non-protease mechanism. Clin Exp Allergy. 2009,
Blume C, Foerster S, Gilles S, Becker WM, Ring J, Behrendt H, Petersen A, Traidl-Hoffmann C: Human epithelial cells of the respiratory tract and the skin differentially internalize grass pollen allergens. J Invest Dermatol. 2009, 129: 1935-1944. 10.1038/jid.2008.459.
Renkonen J, Mattila P, Lehti S, Mäkinen J, Sormunen R, Tervo T, Paavonen T, Renkonen R: Birch pollen allergen Bet v 1 bind to and travels through conjunctival epithelium in allergic patients. Allergy. 2009, 64: 868-875. 10.1111/j.1398-9995.2008.01919.x.
Joenvaara S, Mattila P, Renkonen J, Makitie A, Toppila-Salmi S, Lehtonen M, Salmi P, Lehti S, Makinen J, Sormunen R, Paavonen T, Renkonen R: Caveolar transport through nasal epithelium of birch pollen allergen Bet v 1 in allergic patients. J Allergy Clin Immunol. 2009, 124: 135-142. 10.1016/j.jaci.2008.11.048. e131-121
Zaas DW, Swan ZD, Brown BJ, Li G, Randell SH, Degan S, Sunday ME, Wright JR, Abraham SN: Counteracting signaling activities in lipid rafts associated with the invasion of lung epithelial cells by Pseudomonas aeruginosa. J Biol Chem. 2009, 284: 9955-9964. 10.1074/jbc.M808629200.
Yu LC: The epithelial gatekeeper against food allergy. Pediatr Neonatol. 2009, 50: 247-254. 10.1016/S1875-9572(09)60072-3.
Hens G, Bobic S, Reekmans K, Ceuppens JL, Hellings PW: Rapid systemic uptake of allergens through the respiratory mucosa. J Allergy Clin Immunol. 2007, 120: 472-474. 10.1016/j.jaci.2007.03.047.
Markovic-Housley Z, Degano M, Lamba D, von Roepenack-Lahaye E, Clemens S, Susani M, Ferreira F, Scheiner O, Breiteneder H: Crystal structure of a hypoallergenic isoform of the major birch pollen allergen Bet v 1 and its likely biological function as a plant steroid carrier. J Mol Biol. 2003, 325: 123-133. 10.1016/S0022-2836(02)01197-X.
Mogensen J, Wimmer R, Larsen J, Spangfort M, Otzen D: The major birch allergen, Bet v 1, shows affinity for a broad spectrum of physiological ligands. J Biol Chem. 2002, 277: 23684-23692. 10.1074/jbc.M202065200.
Mogensen JE, Ferreras M, Wimmer R, Petersen SV, Enghild JJ, Otzen DE: The major allergen from birch tree pollen, Bet v 1, binds and permeabilizes membranes. Biochemistry. 2007, 46: 3356-3365. 10.1021/bi062058h.
Mattila K, Renkonen R: Modelling of Bet v 1 binding to lipids. Scand J Immunol. 2009, 70: 116-124. 10.1111/j.1365-3083.2009.02277.x.
Mattila P, Renkonen J, Toppila-Salmi S, Parviainen V, Joenvaara S, Alff-Tuomala S, Nicorici D, Renkonen R: Time-series nasal epithelial transcriptomics during natural pollen exposure in healthy subjects and allergic patients. Allergy. 2010, 65: 175-183. 10.1111/j.1398-9995.2009.02181.x.
Barrias ES, Dutra JM, De Souza W, Carvalho TM: Participation of macrophage membrane rafts in Trypanosoma cruzi invasion process. Biochem Biophys Res Commun. 2007, 363: 828-834. 10.1016/j.bbrc.2007.09.068.
Cantin C, Holguera J, Ferreira L, Villar E, Munoz-Barroso I: Newcastle disease virus may enter cells by caveolae-mediated endocytosis. J Gen Virol. 2007, 88: 559-569. 10.1099/vir.0.82150-0.
Damm EM, Pelkmans L: Systems biology of virus entry in mammalian cells. Cell Microbiol. 2006, 8: 1219-1227. 10.1111/j.1462-5822.2006.00745.x.
Kowalski MP, Dubouix-Bourandy A, Bajmoczi M, Golan DE, Zaidi T, Coutinho-Sledge YS, Gygi MP, Gygi SP, Wiemer EA, Pier GB: Host resistance to lung infection mediated by major vault protein in epithelial cells. Science. 2007, 317: 130-132. 10.1126/science.1142311.
Marsh M, Helenius A: Virus entry: open sesame. Cell. 2006, 124: 729-740. 10.1016/j.cell.2006.02.007.
Moriyama T, Marquez JP, Wakatsuki T, Sorokin A: Caveolar endocytosis is critical for BK virus infection of human renal proximal tubular epithelial cells. J Virol. 2007, 81: 8552-8562. 10.1128/JVI.00924-07.
Parr RD, Storey SM, Mitchell DM, McIntosh AL, Zhou M, Mir KD, Ball JM: The rotavirus enterotoxin NSP4 directly interacts with the caveolar structural protein caveolin-1. J Virol. 2006, 80: 2842-2854. 10.1128/JVI.80.6.2842-2854.2006.
Vroling AB, Jonker MJ, Luiten S, Breit TM, Fokkens WJ, van Drunen CM: Primary Nasal Epithelium Exposed to House Dust Mite Extract Shows Activated Expression in Allergic Individuals. Am J Respir Cell Mol Biol. 2008, 38: 293-299.
Vroling AB, Jonker MJ, Breit TM, Fokkens WJ, van Drunen CM: Comparison of expression profiles induced by dust mite in airway epithelia reveals a common pathway. Allergy. 2008, 63: 461-467. 10.1111/j.1398-9995.2007.01621.x.
van Drunen CM, Vroling AB, Rinia AB, Fokkens WJ: Considerations on the application of microarray analysis in rhinology. Rhinology. 2008, 46: 259-266.
Tesse R, Pandey RC, Kabesch M: Genetic variations in toll-like receptor pathway genes influence asthma and atopy. Allergy. 2011, 66: 307-316. 10.1111/j.1398-9995.2010.02489.x.
Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, Heederik D, Piarroux R, von Mutius E: Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011, 364: 701-709. 10.1056/NEJMoa1007302.
Ege MJ, Strachan DP, Cookson WO, Moffatt MF, Gut I, Lathrop M, Kabesch M, Genuneit J, Buchele G, Sozanska B, Boznanski A, Cullinan P, Horak E, Bieli C, Braun-Fahrlander C, Heederik D, von Mutius E: Gene-environment interaction for childhood asthma and exposure to farming in Central Europe. J Allergy Clin Immunol. 2011, 127: 138-144. 10.1016/j.jaci.2010.09.041. 144 e131-134
Barnes KC: An update on the genetics of atopic dermatitis: scratching the surface in 2009. J Allergy Clin Immunol. 2010, 125: 16-29. 10.1016/j.jaci.2009.11.008. e11-11; quiz 30-11
Holloway JW, Yang IA, Holgate ST: Genetics of allergic disease. J Allergy Clin Immunol. 2010, 125: S81-94. 10.1016/j.jaci.2009.10.071.
Weidinger S, O'Sullivan M, Illig T, Baurecht H, Depner M, Rodriguez E, Ruether A, Klopp N, Vogelberg C, Weiland SK, McLean WH, von Mutius E, Irvine AD, Kabesch M: Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J Allergy Clin Immunol. 2008, 121: 1203-1209. 10.1016/j.jaci.2008.02.014. e1201
Busse WW, Lemanske RF, Gern JE: Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010, 376: 826-834. 10.1016/S0140-6736(10)61380-3.
Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, Bonini S, Bont L, Bossios A, Bousquet J, Braido F, Brusselle G, Canonica GW, Carlsen KH, Chanez P, Fokkens WJ, Garcia-Garcia M, Gjomarkaj M, Haahtela T, Holgate ST, Johnston SL, Konstantinou G, Kowalski M, Lewandowska-Polak A, Lodrup-Carlsen K, Makela M, Malkusova I, Mullol J, Nieto A, Eller E: Viruses and bacteria in acute asthma exacerbations - A GA(2) LEN-DARE* systematic review. Allergy. 2010
Swarr DT, Hakonarson H: Unraveling the complex genetic underpinnings of asthma and allergic disorders. Curr Opin Allergy Clin Immunol. 2010, 10: 434-442. 10.1097/ACI.0b013e32833da71d.
Wang H, Chavali S, Mobini R, Muraro A, Barbon F, Boldrin D, Aberg N, Benson M: A pathway-based approach to find novel markers of local glucocorticoid treatment in intermittent allergic rhinitis. Allergy. 2011, 66: 132-140. 10.1111/j.1398-9995.2010.02444.x.
Broccardo CJ, Mahaffey S, Schwarz J, Wruck L, David G, Schlievert PM, Reisdorph NA, Leung DY: Comparative proteomic profiling of patients with atopic dermatitis based on history of eczema herpeticum infection and Staphylococcus aureus colonization. J Allergy Clin Immunol. 2011, 127: 186-193. 10.1016/j.jaci.2010.10.033. 193 e181-111
Sampaio JL, Gerl MJ, Klose C, Ejsing CS, Beug H, Simons K, Shevchenko A: Membrane lipidome of an epithelial cell line. Proc Natl Acad Sci USA. 2011, 108: 1903-1907. 10.1073/pnas.1019267108.
Acknowledgements
The original work in the Renkonen laboratory referred here was supported in part by research grants from Academy of Finland, Sigrid Juselius Foundation, and Helsinki University Central Hospital Research Funds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors drafted and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mattila, P., Joenväärä, S., Renkonen, J. et al. Allergy as an epithelial barrier disease. Clin Transl Allergy 1, 5 (2011). https://doi.org/10.1186/2045-7022-1-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2045-7022-1-5