Abstract
Background
The amygdala and medial prefrontal cortex (mPFC) comprise a key corticolimbic circuit that helps shape individual differences in sensitivity to threat and the related risk for psychopathology. Although serotonin (5-HT) is known to be a key modulator of this circuit, the specific receptors mediating this modulation are unclear. The colocalization of 5-HT1A and 5-HT2A receptors on mPFC glutamatergic neurons suggests that their functional interactions may mediate 5-HT effects on this circuit through top-down regulation of amygdala reactivity. Using a multimodal neuroimaging strategy in 39 healthy volunteers, we determined whether threat-related amygdala reactivity, assessed with blood oxygen level-dependent functional magnetic resonance imaging, was significantly predicted by the interaction between mPFC 5-HT1A and 5-HT2A receptor levels, assessed by positron emission tomography.
Results
5-HT1A binding in the mPFC significantly moderated an inverse correlation between mPFC 5-HT2A binding and threat-related amygdala reactivity. Specifically, mPFC 5-HT2A binding was significantly inversely correlated with amygdala reactivity only when mPFC 5-HT1A binding was relatively low.
Conclusions
Our findings provide evidence that 5-HT1A and 5-HT2A receptors interact to shape serotonergic modulation of a functional circuit between the amygdala and mPFC. The effect of the interaction between mPFC 5-HT1A and 5-HT2A binding and amygdala reactivity is consistent with the colocalization of these receptors on glutamatergic neurons in the mPFC.
Similar content being viewed by others
Background
Research in human and non-human animal models implicates a corticolimbic circuitry composed of structural and functional connections between the amygdala and regions of the medial prefrontal cortex (mPFC) including the anterior cingulate cortex (ACC) in generating and regulating behavioral and physiological responses to threat-related stimuli [1–4]. Regions of the mPFC are crucially involved in the integration and subsequent regulation of stimulus-driven amygdala response, partly via glutamatergic projections to populations of GABAergic neurons within the amygdala [5–7]. Variability in the structure and function of this corticolimbic circuitry has been associated with interindividual differences in personality measures, reflecting sensitivity to environmental threat and related risk for psychopathology [2, 8–11].
Serotonin (5-hydroxytryptamine, 5-HT) exerts potent modulatory effects on mood, affect, and responsiveness to stress and threat [12]. Neuroimaging studies in humans have mapped interindividual differences in amygdala reactivity to biologically salient environmental stimuli (for example, facial expressions of threat) onto variability in 5-HT signaling within this corticolimbic circuitry [2, 13–21]. However, the role of specific 5-HT-receptor signaling pathways in mediating these effects are not fully understood [12]. Previous work in humans using positron emission tomography (PET) has implicated 5-HT1A and 5-HT2A receptors in modulating mood, affect and threat responsiveness, and in the corticolimbic circuitry supporting these behaviors [22–26]. Importantly, the anatomical localization of these two receptors within prefrontal cortex positions them to mediate effectively the observed effects of 5-HT signaling on corticolimbic circuit dynamics.
In the mPFC, the excitatory 5-HT2A and inhibitory 5-HT1A receptors are colocalized on glutamatergic pyramidal neurons [27]. The 5-HT2A receptor is specifically localized to proximal portions of apical dendrites [28, 29], where convergent inputs are integrated, and is therefore positioned to facilitate mPFC function through second-messenger signaling cascades, resulting in membrane depolarization [27, 30]. By contrast, the 5-HT1A receptor is localized to the initial segment of the axon, where action potentials are typically generated [27–29, 31–34], thus this receptor is positioned to exert an inhibitory effect on mPFC function through 'gating' glutamatergic output via membrane hyperpolarization. Collectively, these two receptors are crucially positioned to mediate effects of 5-HT on glutamatergic neuronal activity and mPFC function, including top-down regulation of amygdala reactivity [27, 35].
In one previous study, we identified an inverse correlation between mPFC 5-HT2A binding and threat-related amygdala reactivity [14]. In another, we reported that 5-H1A autoreceptor binding in the dorsal raphe nucleus was inversely correlated with amygdala reactivity [15]. However, the effects of mPFC 5-HT1A binding on threat-related amygdala reactivity were not explored in either of these studies. More importantly, whether mPFC 5-HT1A binding moderates the previously observed inverse association between mPFC 5-HT2A binding and threat-related amygdala reactivity, as suggested by the aforementioned colocalization of these receptors within the mPFC, has not yet been determined.
In the current study we explored this hypothetical functional interaction using multimodal PET/functional magnetic resonance imaging (fMRI) neuroimaging data in a sample of 39 healthy adult volunteers that partially overlaps with those of our two previous reports [14, 15]. We hypothesized that mPFC 5-HT1A binding would be positively correlated with threat-related amygdala reactivity, reflecting the inhibitory effects of the 5-HT1A receptor on prefrontal pyramidal neurons, which are positioned to exert an inhibitory effect on the amygdala. Consistent with our previous report, we further hypothesized that mPFC 5-HT2A binding would be inversely correlated with amygdala reactivity. Finally, in light of the molecular interactions predicted from the colocalization of 5-HT1A and 5-HT2A receptors, we hypothesized that mPFC 5-HT1A binding would significantly interact with mPFC 5-HT2A binding, so that 5-HT2A binding would be inversely correlated with amygdala reactivity only at relatively low levels of 5-HT1A binding.
Results
Amygdala reactivity
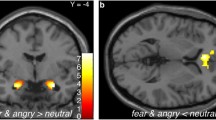
Consistent with previous reports, we observed robust threat-related reactivity in the bilateral amygdala across all participants [36, 37] (Figure 1). The magnitude of right amygdala reactivity, but not left amygdala reactivity, was inversely correlated with age (right amygdala: r2 = 0.19, P = 0.005; left amygdala: r2 = 0.02, P = 0.35). Neither right nor left amygdala reactivity was correlated with gender (r2 values < 0.03, P values > 0.3).
Amygdala reactivity to perceptual processing of fearful and angry facial expressions. Statistical parametric map representing bilateral amygdala clusters exhibiting a significant response to task (faces > shapes; right amygdala: (24, -6, -11), z = 6.28, k = 145 voxels (P < 0.05, corrected); left amygdala: (-18, -7, -15), z = 5.77, k = 146 voxels (P < 0.05, corrected). Color bar indicates t-scores.
Serotonin receptor binding
We focused on quantifying 5-HT1A and 5-HT2A binding within the pregenual and subgenual prefrontal cortex (pgPFC and sgPFC, respectively) because of previous reports supporting an integral structural and functional relationship between the amygdala and these mPFC regions in the context of processing threat that is modulated by 5-HT signaling [1, 2, 6, 14, 38, 39].
Reflecting 5-HT1A binding, we observed specific [11C]WAY100635 binding within both pgPFC (mean ± SD binding potential, non-displaceable (BPND) = 4.32 ± 1.18) and sgPFC (BPND = 4.86 ± 1.41) for all subjects. Reflecting 5-HT2A binding, we observed specific [18F]altanserin binding within both pgPFC (BPND = 1.06 ± 0.37) and sgPFC (BPND = 1.19 ± 0.46). 5-HT1A and 5-HT2A binding between the pgPFC and sgPFC were significantly correlated (5-HT1A BPND: r2 = 0.69, P = 5.87 × 10-11; 5-HT2A BPND: r2 = 0.63, P = 1.80 × 10-9). However, within regions, 5-HT1A binding was not significantly correlated with 5-HT2A binding (pgPFC: r2 = 3.28 × 10-5, P = 0.97; sgPFC: r2 = 0.001, P = 0.88). 5-HT2A binding was significantly inversely correlated with age (pgPFC: r2 = 0.41, P = 1.30 × 10-5; sgPFC: r2 = 0.41, P = 1.29 × 10-5), but 5-HT1A binding did not have a significant correlation with age (pgPFC: r2 = 0.01, P = 0.53, sgPFC: r2 = 0.003, P = 0.73). Neither 5-HT1A nor 5-HT2A binding was significantly correlated with gender (r2 values < 0.01, P values > 0.5).
5-HT1Abinding and amygdala reactivity
Regional 5-HT1A binding was not significantly correlated with amygdala reactivity in either pgPFC nor sgPFC (right amygdala versus pgPFC 5-HT1A BPND: t36 = -0.76, P = 0.94; right amygdala versus sgPFC 5-HT1A BPND: t36 = 0.54, P = 0.60; left amygdala versus pgPFC 5-HT1A BPND: t36 = 0.16, P = 0.88; left amygdala versus sgPFC 5-HT1A BPND: t36 = -0.09, P = 0.93) (Figure 2A,B).
Association between amygdala reactivity and 5-HT 1A BP ND and 5-HT 2A BP ND . (A,B) Plot of non-significant correlation between left and right amygdala reactivity and pgPFC 5-HT1A BPND. (C) Plot of non-significant correlation between left amygdala reactivity and pgPFC 5-HT2A BPND. (D) Plot of significant inverse correlation between right amygdala reactivity and pgPFC 5-HT2A BPND. 5-HT = serotonin; BPND = binding potential, non-displaceable; pgPFC = pregenual prefrontal cortex; sgPFC = subgenual prefrontal cortex.
5-HT2Abinding and amygdala reactivity
Regional 5-HT2A binding was significantly inversely correlated with amygdala reactivity [14]. Specifically, right amygdala reactivity was inversely correlated with 5-HT2A binding within both pgPFC (t36 = -3.44, P = 0.002; Figure 2D) and sgPFC (t36 = -2.49, P = 0.02). There was no significant correlation between left amygdala reactivity and 5-HT2A binding within either pgPFC (t36 = -0.61, P = 0.55; Figure 2C) or sgPFC (t36 = 0.72, P = 0.47). Thus, we focused our analyses on the effects of interaction between 5-HT1A and 5-HT2A binding on right amygdala reactivity.
Interaction between 5-HT1A and 5-HT2Abinding and amygdala reactivity
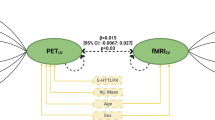
Consistent with our hypothesis, there was a significant interaction between 5-HT1A and 5-HT2A binding in both the pgPFC (t34 = 2.18, P = 0.03) and sgPFC (t34 = 2.72, P = 0.01) in predicting threat-related right amygdala reactivity (Figure 3). Further examination of this interaction effect showed that 5-HT2A binding was significantly inversely correlated with right amygdala reactivity when 5-HT1A binding was < 4.99 (0.6 SDs above the mean) in the pgPFC or < 5.48 (0.4 SDs above the mean) in the sgPFC. It should be noted that the inverse correlations between 5-HT2A binding and right amygdala reactivity remained significant when 5-HT1A binding, the interaction term and age were included in the models (pgPFC: t34 = -3.55, P = 0.001; sgPFC: t34 = -2.72, P = 0.006).
5-HT 1A BP ND significantly moderated the correlation between 5-HT 2A BP ND and right amygdala reactivity. (A) pgPFC 5-HT1A BPND moderated the correlation between pgPFC 5-HT2A BPND and right amygdala reactivity. Lines indicate simple slope between pgPFC 5-HT2A BPND and right amygdala reactivity at three arbitrarily chosen pgPFC 5-HT1A BPND values: low (1 SD below mean (-1 SD), solid black line), mean (equivalent to mean, red dotted line) and high (1 SD above mean (+1 SD), green dotted line). (B) sgPFC 5-HT1A BPND significantly moderated the association between sgPFC 5-HT2A BPND and right amygdala reactivity. Lines indicate simple slope between sgPFC 5-HT2A BPND and right amygdala reactivity at three arbitrarily chosen sgPFC 5-HT1A BPND values: low (-1 SD, solid black line), mean (red dotted line) and high (+1 SD, green dotted line). *Indicates simple slope, P < 0.05; 5-HT = serotonin; a.u. = arbitrary units; BPND = binding potential, non-displaceable; pgPFC = pregenual prefrontal cortex; sgPFC = subgenual prefrontal cortex.
Discussion
Results from our current analyses indicate that the interaction between 5-HT1A and 5-HT2A receptors in the mPFC is crucial for shaping the response of the human amygdala to threat. Specifically, 5-HT2A binding was inversely correlated with threat-related amygdala reactivity, but only when 5-HT1A binding was at mean or relatively low levels. Importantly, these patterns were independent of age and gender, suggesting the general importance and widespread effects that the interaction between mPFC 5-HT1A and 5-HT2A receptors may have on amygdala reactivity. The right lateralized nature of this interaction effect may reflect relatively greater involvement of the right amygdala in the perceptual processing of facial stimuli and, subsequently, greater 5-HT modulation of reactivity in this hemisphere. Although a number of studies have reported asymmetries in monoaminergic modulation of cortical and subcortical circuits [40–43], the biological mechanisms mediating such lateralized effects are difficult to ascertain on the basis of the existing literature.
We explicitly tested for an interaction effect (that is, moderation) between 5-HT1A and 5-HT2A binding because we believe that this represents the most straightforward approach for interpreting how these two systems potentially interact to modulate threat-related amygdala reactivity. Although conceptually and intuitively appealing, we did not employ a metric reflecting the ratio of 5-HT1A and 5-HT2A binding for two reasons: 1) its association with amygdala reactivity would be arbitrarily dependent upon how the ratio term is constructed and 2) testing for the effect of a ratio term (that is, X1 multiplied by the inverse of X2) is essentially a test for an interaction effect in which one of the variables is transformed, which we believe would render interpretation difficult at best. Consequently, we believe our test for an interaction between 5-HT1A and 5-HT2A binding represents the most appropriate and parsimonious statistical test.
These findings are remarkably consistent with the predominant anatomical localization of 5-HT1A and 5-HT2A receptors to the axon hillock and apical dendrites of prefrontal glutamatergic pyramidal neurons, respectively. Given its principal localization on apical dendrites proximal to the soma, the excitatory 5-HT2A receptor is situated to mediate 5-HT depolarization of prefrontal glutamatergic neurons. By contrast, the localization of the inhibitory 5-HT1A receptor to the initial portion of the axon hillock positions it to mediate 5-HT hyperpolarization of these same neurons. Considering the high coexpression of 5-HT1A and 5-HT2A receptors on most prefrontal glutamatergic neurons, this arrangement suggests that the 5-HT1A receptor can effectively (and negatively) gate the depolarizing effects of the 5-HT2A receptors on prefrontal output. In turn, such serotonergic modulation of prefrontal neuron output may shape the capacity of this circuitry to exert an inhibitory effect on amygdala reactivity (Figure 4). We interpret our current findings of an inverse correlation of mPFC 5-HT2A binding with amygdala reactivity but only at mean and low levels of 5-HT1A binding as reflecting the coexpression of these receptors and their role in mediating serotonergic modulation of this circuitry. The absence of a main effect of mPFC 5-HT1A binding on amygdala reactivity is further consistent with this gating model, with the capacity for mPFC 5-HT1A receptors to modulate threat-related amygdala reactivity being dependent upon additional signaling mechanisms such as, but not necessarily limited to, mPFC 5-HT2A receptors. Although interpretation of our findings is consistent with the previously described localization of the 5-HT1A and 5-HT2A receptors within prefrontal cortex, our results reflect only statistical correlation, and do not establish causality. Future studies aimed at establishing a causal link between 5-HT1A and 5-HT2A receptor interactions on prefrontal pyramidal neuron excitability and the response of the amygdala in the context of threat are necessary.
Schematic illustrating mPFC projection neurons that act to regulate amygdala response to threat-related stimuli. 5-HT1A and 5-HT2A in mPFC are positioned to modulate this circuitry by biasing excitability of these mPFC neurons, thereby affecting the capacity to regulate amygdala reactivity. 5-HT = serotonin; mPFC = medial prefrontal cortex; CeL = lateral central nucleus of the amygdala; CeM = medial central nucleus of the amygdala; ITC = intercalated cells.
There is strong evidence suggesting that 5-HT signaling within the amygdala plays an important role in modulating threat-related amygdala reactivity [13, 20, 44], and both 5-HT1A and 5-HT2A receptors are expressed in the amygdala [45–47]. However, we did not observe a significant correlation between either 5-HT1A or 5-HT2A binding in the amygdala and amygdala reactivity (data not shown). Unlike in the mPFC, 5-HT1A and 5-HT2A receptors may be more evenly distributed on both glutamatergic and GABAergic neurons within the amygdala [47, 48]. This potential for both receptor subtypes to cause inhibitory and excitatory modulation of the amygdala complicate efforts to map correlations between estimates of local binding and reactivity in the absence of cell-type specific values, which are beyond the scope of current PET techniques. Finally, additional 5-HT receptor signaling mechanisms within the amygdala, such as the 5-HT3 and 5-HT2C receptors, have been implicated in anxiety-related behavioral phenotypes in animal models, and may have a greater role in mediating the effects of local 5-HT signaling on amygdala function [44, 49–51].
There are important limitations to our study. Our blood oxygen level-dependent (BOLD) fMRI challenge paradigm was explicitly designed to elicit threat-related amygdala reactivity associated with driving behavioral and physiologic arousal in response to environmental stimulation. Our task did not engage any mPFC region involved in regulating amygdala reactivity and overlapping with our PET region of interest (ROI). Thus, we were not able to explore the effects of mPFC 5-HT1A and 5-HT2A binding on mPFC function related to the top-down regulation of amygdala reactivity. Alternative paradigms such as those involving emotion regulation or extinction of conditioned fear responses may help to determine effects of 5-HT1A and 5-HT2A signaling on related mPFC and amygdala reactivity.
BOLD fMRI and PET receptor imaging provide only indirect metrics of amygdala excitation and 5-HT receptor signaling, respectively. The small sample size in our study limited our power to model interaction effects, thus our findings must be interpreted with caution. The interpretation that our findings reflect the interactive effects of 5-HT1A and 5-HT2A receptors on glutamatergic neurons is based on evidence that 1) each of these receptors is predominantly localized to glutamatergic neurons [28, 32], 2) colocalization of 5-HT1A and 5-HT2A receptors within the mPFC is predominantly observed on glutamatergic neurons [27], and 3) projections from the mPFC to the amygdala are composed of glutamatergic neurons [52, 53]. Despite this, the PET technique does not allow identification of binding associated only with neurons that directly innervate the amygdala, thus we could not confirm the causality of this association using methods currently available. Future studies examining these associations in the context of pharmacological challenge (that is, receptor-specific antagonism) could provide more direct evidence implicating the interaction between mPFC 5-HT1A and 5-HT2A receptors in mediating the effects of 5-HT signaling on threat-related amygdala reactivity.
Conclusions
Our current findings provide unique in vivo evidence that 5-HT receptors in the mPFC play an important role in shaping interindividual variability in threat-related amygdala reactivity. Specifically, the data reveal that mPFC 5-HT1A receptors effectively gate the capacity for mPFC 5-HT2A receptors to drive prefrontal pyramidal neuron excitability related to the regulation of threat-related amygdala reactivity. The current work further highlights the effectiveness of multimodal neuroimaging in identifying molecular signaling pathways that modulate neurobiological circuits in humans, and specifically implicates the interaction between mPFC 5-HT1A and 5-HT2A receptors in modulating the response of the human amygdala and possibly mediating the effects of altered 5-HT signaling on mood, affect and related psychopathology.
Methods
The study was approved by the institutional review board of the University of Pittsburgh, and written informed consent was obtained from all participants.
Participants
In total, 39 healthy adult volunteers participated in the study (20 men, 19 women, mean ± SD age 39.1 ± 12.7 years). Subjects were recruited through local advertisements, referrals and ongoing studies. Subjects were generally healthy. Exclusion criteria included 1) current or lifetime mood, anxiety and psychotic disorder as assessed by the Structured Clinical Interview of the fourth edition of the Diagnostic and Statistical Manual (DSM-I) [54], 2) family psychiatric history, 3) history of substance abuse or use of antidepressants, 4) early dementia or mild cognitive impairment according to the Mini Mental State Examination [55], 5) reversed sleep-wake cycle, 6) positive test of urine sample for drugs of abuse assessed on the day of scanning. The association between mPFC 5-HT2A binding and amygdala reactivity has been described previously involving a subset of this cohort (35 people) [14]. Most subjects completed the fMRI and two PET scan sessions on the same day (n = 33). Those subjects who did not complete all three scan sessions on the same day (n = 6) completed them within 1 month.
fMRI
Protocol
The experimental fMRI paradigm consisted of four blocks of a face-processing task interleaved with five blocks of a sensorimotor control task [14, 15]. During the face-processing task, subjects viewed a trio of faces (expressing either anger or fear) and selected one of two faces (bottom) identical to a target face (top). Angry and fearful facial expressions can represent honest indicators of ecologically valid threat, especially that related to conspecific challengers [56]. Based on this, we interpreted the amygdala activation elicited by our task as being threat-related. Subject performance (accuracy and reaction time) was monitored during all scans.
Each sensorimotor control block consisted of six different shapes (circles and vertical and horizontal ellipses) trios. Subjects viewed a shapes trio and selected one of two shapes (bottom) identical to a target shape (top). Each of the six shape trios was presented for 4 seconds with a fixed interstimulus interval (ISI) of 2 seconds, giving a total block length of 36 seconds. Each face-processing block consisted of six face trios, balanced for gender and representing one target affect (angry or fearful) derived from a standard set of pictures of facial affect [57]. Each of the six face trios was presented for 4 seconds with a variable ISI of 2-6 seconds (mean ISI = 4 seconds) for a total block length of 48 seconds. All blocks were preceded by a brief instruction (''Match faces'' or ''Match shapes'') lasting 2 seconds. Total protocol time was 390 seconds.
As we were not interested in neural networks associated with face-specific processing per se, but rather in eliciting a maximal amygdala response across all subjects, we chose not to use neutral faces as control stimuli because neutral faces can be subjectively experienced as affectively laden or ambiguous, and thus engage the amygdala [58, 59].
Acquisition parameters
The acquisition parameters have been described previously [14, 15, 60]. Briefly, each subject was scanned using a head-only scanner (GE Signa 1.5-T; GE Medical Systems, Milwaukee, WI, USA). BOLD functional images were acquired using a reverse spiral sequence covering 28 slices, each 3.8 mm thick, encompassing the entire cerebrum and most of the cerebellum (repetition time (TR) = 2000 ms, echo time (TE) = 35 ms, field of view (FOV) = 240 mm, matrix = 64 × 64, 195 whole-brain volumes acquired). The first two functional volumes acquired were discarded to allow the scanner to reach equilibrium. Scanning parameters were selected to optimize BOLD signal while maintaining enough slices to acquire whole-brain data. Before the acquisition of fMRI data for each subject, localizer scans were acquired and visually inspected for artifacts such as ghosting, and to ensure good signal across the entire volume of acquisition. Before the acquisition of BOLD data, an auto-shimming procedure was conducted in each subject to minimize field inhomogeneities. The fMRI data for all 39 subjects included in this study were cleared of any related problems.
Data analysis
Whole-brain image analysis was completed using the general linear model (GLM) of SPM8 http://www.fil.ion.ucl.ac.uk/spm. Images for each subject were realigned to the first volume in the time series to correct for head motion, spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model (final resolution of functional images = 2 mm isotropic voxels), and smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter, set at 6-mm full-width at half-maximum. Voxel-wise signal intensities were ratio-normalized to the whole-brain global mean. Preprocessed data sets were analyzed using second-level random-effects models that account for both scan-to-scan and participant-to-participant variability to determine task-specific regional responses.
Variability in single-subject whole-brain functional volumes was determined using the software program Artifact Recognition Toolbox http://www.nitrc.org/projects/artifact_detect. Individual whole-brain BOLD fMRI volumes meeting at least one of the following two criteria were excluded from determination of task-specific effects: 1) significant mean volume signal intensity variation (that is, within-volume mean signal greater or less 4 SDs of mean signal of all volumes in time series); and 2) individual volumes with scan-to-scan movement exceeding 2 mm translation or two degrees of rotation in any direction. On average, 2.1 volumes per subject were excluded because of significant variation in mean volume signal intensity (range of volumes excluded per subject = 0-17), and across all subjects, no volumes were excluded because of excessive motion. Only 1% of all volumes were excluded, thus we believe that this approach enhanced our capacity to determine task-specific effects by excluding volumes with substantial variability without compromising our power to detect task-specific effects by excluding a large number of volumes. We believe this method effectively balances the use of available functional neuroimaging data with a reasonable approach towards accounting for effects due to artifacts or movement.
After preprocessing, our GLM, employing canonical hemodynamic response functions, was used to estimate condition-specific and task-specific BOLD activation for each individual (β weights and contrast images, respectively). Individual contrast images (that is, the weighted sum of the β images) were used in second-level random-effects models to determine mean task-specific amygdala reactivity using one-sample t-tests. Group-level effects for our contrast of interest (that is, faces > shapes) were assessed within the amygdala using an ROI constructed from the WFU Pickatlas (version 1.04) [61, 62].
To address the issue of multiple voxel-level comparisons, AlphaSim, a software program within AFNI http://afni.nimh.nih/gov/afni that uses a Monte Carlo simulation method, was used to determine that a voxel-wise statistical threshold of P < 0.05, uncorrected, combined with a cluster extent threshold of k > 56 voxels within our amygdala search volume was sufficiently unlikely (α < 0.05) to have occurred by chance [63]. This threshold was used to assess our main effect of task within the amygdala. Single-subject amygdala-reactivity values for our contrast of interest were extracted from SPM8 using Marsbar (version 0.42) [64]. A sphere of 5 mm radius was centered on the voxel exhibiting the maximal response to our task across all subjects within both the right and left amygdala. Regional 5-HT receptor binding and other variables were regressed against these extracted BOLD values. Neuroimaging data are reported using the coordinate system of Talairach and Tournoux.
General PET methods
Details concerning the MR and PET imaging procedures related to both [11C]WAY100635 and [18F]altanserin are described below, and have also been described previously [14, 15, 65–67] (see previous reports for discussion about the limitations, challenges and methodological attempts to minimize potential artifacts and biases related to these radioligands [25, 67–71]).
Structural MR images (GE Signa 1.5-T scanner) were acquired for each subject using a spoiled-gradient (SPGR) recalled sequence (TR = 25 ms, TE = 5 ms, FOV = 240 mm, slice thickness = 1.5 mm, matrix = 256 × 192) with parameters optimized for contrast between gray matter, white matter and cerebrospinal fluid (CSF).
Catheters were placed in an antecubital vein for radioligand injection and in a radial artery for arterial blood sampling. PET scans were acquired using a PET scanner (ECAT HR+; CTI PET systems, Knoxville, TN) in 3D imaging mode (63 transaxial planes, 2.4 mm thickness, 152 mm FOV). Head movement was minimized by use of a thermoplastic mask immobilization system. A 10 minute transmission scan (rotating 68Ge/68Ga rods) was acquired for attenuation correction of emission data. PET data were further corrected for dead time and scatter.
Each radioligand was administered as a slow bolus over 20 seconds. PET data acquisition and arterial blood sampling was initiated at the start of radioligand injection. The total radioactivity concentration in plasma was determined from approximately 35 0.5-ml hand-drawn blood samples collected over the scanning interval. Additional blood samples were acquired at five to six timepoints during the scan duration for determination of the fraction of the total radioactivity resulting from radiolabeled metabolites of the parent radioligand. Total plasma radioactivity concentration was corrected for radiolabeled metabolites and this 'metabolite-corrected' arterial input function was used for data analysis [69, 71].
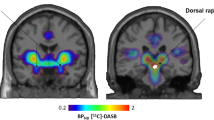
Image reconstruction was performed using filtered back-projection for a final image resolution of about 6 mm. ROIs were drawn on resliced MR images for each subject, and applied to their respective, co-registered PET images (ROIs drawn by SZ and CB). Bilateral ROIs were identified for the sgPFC, pgPFC, amygdala and cerebellum (Figure 5). The cerebellum was used as the reference region for non-displaceable radiotracer uptake (that is, free and nonspecific concentrations, VND) for both [11C]WAY 100635 and [18F]altanserin.
PET data for both radioligands were analyzed using the Logan graphical method [72] to obtain regional volume of distribution values (VT). Regional VT values were used to determine the non-displaceable binding potential, BPND, a measure of specific binding. The BPND is directly proportional to Bavail/Kd, where Bavail is the concentration of receptors available for radiotracer binding (that is, not occupied by endogenous 5-HT), and Kd is the equilibrium dissociation rate constant (that is, inversely related to binding affinity). The PET binding measures were corrected for partial volume effects that arise from atrophy-related CSF dilution using a previously validated two-component MR-based atrophy correction algorithm [66, 73, 74].
[18F]Altanserin specific methods
The radiosynthesis of [18F]altanserin was performed using a modification of the original method [75] that has been used in several studies in our laboratory [14, 67, 76–78]. [18F]Altanserin was administered via intravenous injection (7.23 ± 0.31 mCi), and PET scanning was performed over 90 minutes. The Logan analysis regression was performed over the 12-90 minute post-injection integration intervals (10 points) to obtain regional [18F]altanserin VT and BPND values.
[11C]WAY100635 specific methods
The radiosynthesis of [11C]WAY 100635 was performed as previously described [79], and has been used in several previous studies in our laboratory [15, 65, 71]. [11C]WAY100635 was administered via intravenous injection (14.01 ± 2.10 mCi), and PET scanning was performed over 90 minutes. The Logan analysis regressions were performed over the 14-90 minute post-injection integration interval (13 points) to obtain regional [11C]WAY100635 VT and BPND values.
Regression analyses
The association between threat-related amygdala reactivity and 5-HT1A and 5-HT2A binding was determined using a linear regression analysis between extracted single-subject amygdala BOLD values and ROI-specific 5-HT1A or 5-HT2A binding values in SPSS (version 17.0; SPSS Inc., Chicago, IL, USA). We previously reported within a subset of this cohort that both amygdala reactivity and mPFC 5-HT2A binding are inversely correlated with age [14], and this is consistent with other previous studies [76, 78, 80]. To account for age-related variability in these two measures, age was included as a covariate in all analyses. Consequently, plots indicate the amygdala reactivity values standardized for age effects. These values are the standardized residuals of amygdala reactivity after accounting for effects of age. This procedure was adopted to illustrate more clearly the relationship between regional 5-HT receptor binding and amygdala reactivity, independent of age. The statistics reported reflect the regression analysis results between observed BOLD and binding values including age as a covariate. As gender was not significantly correlated with our neuroimaging data, it was not included in any analyses determining the relationship between prefrontal 5-HT1A or 5-HT2A binding and amygdala reactivity.
The association of the interaction between mPFC 5-HT1A, 5-HT2A binding and threat-related amygdala reactivity was determined using SPSS software and a linear regression model including 5-HT1A binding, 5-HT2A binding, age and the interaction term as covariates. Additional statistics related to the interaction effects were calculated using a previously validated approach http://www.people.ku.edu/~preacher/interact/mlr2.htm that incorporates parameters estimated from our statistical model (for example, regression coefficients, coefficient covariances) [81]. These additional statistics included simple slopes at specified 5-HT1A binding values, significance of simple slopes, and range of 5-HT1A binding values over which association between 5-HT2A binding and amygdala reactivity was significant.
References
Hariri AR, Drabant EM, Weinberger DR: Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biological Psychiatry. 2006, 59 (10): 888-10.1016/j.biopsych.2005.11.005.
Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR: 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005, 8 (6): 828-34. 10.1038/nn1463.
Phelps EA, Delgado MR, Nearing KI, LeDoux JE: Extinction Learning in Humans: Role of the Amygdala and vmPFC. Neuron. 2004, 43 (6): 897-10.1016/j.neuron.2004.08.042.
Quirk GJ, Mueller D: Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008, 33 (1): 56-72. 10.1038/sj.npp.1301555.
Likhtik E, Pelletier JG, Paz R, Pare D: Prefrontal control of the amygdala. J Neurosci. 2005, 25 (32): 7429-37. 10.1523/JNEUROSCI.2314-05.2005.
Quirk GJ, Likhtik E, Pelletier JG, Pare D: Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003, 23 (25): 8800-7.
Sesack SR, Deutch AY, Roth RH, Bunney BS: Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989, 290 (2): 213-42. 10.1002/cne.902900205.
Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL: A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005, 62 (3): 273-81. 10.1001/archpsyc.62.3.273.
Fakra E, Hyde LW, Gorka A, Fisher PM, Munoz KE, Kimak M, Halder I, Ferrell RE, Manuck SB, Hariri AR: Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Arch Gen Psychiatry. 2009, 66 (1): 33-40. 10.1001/archpsyc.66.1.33.
Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, Egan MF, Mattay VS, Weinberger DR, Meyer-Lindenberg A: Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Mol Psychiatry. 2008, 13 (3): 313-24. 10.1038/sj.mp.4002020.
Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J: Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004, 44 (6): 1043-55. 10.1016/j.neuron.2004.12.006.
Holmes A: Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neuroscience & Biobehavioral Reviews. 2008, 32 (7): 1293-1314. 10.1016/j.neubiorev.2008.03.006.
Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR: Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008, 33 (13): 3221-5. 10.1038/npp.2008.52. Epub 2008 May 7
Fisher PM, Meltzer CC, Price JC, Coleman RL, Ziolko SK, Becker C, Moses-Kolko EL, Berga SL, Hariri AR: Medial prefrontal cortex 5-HT2A density is correlated with amygdala reactivity, response habituation, and functional coupling. Cereb Cortex. 2009, bhp022
Fisher PM, Meltzer CC, Ziolko SK, Price JC, Hariri AR: Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nature Neuroscience. 2006, 9 (11): 1362-3. 10.1038/nn1780.
Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR: Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002, 297 (5580): 400-3. 10.1126/science.1071829.
Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C: Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005, 8 (1): 20-1. 10.1038/nn1366.
Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR: A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000, 47 (7): 643-9. 10.1016/S0006-3223(99)00171-7.
Munafo MR, Brown SM, Hariri AR: Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008, 63 (9): 852-7. 10.1016/j.biopsych.2007.08.016.
Rhodes RA, Murthy NV, Dresner MA, Selvaraj S, Stavrakakis N, Babar S, Cowen PJ, Grasby PM: Human 5-HT transporter availability predicts amygdala reactivity in vivo. J Neurosci. 2007, 27 (34): 9233-9237. 10.1523/JNEUROSCI.1175-07.2007.
Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM: Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biological Psychiatry. 2006, 59 (9): 816-10.1016/j.biopsych.2005.10.015.
Frokjaer VG, Mortensen EL, Nielsen FÅ, Haugbol S, Pinborg LH, Adams KH, Svarer C, Hasselbalch SG, Holm S, Paulson OB, Knudsen GM: Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biological Psychiatry. 2008, 63 (6): 569-10.1016/j.biopsych.2007.07.009.
Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby P: Increased 5-HT2A receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [11C]MDL 100,907. Am J Psychiatry. 2006, 163 (9): 1580-1587. 10.1176/appi.ajp.163.9.1580.
Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S: Inverse relationship between serotonin 5-HT(1A) receptor binding and anxiety: a [(11)C]WAY-100635 PET investigation in healthy volunteers. Am J Psychiatry. 2001, 158 (8): 1326-8. 10.1176/appi.ajp.158.8.1326.
Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ: Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006, 31 (8): 1745-9. 10.1038/sj.npp.1300992.
Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, Meltzer HY, Konick LC, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA, Austin MC: Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol. 2009, 12 (2): 155-68. 10.1017/S1461145708009012.
Amargos-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F: Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004, 14 (3): 281-99. 10.1093/cercor/bhg128.
Jakab RL, Goldman-Rakic PS: 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proceedings of the National Academy of Sciences of the United States of America. 1998, 95 (2): 735-40. 10.1073/pnas.95.2.735.
de Almeida J, Mengod G: Quantitative analysis of glutamatergic and GABAergic neurons expressing 5-HT2A receptors in human and monkey prefrontal cortex. Journal of Neurochemistry. 2007, 103 (2): 475-486. 10.1111/j.1471-4159.2007.04768.x.
Puig MV, Artigas F, Celada P: Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex. 2005, 15 (1): 1-14.
Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM: Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996, 14 (1): 35-46. 10.1016/S0893-133X(96)80057-1.
Cruz DA, Eggan SM, Azmitia EC, Lewis DA: Serotonin1A receptors at the axon initial segment of prefrontal pyramidal neurons in schizophrenia. Am J Psychiatry. 2004, 161 (4): 739-42. 10.1176/appi.ajp.161.4.739.
de Almeida J, Mengod G: Serotonin 1A receptors in human and monkey prefrontal cortex are mainly expressed in pyramidal neurons and in a GABAergic interneuron subpopulation: implications for schizophrenia and its treatment. J Neurochem. 2008, 107 (2): 488-96. 10.1111/j.1471-4159.2008.05649.x.
Miner LAH, Backstrom JR, Sanders-Bush E, Sesack SR: Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003, 116 (1): 107-10.1016/S0306-4522(02)00580-8.
Puig MV, Celada P, Diaz-Mataix L, Artigas F: In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex. 2003, 13 (8): 870-82. 10.1093/cercor/13.8.870.
Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR: A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005, 62 (2): 146-52. 10.1001/archpsyc.62.2.146.
Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR: The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002, 17 (1): 317-23. 10.1006/nimg.2002.1179.
Barbas H: Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev. 1995, 19 (3): 499-510. 10.1016/0149-7634(94)00053-4.
Kim MJ, Whalen PJ: The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009, 29 (37): 11614-8. 10.1523/JNEUROSCI.2335-09.2009.
Besson C, Louilot A: Asymmetrical involvement of mesolimbic dopaminergic neurons in affective perception. Neuroscience. 1995, 68 (4): 963-8. 10.1016/0306-4522(95)00255-H.
Merali Z, McIntosh J, Anisman H: Anticipatory cues differentially provoke in vivo peptidergic and monoaminergic release at the medial prefrontal cortex. Neuropsychopharmacology. 2004, 29 (8): 1409-18. 10.1038/sj.npp.1300441.
Sullivan RM, Dufresne MM: Mesocortical dopamine and HPA axis regulation: role of laterality and early environment. Brain Res. 2006, 1076 (1): 49-59. 10.1016/j.brainres.2005.12.100.
Young EJ, Williams CL: Valence dependent asymmetric release of norepinephrine in the basolateral amygdala. Behav Neurosci. 2010, 124 (5): 633-44.
Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF: 5-Hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biological Psychiatry. 2010, 67 (4): 339-345. 10.1016/j.biopsych.2009.09.011.
Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Verge D: Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol. 1996, 365 (2): 289-305. 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1.
Barnes NM, Sharp T: A review of central 5-HT receptors and their function. Neuropharmacology. 1999, 38 (8): 1083-10.1016/S0028-3908(99)00010-6.
McDonald AJ, Mascagni F: Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience. 2007, 146 (1): 306-10.1016/j.neuroscience.2007.01.047.
Aznar S, Qian Z, Shah R, Rahbek B, Knudsen GM: The 5-HT1A serotonin receptor is located on calbindin- and parvalbumin-containing neurons in the rat brain. Brain Research. 2003, 959 (1): 58-10.1016/S0006-8993(02)03727-7.
Burghardt NS, Bush DEA, McEwen BS, LeDoux JE: Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT2C receptor antagonist. Biological Psychiatry. 2007, 62 (10): 1111-10.1016/j.biopsych.2006.11.023.
Bhatnagar S, Sun LM, Raber J, Maren S, Julius D, Dallman MF: Changes in anxiety-related behaviors and hypothalamic-pituitary-adrenal activity in mice lacking the 5-HT-3A receptor. Physiol Behav. 2004, 81 (4): 545-55. 10.1016/j.physbeh.2004.01.018.
Clark MS, Vincow ES, Sexton TJ, Neumaier JF: Increased expression of 5-HT1B receptor in dorsal raphe nucleus decreases fear-potentiated startle in a stress dependent manner. Brain Res. 2004, 1007 (1-2): 86-97. 10.1016/j.brainres.2004.01.070.
Smith Y, Pare JF, Pare D: Differential innervation of parvalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. Journal of Comparative Neurology. 2000, 416 (4): 496-508. 10.1002/(SICI)1096-9861(20000124)416:4<496::AID-CNE6>3.0.CO;2-N.
Ghashghaei HT, Barbas H: Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002, 115 (4): 1261-79. 10.1016/S0306-4522(02)00446-3.
First MB, Spitzer RL, Gibbon M, Williams JBM: Structured Clinical Interview for DSM-IV Axis I Disorders: Research Version, Non-Patient Edition. 1996
Folstein MF, Folstein SE, McHugh PR: "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975, 12 (3): 189-98. 10.1016/0022-3956(75)90026-6.
Darwin C, Ekman P: The expression of the emotions in man and animals. 1998, New York: Oxford University Press, xxxvi: 472-3
Ekman P, Friesen WV: Pictures of Facial Affect. 1976, Palo Alto: Consulting Psychologists Press
Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL: Inhibited and uninhibited infants "grown up": adult amygdalar response to novelty. Science. 2003, 300 (5627): 1952-3. 10.1126/science.1083703.
Wright CI, Martis B, Schwartz CE, Shin LM, Fischer HH, McMullin K, Rauch SL: Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage. 2003, 18 (3): 660-9. 10.1016/S1053-8119(02)00037-X.
Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, Rauch SL: Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biol Psychiatry. 2003, 53 (10): 854-62. 10.1016/S0006-3223(02)01906-6.
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT: Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000, 10 (3): 120-31. 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH: An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003, 19 (3): 1233-9. 10.1016/S1053-8119(03)00169-1.
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC: Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995, 33 (5): 636-647. 10.1002/mrm.1910330508.
Brett M, Anton J, Valabregue R, Poline J: Region of interest analysis using an SPM toolbox. NeuroImage. 2002, 16 (2): S497-
Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Mathis CA, Wagner A, Thornton L, Hoge J, Ziolko SK, Becker CR, McConaha CW, Kaye WH: Exaggerated 5-HT1A but normal 5-HT2A receptor activity in individuals ill with anorexia nervosa. Biological Psychiatry. 2007, 61 (9): 1090-10.1016/j.biopsych.2006.07.018.
Cidis Meltzer C, Drevets WC, Price JC, Mathis CA, Lopresti B, Greer PJ, Villemagne VL, Holt D, Mason NS, Houck PR, Reynolds CF, DeKosky ST: Gender-specific aging effects on the serotonin 1A receptor. Brain Research. 2001, 895 (1-2): 9-17. 10.1016/S0006-8993(00)03211-X.
Soloff PH, Price JC, Mason NS, Becker C, Meltzer CC: Gender, personality, and serotonin-2A receptor binding in healthy subjects. Psychiatry Res. 2010, 181 (1): 77-84. 10.1016/j.pscychresns.2009.08.007.
Price JC, Lopresti BJ, Mason NS, Holt DP, Huang Y, Mathis CA: Analyses of [18F]altanserin bolus injection PET data. I: Consideration of radiolabeled metabolites in baboons. Synapse. 2001, 41 (1): 1-10. 10.1002/syn.1054.
Price JC, Lopresti BJ, Meltzer CC, Smith GS, Mason NS, Huang Y, Holt DP, Gunn RN, Mathis CA: Analyses of [18F]altanserin bolus injection PET data. II: Consideration of radiolabeled metabolites in humans. Synapse. 2001, 41 (1): 11-21. 10.1002/syn.1055.
Parsey RV, Slifstein M, Hwang DR, Abi-Dargham A, Simpson N, Mawlawi O, Guo NN, Van Heertum R, Mann JJ, Laruelle M: Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tisssue input functions. J Cereb Blood Flow Metab. 2000, 20 (7): 1111-33.
Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, Mazumdar S, Mulsant BH, Houck PR, Lopresti BJ, Weissfeld LA, Reynolds CF: Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004, 29 (12): 2258-65. 10.1038/sj.npp.1300556.
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, et al: Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. Journal of Cerebral Blood Flow & Metabolism. 1990, 10 (5): 740-7. 10.1038/jcbfm.1990.127.
Meltzer CC, Kinahan PE, Greer PJ, Nichols TE, Comtat C, Cantwell MN, Lin MP, Price JC: Comparative evaluation of MR-based partial-volume correction schemes for PET. Journal of Nuclear Medicine. 1999, 40 (12): 2053-65.
Meltzer CC, Leal JP, Mayberg HS, Wagner HN, Frost JJ: Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. Journal of Computer Assisted Tomography. 1990, 14 (4): 561-70. 10.1097/00004728-199007000-00011.
Lemaire C, Cantineau R, Guillaume M, Plenevaux A, Christiaens L: Fluorine-18-Altanserin: A Radioligand for the Study of Serotonin Receptors with PET: Radiolabeling and In Vivo Biologic Behavior in Rats. J Nucl Med. 1991, 32 (12): 2266-2272.
Meltzer CC, Smith G, Price JC, Reynolds CF, Mathis CA, Greer P, Lopresti B, Mintun MA, Pollock BG, Ben-Eliezer D, Cantwell MN, Kaye W, DeKosky ST: Reduced binding of altanserin to serotonin type 2A receptors in aging: persistence of effect after partial volume correction. Brain Research. 1998, 813 (1): 167-10.1016/S0006-8993(98)00909-3.
Smith GS, Price JC, Lopresti BJ, Huang Y, Simpson N, Holt D, Mason NS, Meltzer CC, Sweet RA, Nichols T, Sashin D, Mathis CA: Test-retest variability of serotonin 5-HT2A receptor binding measured with positron emission tomography and [18F]altanserin in the human brain. Synapse. 1998, 30 (4): 380-392. 10.1002/(SICI)1098-2396(199812)30:4<380::AID-SYN5>3.0.CO;2-U.
Bailer UF, Price JC, Meltzer CC, Mathis CA, Frank GK, Weissfeld L, McConaha CW, Henry SE, Brooks-Achenbach S, Barbarich NC, Kaye WH: Altered 5-HT(2A) receptor binding after recovery from bulimia-type anorexia nervosa: relationships to harm avoidance and drive for thinness. Neuropsychopharmacology. 2004, 29 (6): 1143-55. 10.1038/sj.npp.1300430.
McCarron JA, Turton DR, Pike VW, Poole KG: Remotely-controlled production of the 5-HT(1A) receptor radioligand, [carbonyl-11C]WAY-100635, via 11C-carboxylation of an immobilized Grignard reagent. Journal of Labelled Compounds and Radiopharmaceuticals. 1996, 38 (10): 941-10.1002/(SICI)1099-1344(199610)38:10<941::AID-JLCR906>3.0.CO;2-Y.
Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, Mattay VS: Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Research: Neuroimaging. 2005, 139 (1): 9-10.1016/j.pscychresns.2005.02.009.
Preacher KJ, Curran PJ, Bauer DJ: Computational tool for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006, 31 (4): 437-448. 10.3102/10769986031004437.
Acknowledgements
We thank S. Ziolko, R. Coleman, S. Hulland, M. Lightfoot and A. Saul for technical assistance. We also thank the University of Pittsburgh Medical Center PET facility and Magnetic Resonance Research Center for imaging resources. We are grateful to our funding sources including the Multi-modal neuroimaging training program fellowship from the National Institute of Drug Addiction (R90 DA023420 to PMF), National Institute of Mental Health (MH067602 and MH064625 to CCM and MH072837 to ARH) and National Alliance for Research on Schizophrenia and Depression (Young Investigator Award to ARH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PMF designed the study, and participated in data collection, analysis and interpretation and drafting of manuscript. JCP participated in data analysis, interpretation and drafting of manuscript. CCM acquired related funding and participated in interpretation of data. ELMK acquired related funding and participated in interpretation of data. CB participated in data analysis. SLB acquired related funding. ARH designed the study, acquired related funding, and participated in data analysis, interpretation and drafting of manuscript. All authors provided comments and suggestions during manuscript preparation. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Fisher, P.M., Price, J.C., Meltzer, C.C. et al. Medial prefrontal cortex serotonin 1A and 2A receptor binding interacts to predict threat-related amygdala reactivity. Biol Mood Anxiety Disord 1, 2 (2011). https://doi.org/10.1186/2045-5380-1-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2045-5380-1-2