Abstract
MicroRNAs (miRNAs) are small non-coding RNA transcripts that affect various cellular pathways by serving as regulators of gene expression at the translational and transcriptional level. Nuclear receptors (NRs) are ligand-activated transcription factors that regulate gene transcription by binding to the promoter region or by interacting with other transcription factors. NRs can regulate miRNA expression either at the transcriptional level, or through posttranscriptional maturation by interacting with miRNA processing factors. This review will summarize recent advances in knowledge of the modulation of miRNA expression by NRs. Increased understanding of the molecular basis of miRNA expression may enable new therapeutic interventions that modulate miRNA activities through NR-mediated signaling.
Similar content being viewed by others
Introduction
The binding of microRNA (miRNA, or miR) to the 3'-untranslated region of target mRNAs causes transcript degradation or interferes with translation initiation. This posttranscriptional inhibitory mechanism is of critical importance in fundamental cell processes, including development [1], proliferation [2], survival and death. During the past decade, much effort has been focused on elucidating the mechanism of miRNA target gene regulation, however, relatively little is known about the regulation of miRNA genes themselves [3]. A number of large-scale expression profiling studies have shown that the expression of miRNAs is dysregulated under various pathological conditions. Many miRNAs are expressed in a tissue-specific or developmental-stage-specific manner, thereby contributing greatly to cell-type-specific profiles of protein expression [4, 5]. Growing evidence suggests that miRNAs can be regulated extensively at the levels of promoter transcription, methylation, miRNA processing, RNA editing, and miRNA-target interactions [6]. Transcriptional regulation by nuclear receptors is the primary level of control for miRNA expression (Table 1). Elucidation of the underlying mechanisms is crucial to understanding the pathways governing the miRNA network [7].
1.1 microRNA
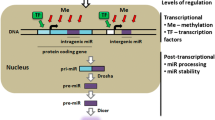
miRNAs comprise a class of short (approximately 19-24 nucleotides) single-stranded non-coding RNAs that regulate gene expression through post-transcriptional mechanisms [8]. Since the discovery of RNA interference (RNAi) in 1993, efforts to identify endogenous small RNAs have led to the discovery of thousands of miRNAs in different species [9, 10]. The newest database contains 16772 entries representing hairpin precursor miRNAs and expressing 19724 mature miRNA products in 153 species http://www.mirbase.org. Most of the miRNAs are conserved in closely related species and many have homologs in distant species, suggesting that their functions could also be conserved [11]. Accumulating evidence indicates that miRNAs play a central role in controlling a broad range of biological activities including embryonic development, cell proliferation, metabolic homeostasis, and apoptosis [12]. According to their locations in the genome, miRNA genes are classified into intragenic and intergenic regions. Although some intronic miRNAs are reported to have their own promoters, a significant percentage of miRNAs are embedded within introns or exons of protein coding genes and share the same transcriptional control of the host gene [13]. Mirtron is a notable exception that is spliced out of the host transcripts into the direct substrate of Dicer [14–16].
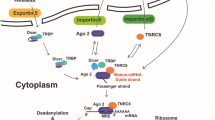
The biogenesis of miRNA starting in the nucleus is found to be quite complex, involving a vast group of protein complexes [17]. In general, miRNA genes are transcribed by Polymerase II as primary-miRNAs (pri-miRs). These large RNA precursors, which are several hundred base pairs in length, are often capped, spliced, and polyadenylated, and can encode sequences for multiple miRNA genes [6]. These precursors are processed by the RNAse-III-type endonuclease Drosha in association with the DiGeorge syndrome critical region gene 8 (DGCR8) (or Pasha in Drosophila, PASH-1 in Caenorhabditis elegans) into hairpin-shaped stem-loop structures of approximately 60-70 nt named pre-miRNAs. The pre-miRNAs are then exported out of the nucleus to the cytoplasm by Expertin-5, a Ran-GFP dependent transporter that specifically recognizes dsRNAs. In the cytoplasm, the pre-miRNAs undergo further processing by a second RNase III enzyme, named Dicer, into a miRNA-miRNA* duplex of variable length (~18-25 nt). Only one strand of the miRNA duplex, designated as the "guide" strand, is preferentially loaded into a large multi-protein miRNA ribonucleoprotein complex (miRNP, also referred to as the miRISC complex), and is used to modulate target gene expression. The "passenger" strand of the miRNA-miRNA* duplex (referred to as miRNA*) is excluded from the miRNP complex and degraded.
The well-known "seed" region, positions 2-8 nt from the 5'miRNA, is extremely important for miRNA targeting [18, 19]. Depending on the degree of complementarity to the target mRNA, the mechanism of silencing target mRNA expression will be one of the following: 1) if there is perfect complementarity to a target mRNA, there will be target mRNA cleavage; and 2) in the case of incomplete complementarity, translational repression or alteration of mRNA stability will occur [20]. Bioinformatical prediction is based on the degree of seed region match with the 3'UTR of target genes. Furthermore, other "non-canonical" miRNA-mediated mechanisms of mRNA expression modulation are emerging [21–23]. Some miRNAs can bind to the open reading frame or 5'UTR of target genes; moreover, they have been shown to activate, rather than to inhibit, gene expression [21, 24, 25].
1.2 microRNA regulation by nuclear receptors
1.2.1 Nuclear receptors
Nuclear receptors (NRs) are ligand-activated transcription factors that regulate the expression of target genes by binding to cis-acting DNA sequences. The superfamily of nuclear receptors contains 48 human members that include classical receptors, adopted orphan receptors and orphan receptors. Classical receptors are regulated by an extensively studied group of endocrine ligands, such as the estrogen receptor (ER), androgen receptor (AR), progesterone receptor (PR), and glucocorticoid receptor (GR). Orphan nuclear receptors, like small heterodimer partner (SHP), have no natural ligands, and they behave like normal transcription factors. In the past few years, a class of so-called "adopted" orphan receptors (for which either natural or synthetic ligands have been identified) has arisen, such as peroxisome proliferator-activated receptors (PPARs) and liver × receptors (LXRs). A typical nuclear receptor usually contains five functional regions: the A/B region that contains an N-terminal activation function-1 (AF1) domain, the central C region that contains a DNA-binding domain (DBD), the C terminal E region that contains a ligand-binding domain (LBD) and activation function-2 (AF-2) domain, and the D hinge region that links the DBD to the LBD. Interestingly, the nuclear receptors Dax-1 and SHP only have LBDs, but they can interact with other transcription factors and function as corepressors in regulating their target genes. Nuclear receptors can activate or repress target genes by binding directly to DNA response elements as homo- or heterodimers, or by binding to other classes of DNA-bound transcription factors. Two groups of regulators, coactivators and corepressors, are recruited by NRs and in turn regulate the expression of downstream target genes.
1.2.2 Estrogen receptor (ER)
Since miRNAs are encoded by genes that are mainly transcribed by RNA polymerase II, their transcription can be regulated by a variety of transcription factors including NRs [26]. After estrogenic activation, ERs mediate transcription by interacting directly with specific estrogen response elements (EREs) located in the promoter/enhancer region of target genes, followed by recruitment of additional cofactors that have either activator or repressor functions on target genes [27]. For example, ERα binds directly to the promoter region of miR-221/222 and recruits NCoR and SMRT to suppress miR-221/222 expression [28]. The miR-221/222 may play a role in tamoxifen resistance because they have high expression levels in tamoxifen resistant breast cancer. In addition, c-Myc, induced by estrogen, can bind to the miR-17-92 locus in an estrogen-dependent manner [29]. E2 induces the expression of let-7 family members, as well as other miRNAs including miR-98 and miR-21, which reduce the levels of c-Myc and E2F2 proteins [30].
Apart from regulating the expression of miRNAs at the transcriptional level, ERα appears to be able to regulate the biogenesis of miRNAs. Drosha is directly inhibited by ERα [31]. Exportin 5, which controls the translocation of precursors, is induced by estradiol and progestins [32]. The expression levels of Dicer are induced by estradiol and progestins and are higher in ERα positive versus negative breast cancers [33, 34]. Ago2, a component of RISC, is induced by estradiol. Ago1 and Ago2 are low in ERα positive breast cancers [33].
1.2.3 Androgen receptor (AR)
AR is a ligand-dependent transcription factor that regulates the expression of androgen target genes. Several miRNAs have been implicated in prostate cancer (CaP) development, including miR-125b [35], miR-21, miR-10a, miR-141, miR-150*, and miR-1225-5p [36, 37], miR-205 and miR-200c [38], miR-146a [39], miR-221 and miR-222 [40], miR-101 and miR-26a [41], and the miR-15a-miR-16-1 locus [42]. Transfection of synthetic miR-125b, miR-21 or miR-141 stimulates androgen-independent growth of CaP cells [43], while miR-146a markedly reduces cell proliferation, invasion, and metastasis [39]. The expression of miR-125b, an androgen induced miRNA, is high in malignant prostate tissues. The miR-15a/miR-16-1 locus was homozygously deleted in a subset of prostate cancers leading to the abolishment of miR-15a, but not miR-16, expression [42]. The recruitment of AR to the 5'DNA region of miR-125b and miR-21 has been confirmed by ChIP analysis [37, 43].
ARs can also bind to the promoter region of miR-221 to repress miR-221 expression in LNCaP cells [44]. Knocking-down miR-221 increases LNCaP cell migration and invasion by targeting DVL2 [45]. The circulating level of miR-21, miR-141 and miR-221 in the bloodstream might be useful as a prognostic marker in patients with prostate cancer [46, 47]. miR-616 is over-expressed specifically in malignant prostate tissues, not in benign prostate specimens. Stable miR-616 overexpression in LNCaP cells stimulates prostate cancer cell proliferation and castration resistance [48].
1.2.4 Progesterone receptor (PR)
In mammalian pregnancy, uterine quiescence is maintained by elevated circulating progesterone (P4) acting via PR. The miR-200 family, including miR-200b/c/429 and miR-200a/141, is upregulated during late gestation and labor [49]. P4 injection causes a modest decrease in myometrial expression of miR-200b/429, yet it also significantly increases ZEB1 mRNA and protein, a target of the miR-200 family. Further studies show that ZEB2 rather than ZEB1 is acting as a transcriptional repressor on the miR-200c/141 promoter [50]. In SKOV-3 cells, the expression of let-7, which targets PGRMC1 (progesterone receptor membrane component 1), is increased after stimulation with progesterone [51]. Progesterone is also reported to regulate miR-320 expression [52]. Conversely, miR-126-3p inhibits PR protein expression as well as the proliferation of mammary epithelial cells by targeting the PR 3'UTR directly [53].
1.2.5 Hepatocyte nuclear factor-4α (HNF4α)
HNF-4α is a highly conserved nuclear receptor that is expressed in the liver, kidney, intestine, and pancreas. HNF-4α is a key regulator of energy metabolism, glucose and lipid homeostasis [54]. A putative binding site for HNF-4α is found in the conserved core element of the hpri-miR-122 promoter. The miR-122 promoter activation by HNF-4α is further enhanced by the addition of PGC1α, a well-recognized co-activator of HNF4α [55].
1.2.6 Peroxisome proliferator-activated receptor (PPAR)
MiR-29a and 29c levels are decreased in the rat heart after 7-day treatment with PIO, a peroxisome proliferator-activated receptor (PPAR)-γ agonist [56]. Wy-14,643, a specific PPARα agonist, inhibits microRNA let-7c expression via a PPARα-dependent pathway. The lack of any significant difference in basal let-7c expression between the WT and PPARα-null mice suggests an active transrepression mechanism, where the receptor is recruited to the genomic regulatory region of let-7c following ligand treatment [57]. Because let-7c targets the c-Myc 3'UTR for degradation, the PPARα-mediated induction of c-Myc via let-7c subsequently increases the expression of oncogenic miR-17-92 clusters [58].
1.2.7 Farnesoid × receptor (FXR)
FXR is the primary biosensor for endogenous bile acids and regulates the expression of numerous genes involved in lipid and glucose metabolism. miRNAs regulated by FXR were detected by miRNA microarray analysis with hepatic RNAs of wild type or FXR-null mice. Of the miRNAs tested, the level of miR-34a is upregulated in FXR-null mice. The mechanism of this regulation is as follows: activation of FXR induces SHP, which in turn suppresses miR-34a gene transcription by inhibiting p53 binding to the miR-34a promoter [59]. Treatment with GW4064, a synthetic FXR ligand, upregulates miR-29a in hematopoietic stem cells (HSCs) isolated from wild-type mice, rats, and humans but not from FXR-null mice. A FXR-responsive element has been identified in the miR-29a promoter, which is involved in the regulation of extracellular matrix (ECM) production in liver [60]. The expression of miR-29a is also negatively regulated by TGF-β, c-Myc, hedgehog or NF-κB signaling in liver and lung fibrosis [61–63].
1.2.8 Liver × receptor (LXR)
LXR plays an important role in the metabolism and homeostasis of cholesterol, lipids, bile acids, and steroid hormones [64]. After endogenous or synthetic ligand binding, LXR forms a heterodimer with retinoid × receptor (RXR) and binds to LXR response elements (LXREs) in the promoters of LXR target genes. Treatment with GW3965, a LXR ligand, induces the expression of mature hsa-miR-613 in primary human hepatocytes as well as in human hepatoma HepG2 and Huh7 cells. The positive regulation of hsa-miR-613 by LXR is mediated by the sterol regulatory element binding protein (SREBP)-1c, a known LXR target gene [65]. Interestingly, hsa-miR-613 can target the 3'UTR of endogenous LXRα. The negative regulation mediated by hsa-miR-613 and SREBP-1c and the previously reported positive regulation mediated by an LXRE constitute a negative autoregulatory feedback loop to ensure a tight regulation of LXRα [66].
1.2.9 Pregnane × receptor (PXR)
PXR is a crucial regulator of drug metabolism in liver and small intestine. PXR dimerizes with RXRα and binds to the response elements of its target genes, including CYP3A4 [67]. Eleven out of the three hundred human miRNAs that have been identified exhibit a more than three-fold altered expression in HepG2 cells treated with the PXR ligand rifampicin. MiR-31 is 5.4-fold down-regulated, whereas all others, namely miR-193a -5p, miR-296-5p, miR-324-5p, miR-379, miR-411, miR-489, miR505, miR-519a, miR-545 and miR-548b-5p, exhibit up to 5.8-fold higher expression levels [68, 69]. Interestingly, miRNA can regulate PXR expression too. For example, miR-148a post-transcriptionally regulates human PXR, resulting in the modulation of the inducible and/or constitutive levels of CYP3A4 in human liver [67].
1.2.10 Retinoic acid receptor (RAR) and retinoic × receptor (RXR)
To identify potential transcription factor binding sites (TFBS) in the promoter regions of 247 human intergenic microRNAs (from a miRNA promoter dataset provided by Mahony et al [70]), NHR-Scan was used for the PML-RARA fusion protein [69]. Sixty-five microRNA promoters contain a PML-RARA predicted site. Further investigations are focused on the miR-210 and cluster of miR23a/24-2. After treating with the RAR agonist all-trans retinoic acid (ATRA), expression of miR-23a and miR-210 is increased. ChIP experiments directed against both RARα and RXRα in 293T cells show that RARα/RXRα heterodimers bind miR-210 and miR-23a/24-2 promoters. This method which is start with prediction of NR binding site in miRNA promoter database, is different from other microRNA array first analysis [69].
1.2.11 Nuclear receptor TLX (homologue of the Drosophila tailless gene)
Orphan nuclear receptor TLX, which is expressed in the neuroepithelium of the embryonic mouse brain and in adult neurogenic regions, is essential for neural stem cell (NSC) proliferation [71]. TLX binds to downstream of the miR-9 sequence at the miR-9-1 locus and represses miR-9 at the transcriptional level. Meanwhile, miR-9 targets TLX mRNA for destabilization and/or translational inhibition, reducing TLX protein levels. MiR-9 and TLX thus form a feedback loop to regulate the switch of neural stem cell proliferation and differentiation [72].
1.2.12 Small heterodimer partner (SHP)
SHP is an orphan nuclear receptor that contains the ligand-binding domain (LBD), but lacks the DNA binding domain (DBD) [73]. SHP functions as a transcriptional repressor though interacting with other NRs, and plays important roles in several metabolic diseases and in liver carcinogenesis [74–77]. SHP interacts with ERRγ to control the expression of miR-433 and miR-127 in several mammalian species [78–80]. SHP also activates miR-206 expression through a ''dual inhibitory'' mechanism [81], which in turn targets Notch3 for degradation [82]. The SHP/FXR signaling is important in controlling the expression of miR-34α and its target SIRT1 [83].
1.2.13 Other NRs
Other members of the NR family are also involved in miRNA expression regulation. Glucocorticoids (GC) bind to both the glucocorticoid receptor (GR) and mineralocorticoid (MR). Both receptor types act through transactivation at glucocorticoid response elements (GREs) [84]. GR controls a variety of physiological functions, such as metabolism, development, and reproduction; whereas MR is critical for controlling sodium and potassium transport, pathophysiology of hypertension, and cardiac fibrosis [85]. Several miRNAs/mirtrons are regulated by GC in patients and cell lines, including the myeloid-specific miR-223 and the apoptosis and cell cycle arrest-inducing miR-15/16 clusters. miR-15b/16 increases GC sensitivity in leukemia cell lines, further suggesting that miRNA regulation is a vital component of GC signaling [86]. In addition, miR-208 potentiates βMHC expression through a mechanism involving the thyroid hormone receptor [87].
1.3 Conclusion
NRs appear to regulate miRNA expression via three means: direct binding to the promoter regions of miRNAs, indirect regulation of miRNA expression through NR target genes, and involvement in regulation of miRNA biogenesis (Figure 1). Microarray analyses has revealed large numbers of miRNAs that are differentially regulated by NRs or their ligands, but the detailed regulatory mechanisms remain to be elucidated. Future exploration of the interactions between NRs and miRNAs in the regulation of gene expression networks is needed for better understanding of miRNA modulation and function by NR signaling.
Nuclear receptor regulation of miRNA expression and biogenesis. 1. The miRNA genes are transcribed by RNA polymerase II to produce pri-miRNAs. NRs bind directly to the miRNA gene promoters or interact with other transcription factors that bind to the target miRNA promoters. 2. The pri-miRNAs are cleaved by Drosha and DGCR8 to become pre-miRNAs, which have hairpin-shaped stem-loop structures. ERα inhibits Drosha by direct interaction. 3. The pre-miRNAs are exported to the cytoplasm by Expertin-5 that is induced by estradiol and progestins. 4. The pre-miRNAs undergo further processing by Dicer to become mature miRNAs. The expression of Dicer is increased by estradiol and progestins. 5. The mature miRNAs are loaded to the miRISC to regulate target gene expression. Ago2, a component of the RISC, is induced by estradiol.
References
Liu N, Olson EN: MicroRNA regulatory networks in cardiovascular development. Dev Cell. 2010, 18: 510-525. 10.1016/j.devcel.2010.03.010
Belver L, Papavasiliou FN, Ramiro AR: MicroRNA control of lymphocyte differentiation and function. Curr Opin Immunol. 2011, 23: 368-373. 10.1016/j.coi.2011.02.001
Megraw M, Baev V, Rusinov V, Jensen ST, Kalantidis K, Hatzigeorgiou AG: MicroRNA promoter element discovery in Arabidopsis. RNA. 2006, 12: 1612-1619. 10.1261/rna.130506
Krol J, Loedige I, Filipowicz W: The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010, 11: 597-610.
Davis BN, Hata A: microRNA in Cancer---The involvement of aberrant microRNA biogenesis regulatory pathways. Genes Cancer. 2010, 1: 1100-1114. 10.1177/1947601910396213
Breving K, Esquela-Kerscher A: The complexities of microRNA regulation: mirandering around the rules. Int J Biochem Cell Biol. 2010, 42: 1316-1329. 10.1016/j.biocel.2009.09.016
Ozsolak F, Poling LL, Wang Z, Liu H, Liu XS, Roeder RG, Zhang X, Song JS, Fisher DE: Chromatin structure analyses identify miRNA promoters. Genes Dev. 2008, 22: 3172-3183. 10.1101/gad.1706508
Bartel DP: MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004, 116: 281-297. 10.1016/S0092-8674(04)00045-5
Lee RC, Feinbaum RL, Ambros V: The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993, 75: 843-854. 10.1016/0092-8674(93)90529-Y
Wightman B, Ha I, Ruvkun G: Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993, 75: 855-862. 10.1016/0092-8674(93)90530-4
Kim VN, Nam JW: Genomics of microRNA. Trends Genet. 2006, 22: 165-173. 10.1016/j.tig.2006.01.003
Stefani G, Slack FJ: Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008, 9: 219-230. 10.1038/nrm2347
Xiong H, Qian J, He T, Li F: Independent transcription of miR-281 in the intron of ODA in Drosophila melanogaster. Biochem Biophys Res Commun. 2009, 378: 883-889. 10.1016/j.bbrc.2008.12.010
Berezikov E, Chung WJ, Willis J, Cuppen E, Lai EC: Mammalian mirtron genes. Mol Cell. 2007, 28: 328-336. 10.1016/j.molcel.2007.09.028
Okamura K, Hagen JW, Duan H, Tyler DM, Lai EC: The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell. 2007, 130: 89-100. 10.1016/j.cell.2007.06.028
Ruby JG, Jan CH, Bartel DP: Intronic microRNA precursors that bypass Drosha processing. Nature. 2007, 448: 83-86. 10.1038/nature05983
Lima RT, Busacca S, Almeida GM, Gaudino G, Fennell DA, Vasconcelos MH: MicroRNA regulation of core apoptosis pathways in cancer. Eur J Cancer. 2011, 47: 163-174. 10.1016/j.ejca.2010.11.005
Garzon R, Marcucci G, Croce CM: Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010, 9: 775-789. 10.1038/nrd3179
Bartel DP: MicroRNAs: target recognition and regulatory functions. Cell. 2009, 136: 215-233. 10.1016/j.cell.2009.01.002
Liu J: Control of protein synthesis and mRNA degradation by microRNAs. Curr Opin Cell Biol. 2008, 20: 214-221. 10.1016/j.ceb.2008.01.006
Orom UA, Nielsen FC, Lund AH: MicroRNA-10a binds the 5'UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell. 2008, 30: 460-471. 10.1016/j.molcel.2008.05.001
Eiring AM, Harb JG, Neviani P, Garton C, Oaks JJ, Spizzo R, Liu S, Schwind S, Santhanam R, Hickey CJ: miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell. 2010, 140: 652-665. 10.1016/j.cell.2010.01.007
Beitzinger M, Meister G: Preview. MicroRNAs: from decay to decoy. Cell. 2010, 140: 612-614. 10.1016/j.cell.2010.02.020
Stark A, Lin MF, Kheradpour P, Pedersen JS, Parts L, Carlson JW, Crosby MA, Rasmussen MD, Roy S, Deoras AN: Discovery of functional elements in 12 Drosophila genomes using evolutionary signatures. Nature. 2007, 450: 219-232. 10.1038/nature06340
Kim DH, Saetrom P, Snove O, Rossi JJ: MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc Natl Acad Sci USA. 2008, 105: 16230-16235. 10.1073/pnas.0808830105
Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN: MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23: 4051-4060. 10.1038/sj.emboj.7600385
Lonard DM, O'Malley BW: Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell. 2007, 27: 691-700. 10.1016/j.molcel.2007.08.012
Di Leva G, Gasparini P, Piovan C, Ngankeu A, Garofalo M, Taccioli C, Iorio MV, Li M, Volinia S, Alder H: MicroRNA cluster 221-222 and estrogen receptor alpha interactions in breast cancer. J Natl Cancer Inst. 2010, 102: 706-721. 10.1093/jnci/djq102
Castellano L, Giamas G, Jacob J, Coombes RC, Lucchesi W, Thiruchelvam P, Barton G, Jiao LR, Wait R, Waxman J: The estrogen receptor-alpha-induced microRNA signature regulates itself and its transcriptional response. Proc Natl Acad Sci USA. 2009, 106: 15732-15737. 10.1073/pnas.0906947106
Bhat-Nakshatri P, Wang G, Collins NR, Thomson MJ, Geistlinger TR, Carroll JS, Brown M, Hammond S, Srour EF, Liu Y, Nakshatri H: Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res. 2009, 37: 4850-4861. 10.1093/nar/gkp500
Yamagata K, Fujiyama S, Ito S, Ueda T, Murata T, Naitou M, Takeyama K, Minami Y, O'Malley BW, Kato S: Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol Cell. 2009, 36: 340-347. 10.1016/j.molcel.2009.08.017
Nothnick WB, Healy C, Hong X: Steroidal regulation of uterine miRNAs is associated with modulation of the miRNA biogenesis components Exportin-5 and Dicer1. Endocrine. 2010, 37: 265-273. 10.1007/s12020-009-9293-9
Cheng C, Fu X, Alves P, Gerstein M: mRNA expression profiles show differential regulatory effects of microRNAs between estrogen receptor-positive and estrogen receptor-negative breast cancer. Genome Biol. 2009, 10: R90. 10.1186/gb-2009-10-9-r90
Grelier G, Voirin N, Ay AS, Cox DG, Chabaud S, Treilleux I, Leon-Goddard S, Rimokh R, Mikaelian I, Venoux C: Prognostic value of Dicer expression in human breast cancers and association with the mesenchymal phenotype. Br J Cancer. 2009, 101: 673-683. 10.1038/sj.bjc.6605193
Ozen M, Creighton CJ, Ozdemir M, Ittmann M: Widespread deregulation of microRNA expression in human prostate cancer. Oncogene. 2008, 27: 1788-1793. 10.1038/sj.onc.1210809
Waltering KK, Porkka KP, Jalava SE, Urbanucci A, Kohonen PJ, Latonen LM, Kallioniemi OP, Jenster G, Visakorpi T: Androgen regulation of micro-RNAs in prostate cancer. Prostate. 2011, 71: 604-614. 10.1002/pros.21276
Ribas J, Ni X, Haffner M, Wentzel EA, Salmasi AH, Chowdhury WH, Kudrolli TA, Yegnasubramanian S, Luo J, Rodriguez R: miR-21: an androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res. 2009, 69: 7165-7169. 10.1158/0008-5472.CAN-09-1448
Tang X, Gal J, Kyprianou N, Zhu H, Tang G: Detection of microRNAs in prostate cancer cells by microRNA array. Methods Mol Biol. 2011, 732: 69-88. 10.1007/978-1-61779-083-6_6
Lin SL, Chiang A, Chang D, Ying SY: Loss of mir-146a function in hormone-refractory prostate cancer. RNA. 2008, 14: 417-424. 10.1261/rna.874808
Sun T, Wang Q, Balk S, Brown M, Lee GS, Kantoff P: The role of microRNA-221 and microRNA-222 in androgen-independent prostate cancer cell lines. Cancer Res. 2009, 69: 3356-3363. 10.1158/0008-5472.CAN-08-4112
Cao P, Deng Z, Wan M, Huang W, Cramer SD, Xu J, Lei M, Sui G: MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1alpha/HIF-1beta. Mol Cancer. 2010, 9: 108. 10.1186/1476-4598-9-108
Porkka KP, Ogg EL, Saramaki OR, Vessella RL, Pukkila H, Lahdesmaki H, van Weerden WM, Wolf M, Kallioniemi OP, Jenster G, Visakorpi T: The miR-15a-miR-16-1 locus is homozygously deleted in a subset of prostate cancers. Genes Chromosomes Cancer. 2011, 50: 499-509. 10.1002/gcc.20873
Shi XB, Xue L, Yang J, Ma AH, Zhao J, Xu M, Tepper CG, Evans CP, Kung HJ, deVere White RW: An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc Natl Acad Sci USA. 2007, 104: 19983-19988. 10.1073/pnas.0706641104
Ambs S, Prueitt RL, Yi M, Hudson RS, Howe TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA: Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008, 68: 6162-6170. 10.1158/0008-5472.CAN-08-0144
Zheng C, Yinghao S, Li J: MiR-221 expression affects invasion potential of human prostate carcinoma cell lines by targeting DVL2. Med Oncol. 2011.
Kanemaru H, Fukushima S, Yamashita J, Honda N, Oyama R, Kakimoto A, Masuguchi S, Ishihara T, Inoue Y, Jinnin M, Ihn H: The circulating microRNA-221 level in patients with malignant melanoma as a new tumor marker. J Dermatol Sci. 2011, 61: 187-193. 10.1016/j.jdermsci.2010.12.010
Yaman Agaoglu F, Kovancilar M, Dizdar Y, Darendeliler E, Holdenrieder S, Dalay N, Gezer U: Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumour Biol. 2011, 32: 583-588. 10.1007/s13277-011-0154-9
Ma S, Chan YP, Kwan PS, Lee TK, Yan M, Tang KH, Ling MT, Vielkind JR, Guan XY, Chan KW: MicroRNA-616 induces androgen-independent growth of prostate cancer cells by suppressing expression of tissue factor pathway inhibitor TFPI-2. Cancer Res. 2011, 71: 583-592. 10.1158/0008-5472.CAN-10-2587
Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR: miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci USA. 2010, 107: 20828-20833. 10.1073/pnas.1008301107
Bendoraite A, Knouf EC, Garg KS, Parkin RK, Kroh EM, O'Briant KC, Ventura AP, Godwin AK, Karlan BY, Drescher CW: Regulation of miR-200 family microRNAs and ZEB transcription factors in ovarian cancer: evidence supporting a mesothelial-to-epithelial transition. Gynecol Oncol. 2010, 116: 117-125. 10.1016/j.ygyno.2009.08.009
Wendler A, Keller D, Albrecht C, Peluso JJ, Wehling M: Involvement of let-7/miR-98 microRNAs in the regulation of progesterone receptor membrane component 1 expression in ovarian cancer cells. Oncol Rep. 2011, 25: 273-279.
Xia HF, Jin XH, Song PP, Cui Y, Liu CM, Ma X: Temporal and spatial regulation of miR-320 in the uterus during embryo implantation in the rat. Int J Mol Sci. 2010, 11: 719-730. 10.3390/ijms11020719
Cui W, Li Q, Feng L, Ding W: MiR-126-3p regulates progesterone receptors and involves development and lactation of mouse mammary gland. Mol Cell Biochem. 2011.
Watt AJ, Garrison WD, Duncan SA: HNF4: a central regulator of hepatocyte differentiation and function. Hepatology. 2003, 37: 1249-1253. 10.1053/jhep.2003.50273
Li ZY, Xi Y, Zhu WN, Zeng C, Zhang ZQ, Guo ZC, Hao DL, Liu G, Feng L, Chen HZ: Positive regulation of hepatic miR-122 expression by HNF4alpha. J Hepatol. 2011.
Ye Y, Hu Z, Lin Y, Zhang C, Perez-Polo JR: Downregulation of microRNA-29 by antisense inhibitors and a PPAR-gamma agonist protects against myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2010, 87: 535-544. 10.1093/cvr/cvq053
Shah YM, Morimura K, Yang Q, Tanabe T, Takagi M, Gonzalez FJ: Peroxisome proliferator-activated receptor alpha regulates a microRNA-mediated signaling cascade responsible for hepatocellular proliferation. Mol Cell Biol. 2007, 27: 4238-4247. 10.1128/MCB.00317-07
O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT: c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005, 435: 839-843. 10.1038/nature03677
Lee J, Padhye A, Sharma A, Song G, Miao J, Mo YY, Wang L, Kemper JK: A pathway involving farnesoid × receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J Biol Chem. 2010, 285: 12604-12611. 10.1074/jbc.M109.094524
Li J, Zhang Y, Kuruba R, Gao X, Gandhi CR, Xie W, Li S: Roles of miR-29a in the Antifibrotic Effect of FXR in Hepatic Stellate Cells. Mol Pharmacol. 2011.
Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lu J: MIR-29 is a Major Regulator of Genes Associated with Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2010.
Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M: Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011, 53: 209-218. 10.1002/hep.23922
Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME: Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem. 2010, 110: 1155-1164. 10.1002/jcb.22630
Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ: Role of LXRs in control of lipogenesis. Genes Dev. 2000, 14: 2831-2838. 10.1101/gad.850400
Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ: Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000, 14: 2819-2830. 10.1101/gad.844900
Ou Z, Wada T, Gramignoli R, Li S, Strom SC, Huang M, Xie W: MicroRNA hsa-miR-613 targets the human LXRalpha gene and mediates a feedback loop of LXRalpha autoregulation. Mol Endocrinol. 2011, 25: 584-596. 10.1210/me.2010-0360
Takagi S, Nakajima M, Mohri T, Yokoi T: Post-transcriptional regulation of human pregnane × receptor by micro-RNA affects the expression of cytochrome P450 3A4. J Biol Chem. 2008, 283: 9674-9680. 10.1074/jbc.M709382200
Mahony S, Corcoran DL, Feingold E, Benos PV: Regulatory conservation of protein coding and microRNA genes in vertebrates: lessons from the opossum genome. Genome Biol. 2007, 8: R84. 10.1186/gb-2007-8-5-r84
Saumet A, Vetter G, Bouttier M, Portales-Casamar E, Wasserman WW, Maurin T, Mari B, Barbry P, Vallar L, Friederich E: Transcriptional repression of microRNA genes by PML-RARA increases expression of key cancer proteins in acute promyelocytic leukemia. Blood. 2009, 113: 412-421.
Sandelin A, Wasserman WW: Prediction of nuclear hormone receptor response elements. Mol Endocrinol. 2005, 19: 595-606.
Denli AM, Cao X, Gage FH: miR-9 and TLX: chasing tails in neural stem cells. Nat Struct Mol Biol. 2009, 16: 346-347. 10.1038/nsmb0409-346
Zhao C, Sun G, Li S, Shi Y: A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009, 16: 365-371. 10.1038/nsmb.1576
Zhang Y, Hagedorn CH, Wang L: Role of nuclear receptor SHP in metabolism and cancer. Biochim Biophys Acta. 2011, 1812: 893-908.
Wang L, Liu J, Saha P, Huang J, Chan L, Spiegelman B, Moore DD: The orphan nuclear receptor SHP regulates PGC-1alpha expression and energy production in brown adipocytes. Cell Metab. 2005, 2: 227-238. 10.1016/j.cmet.2005.08.010
Huang J, Iqbal J, Saha PK, Liu J, Chan L, Hussain MM, Moore DD, Wang L: Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology. 2007, 46: 147-157. 10.1002/hep.21632
He N, Park K, Zhang Y, Huang J, Lu S, Wang L: Epigenetic inhibition of nuclear receptor small heterodimer partner is associated with and regulates hepatocellular carcinoma growth. Gastroenterology. 2008, 134: 793-802. 10.1053/j.gastro.2008.01.006
Zhang Y, Xu P, Park K, Choi Y, Moore DD, Wang L: Orphan receptor small heterodimer partner suppresses tumorigenesis by modulating cyclin D1 expression and cellular proliferation. Hepatology. 2008, 48: 289-298.
Song G, Wang L: A conserved gene structure and expression regulation of miR-433 and miR-127 in mammals. PLoS One. 2009, 4: e7829. 10.1371/journal.pone.0007829
Song G, Wang L: MiR-433 and miR-127 arise from independent overlapping primary transcripts encoded by the miR-433-127 locus. PLoS One. 2008, 3: e3574. 10.1371/journal.pone.0003574
Song G, Wang L: Transcriptional mechanism for the paired miR-433 and miR-127 genes by nuclear receptors SHP and ERRgamma. Nucleic Acids Res. 2008, 36: 5727-5735. 10.1093/nar/gkn567
Song G, Wang L: Nuclear receptor SHP activates miR-206 expression via a cascade dual inhibitory mechanism. PLoS One. 2009, 4: e6880. 10.1371/journal.pone.0006880
Song G, Zhang Y, Wang L: MicroRNA-206 targets notch3, activates apoptosis, and inhibits tumor cell migration and focus formation. J Biol Chem. 2009, 284: 31921-31927. 10.1074/jbc.M109.046862
Lee J, Kemper JK: Controlling SIRT1 expression by microRNAs in health and metabolic disease. Aging (Albany NY). 2010, 2: 527-534.
de Kloet ER, Fitzsimons CP, Datson NA, Meijer OC, Vreugdenhil E: Glucocorticoid signaling and stress-related limbic susceptibility pathway: about receptors, transcription machinery and microRNA. Brain Res. 2009, 1293: 129-141.
Cochrane DR, Cittelly DM, Richer JK: Steroid receptors and microRNAs: relationships revealed. Steroids. 2011, 76: 1-10. 10.1016/j.steroids.2010.11.003
Rainer J, Ploner C, Jesacher S, Ploner A, Eduardoff M, Mansha M, Wasim M, Panzer-Grumayer R, Trajanoski Z, Niederegger H, Kofler R: Glucocorticoid-regulated microRNAs and mirtrons in acute lymphoblastic leukemia. Leukemia. 2009, 23: 746-752. 10.1038/leu.2008.370
van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN: Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007, 316: 575-579. 10.1126/science.1139089
Osada H, Takahashi T: let-7 and miR-17-92: small-sized major players in lung cancer development. Cancer Sci. 2011, 102: 9-17. 10.1111/j.1349-7006.2010.01707.x
Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, Jacks T: Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci USA. 2008, 105: 3903-3908. 10.1073/pnas.0712321105
Lee YS, Dutta A: The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007, 21: 1025-1030. 10.1101/gad.1540407
Tsang WP, Kwok TT: Let-7a microRNA suppresses therapeutics-induced cancer cell death by targeting caspase-3. Apoptosis. 2008, 13: 1215-1222. 10.1007/s10495-008-0256-z
Christensen BC, Moyer BJ, Avissar M, Ouellet LG, Plaza SL, McClean MD, Marsit CJ, Kelsey KT: A let-7 microRNA-binding site polymorphism in the KRAS 3' UTR is associated with reduced survival in oral cancers. Carcinogenesis. 2009, 30: 1003-1007. 10.1093/carcin/bgp099
Graziano F, Canestrari E, Loupakis F, Ruzzo A, Galluccio N, Santini D, Rocchi M, Vincenzi B, Salvatore L, Cremolini C: Genetic modulation of the Let-7 microRNA binding to KRAS 3'-untranslated region and survival of metastatic colorectal cancer patients treated with salvage cetuximab-irinotecan. Pharmacogenomics J. 2010, 10: 458-464. 10.1038/tpj.2010.9
Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ: RAS is regulated by the let-7 microRNA family. Cell. 2005, 120: 635-647. 10.1016/j.cell.2005.01.014
Hossain A, Kuo MT, Saunders GF: Mir-17-5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol Cell Biol. 2006, 26: 8191-8201. 10.1128/MCB.00242-06
Yu Z, Wang C, Wang M, Li Z, Casimiro MC, Liu M, Wu K, Whittle J, Ju X, Hyslop T: A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J Cell Biol. 2008, 182: 509-517. 10.1083/jcb.200801079
Bourguignon LY, Earle C, Wong G, Spevak CC, Krueger K: Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene. 2011.
Kida K, Nakajima M, Mohri T, Oda Y, Takagi S, Fukami T, Yokoi T: PPARalpha Is Regulated by miR-21 and miR-27b in Human Liver. Pharm Res. 2011.
Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH: MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS One. 2011, 6: e19139. 10.1371/journal.pone.0019139
Gaur AB, Holbeck SL, Colburn NH, Israel MA: Downregulation of Pdcd4 by mir-21 facilitates glioblastoma proliferation in vivo. Neuro Oncol. 2011, 13: 580-590. 10.1093/neuonc/nor033
Polytarchou C, Iliopoulos D, Hatziapostolou M, Kottakis F, Maroulakou I, Struhl K, Tsichlis PN: Akt2 regulates all Akt isoforms and promotes resistance to hypoxia through induction of miR-21 upon oxygen deprivation. Cancer Res. 2011.
Chun-Zhi Z, Lei H, An-Ling Z, Yan-Chao F, Xiao Y, Guang-Xiu W, Zhi-Fan J, Pei-Yu P, Qing-Yu Z, Chun-Sheng K: MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer. 2010, 10: 367. 10.1186/1471-2407-10-367
Zhang C, Zhang J, Zhang A, Wang Y, Han L, You Y, Pu P, Kang C: PUMA is a novel target of miR-221/222 in human epithelial cancers. Int J Oncol. 2010, 37: 1621-1626.
Kim D, Song J, Jin EJ: MicroRNA-221 regulates chondrogenic differentiation through promoting proteosomal degradation of slug by targeting Mdm2. J Biol Chem. 2010, 285: 26900-26907. 10.1074/jbc.M110.115105
Galardi S, Mercatelli N, Farace MG, Ciafre SA: NF-kB and c-Jun induce the expression of the oncogenic miR-221 and miR-222 in prostate carcinoma and glioblastoma cells. Nucleic Acids Res. 2011, 39: 3892-3902. 10.1093/nar/gkr006
Zhao JJ, Lin J, Yang H, Kong W, He L, Ma X, Coppola D, Cheng JQ: MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J Biol Chem. 2008, 283: 31079-31086. 10.1074/jbc.M806041200
Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A: The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009, 11: 1487-1495. 10.1038/ncb1998
Paik JH, Jang JY, Jeon YK, Kim WY, Kim TM, Heo DS, Kim CW: MicroRNA-146a downregulates NF{kappa}B activity via targeting TRAF6, and functions as a tumor suppressor having strong prognostic implications in NK/T cell lymphoma. Clin Cancer Res. 2011.
Garcia AI, Buisson M, Bertrand P, Rimokh R, Rouleau E, Lopez BS, Lidereau R, Mikaelian I, Mazoyer S: Down-regulation of BRCA1 expression by miR-146a and miR-146b-5p in triple negative sporadic breast cancers. EMBO Mol Med. 2011, 3: 279-290. 10.1002/emmm.201100136
Chen T, Li Z, Jing T, Zhu W, Ge J, Zheng X, Pan X, Yan H, Zhu J: MicroRNA-146a regulates the maturation process and pro-inflammatory cytokine secretion by targeting CD40L in oxLDL-stimulated dendritic cells. FEBS Lett. 2011, 585: 567-573. 10.1016/j.febslet.2011.01.010
Taganov KD, Boldin MP, Chang KJ, Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006, 103: 12481-12486. 10.1073/pnas.0605298103
Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, Yoshimura A, Baltimore D, Rudensky AY: Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010, 142: 914-929. 10.1016/j.cell.2010.08.012
Lu J, He ML, Wang L, Chen Y, Liu X, Dong Q, Chen YC, Peng Y, Yao KT, Kung HF, Li XP: MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011, 71: 225-233. 10.1158/0008-5472.CAN-10-1850
Zhang B, Liu XX, He JR, Zhou CX, Guo M, He M, Li MF, Chen GQ, Zhao Q: Pathologically decreased miR-26a antagonizes apoptosis and facilitates carcinogenesis by targeting MTDH and EZH2 in breast cancer. Carcinogenesis. 2011, 32: 2-9. 10.1093/carcin/bgq209
Buechner J, Tomte E, Haug BH, Henriksen JR, Lokke C, Flaegstad T, Einvik C: Tumour-suppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br J Cancer. 2011.
Hao Y, Gu X, Zhao Y, Greene S, Sha W, Smoot D, Califano JA, Wu TC, Pang X: Enforced Expression of miR-101 inhibits prostate cancer cell growth by modulating cyclooxygenase-2 pathway in vivo. Cancer Prev Res (Phila). 2011.
Sachdeva M, Wu H, Ru P, Hwang L, Trieu V, Mo YY: MicroRNA-101-mediated Akt activation and estrogen-independent growth. Oncogene. 2011, 30: 822-831. 10.1038/onc.2010.463
Tanaka T, Haneda S, Imakawa K, Sakai S, Nagaoka K: A microRNA, miR-101a, controls mammary gland development by regulating cyclooxygenase-2 expression. Differentiation. 2009, 77: 181-187. 10.1016/j.diff.2008.10.001
Zheng SQ, Li YX, Zhang Y, Li X, Tang H: MiR-101 regulates HSV-1 replication by targeting ATP5B. Antiviral Res. 2011, 89: 219-226. 10.1016/j.antiviral.2011.01.008
Zhu QY, Liu Q, Chen JX, Lan K, Ge BX: MicroRNA-101 targets MAPK phosphatase-1 to regulate the activation of MAPKs in macrophages. J Immunol. 2010, 185: 7435-7442. 10.4049/jimmunol.1000798
Zhou R, Hu G, Gong AY, Chen XM: Binding of NF-kappaB p65 subunit to the promoter elements is involved in LPS-induced transactivation of miRNA genes in human biliary epithelial cells. Nucleic Acids Res. 2010, 38: 3222-3232. 10.1093/nar/gkq056
Ge Y, Sun Y, Chen J: IGF-II is regulated by microRNA-125b in skeletal myogenesis. J Cell Biol. 2011, 192: 69-81. 10.1083/jcb.201007165
Rajabi H, Joshi MD, Jin C, Ahmad R, Kufe D: Androgen receptor regulates expression of the MUC1-C oncoprotein in human prostate cancer cells. Prostate. 2011.
Alpini G, Glaser SS, Zhang JP, Francis H, Han Y, Gong J, Stokes A, Francis T, Hughart N, Hubble L: Regulation of placenta growth factor by microRNA-125b in hepatocellular cancer. J Hepatol. 2011.
Xu N, Brodin P, Wei T, Meisgen F, Eidsmo L, Nagy N, Kemeny L, Stahle M, Sonkoly E, Pivarcsi A: MiR-125b, a MicroRNA Downregulated in Psoriasis, Modulates Keratinocyte Proliferation by Targeting FGFR2. J Invest Dermatol. 2011, 131: 1521-1529. 10.1038/jid.2011.55
Le MT, Xie H, Zhou B, Chia PH, Rizk P, Um M, Udolph G, Yang H, Lim B, Lodish HF: MicroRNA-125b promotes neuronal differentiation in human cells by repressing multiple targets. Mol Cell Biol. 2009, 29: 5290-5305. 10.1128/MCB.01694-08
Castoldi M, Vujic Spasic M, Altamura S, Elmen J, Lindow M, Kiss J, Stolte J, Sparla R, D'Alessandro LA, Klingmuller U: The liver-specific microRNA miR-122 controls systemic iron homeostasis in mice. J Clin Invest. 2011, 121: 1386-1396. 10.1172/JCI44883
Burns DM, D'Ambrogio A, Nottrott S, Richter JD: CPEB and two poly(A) polymerases control miR-122 stability and p53 mRNA translation. Nature. 2011, 473: 105-108. 10.1038/nature09908
Wilson JA, Zhang C, Huys A, Richardson CD: Human Ago2 is required for efficient microRNA 122 regulation of hepatitis C virus RNA accumulation and translation. J Virol. 2011, 85: 2342-2350. 10.1128/JVI.02046-10
Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, Xu C, Mason WS, Moloshok T, Bort R: miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004, 1: 106-113. 10.4161/rna.1.2.1066
Gatfield D, Le Martelot G, Vejnar CE, Gerlach D, Schaad O, Fleury-Olela F, Ruskeepaa AL, Oresic M, Esau CC, Zdobnov EM, Schibler U: Integration of microRNA miR-122 in hepatic circadian gene expression. Genes Dev. 2009, 23: 1313-1326. 10.1101/gad.1781009
Teichler S, Illmer T, Roemhild J, Ovcharenko D, Stiewe T, Neubauer A: MicroRNA29a regulates the expression of the nuclear oncogene Ski. Blood. 2011.
Pullen TJ, da Silva Xavier G, Kelsey G, Rutter GA: miR-29a and miR-29b contribute to pancreatic {beta}-cell specific silencing of Monocarboxylate Transporter 1 (Mct1/slc16a1). Mol Cell Biol. 2011.
Kong G, Zhang J, Zhang S, Shan C, Ye L, Zhang X: Upregulated MicroRNA-29a by Hepatitis B Virus × Protein Enhances Hepatoma Cell Migration by Targeting PTEN in Cell Culture Model. PLoS One. 2011, 6: e19518. 10.1371/journal.pone.0019518
Zhao JJ, Lin J, Lwin T, Yang H, Guo J, Kong W, Dessureault S, Moscinski LC, Rezania D, Dalton WS: microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010, 115: 2630-2639. 10.1182/blood-2009-09-243147
Chan SY, Loscalzo J: MicroRNA-210: A unique and pleiotropic hypoxamir. Cell Cycle. 2010, 9: 1072-1083. 10.4161/cc.9.6.11006
Huang X, Le QT, Giaccia AJ: MiR-210--micromanager of the hypoxia pathway. Trends Mol Med. 2010, 16: 230-237. 10.1016/j.molmed.2010.03.004
Tsuchiya S, Fujiwara T, Sato F, Shimada Y, Tanaka E, Sakai Y, Shimizu K, Tsujimoto G: MicroRNA-210 regulates cancer cell proliferation through targeting fibroblast growth factor receptor-like 1 (FGFRL1). J Biol Chem. 2011, 286: 420-428. 10.1074/jbc.M110.170852
Zhang Y, Xie RL, Croce CM, Stein JL, Lian JB, van Wijnen AJ, Stein GS: A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Natl Acad Sci USA. 2011, 108: 9863-9868. 10.1073/pnas.1018493108
Siegel C, Li J, Liu F, Benashski SE, McCullough LD: miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc Natl Acad Sci USA. 2011.
Zhu LH, Liu T, Tang H, Tian RQ, Su C, Liu M, Li X: MicroRNA-23a promotes the growth of gastric adenocarcinoma cell line MGC803 and downregulates interleukin-6 receptor. FEBS J. 2010, 277: 3726-3734. 10.1111/j.1742-4658.2010.07773.x
Zhang J, Chintalgattu V, Shih T, Ai D, Xia Y, Khakoo AY: MicroRNA-9 is an activation-induced regulator of PDGFR-beta expression in cardiomyocytes. J Mol Cell Cardiol. 2011.
Shibata M, Nakao H, Kiyonari H, Abe T, Aizawa S: MicroRNA-9 regulates neurogenesis in mouse telencephalon by targeting multiple transcription factors. J Neurosci. 2011, 31: 3407-3422. 10.1523/JNEUROSCI.5085-10.2011
Otaegi G, Pollock A, Hong J, Sun T: MicroRNA miR-9 modifies motor neuron columns by a tuning regulation of FoxP1 levels in developing spinal cords. J Neurosci. 2011, 31: 809-818. 10.1523/JNEUROSCI.4330-10.2011
Arora H, Qureshi R, Jin S, Park AK, Park WY: miR-9 and let-7g enhance the sensitivity to ionizing radiation by suppression of NFkappaB1. Exp Mol Med. 2011, 43: 298-304. 10.3858/emm.2011.43.5.031
Craig VJ, Cogliatti SB, Imig J, Renner C, Neuenschwander S, Rehrauer H, Schlapbach R, Dirnhofer S, Tzankov A, Muller A: Myc-mediated repression of microRNA-34a promotes high-grade transformation of B-cell lymphoma by dysregulation of FoxP1. Blood. 2011, 117: 6227-6236. 10.1182/blood-2010-10-312231
Hermeking H: The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010, 17: 193-199. 10.1038/cdd.2009.56
Simon D, Laloo B, Barillot M, Barnetche T, Blanchard C, Rooryck C, Marche M, Burgelin I, Coupry I, Chassaing N: A mutation in the 3'-UTR of the HDAC6 gene abolishing the post-transcriptional regulation mediated by hsa-miR-433 is linked to a new form of dominant X-linked chondrodysplasia. Hum Mol Genet. 2010, 19: 2015-2027. 10.1093/hmg/ddq083
Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA, Jones PA: Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006, 9: 435-443. 10.1016/j.ccr.2006.04.020
Chen JF, Tao Y, Li J, Deng Z, Yan Z, Xiao X, Wang DZ: microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol. 2010, 190: 867-879. 10.1083/jcb.200911036
Winbanks CE, Wang B, Beyer C, Koh P, White L, Kantharidis P, Gregorevic P: TGF-beta regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J Biol Chem. 2011, 286: 13805-13814. 10.1074/jbc.M110.192625
Lin CC, Liu LZ, Addison JB, Wonderlin WF, Ivanov AV, Ruppert JM: A KLF4-miRNA-206 Autoregulatory Feedback Loop Can Promote or Inhibit Protein Translation Depending upon Cell Context. Mol Cell Biol. 2011, 31: 2513-2527. 10.1128/MCB.01189-10
Dey BK, Gagan J, Dutta A: miR-206 and -486 induce myoblast differentiation by downregulating Pax7. Mol Cell Biol. 2011, 31: 203-214. 10.1128/MCB.01009-10
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests. All authors read and approved the final manuscript.
Authors' contributions
ZY prepared the draft, LW made the final version. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yang, Z., Wang, L. Regulation of microRNA expression and function by nuclear receptor signaling. Cell Biosci 1, 31 (2011). https://doi.org/10.1186/2045-3701-1-31
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2045-3701-1-31