Abstract
Background
Lactic acid bacteria are considered important probiotics for prevention of some infections. The aim of this work was to investigate the effect of selenium dioxide on the antifungal activity of Lactobacillus plantarum and L. johnsonii against Candida albicans.
Methods
Lactobacillus plantarum and L. johnsonii cells, grown in the presence and absence of selenium dioxide, and their cell-free spent culture media were tested for antifungal activity against C. albicans ATCC 14053 by a hole-plate diffusion method and a time-kill assay.
Results
Both L. plantarum and L. johnsonii reduced selenium dioxide to cell-associated elemental selenium nanoparticles. The cell-free spent culture media, from both Lactobacillus species that had been grown with selenium dioxide for 48 h, showed enhanced antifungal activity against C. albicans. Enhanced antifungal activity of cell biomass against C. albicans was also observed in cultures grown with selenium dioxide.
Conclusions
Selenium dioxide-treated Lactobacillus spp. or their cell-free spent broth inhibited the growth of C. albicans and should be investigated for possible use in anti-Candida probiotic formulations in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Candida albicans, although it is a commensal yeast in the oral cavity, gastrointestinal tract and urogenital tract, can cause a variety of mild to serious infections. C. albicans usually infects immunocompromised patients or others who use antibiotics for a long time [1]. One reason for the overgrowth of C. albicans and infection is disequilibrium in the microbiota [2, 3]. Probiotics are microorganisms which, when consumed in adequate amounts, can improve intestinal microbial balance and provide benefits for human health [4]. Lactic acid bacteria (LAB) are important probiotics and also part of the normal Gram-positive microflora inhabiting the intestinal mucosa. They aid in prevention of colonization by pathogenic microorganisms [5]. In the vagina, normal Lactobacillus species have a critical role in protection against vaginal infections and the transmission of pathogens responsible for sexually transmitted diseases [4–8]. These bacteria produce lactic acid, acetic acid, hydrogen peroxide, and other antimicrobial substances, which allow them to prevent the colonization of pathogens [8, 9]. Some LAB strains can protect the human vagina from candidiasis through the production of these exometabolites [10–14]. As yet uncharacterized metabolites from selenium-enriched probiotics have recently been shown to exert an antibacterial effect against Escherichia coli[15]. In the present work, we aimed to study the antimicrobial effect of two selenium-enriched Lactobacillus spp. cultures and their exometabolites against C. albicans ATCC 14053 and to compare these results with anti-Candida effects of spent broth of non-selenium-enriched Lactobacillus cultures.
Materials and methods
Microbial strains
L. plantarum (ATCC 8014) and C. albicans (ATCC 14053) were obtained from the American Type Culture Collection (ATCC). The other Lactobacillus was a clinical isolate, which was identified as L. johnsonii during a previous study [16].
Effect of Lactobacillus species on selenium dioxide
One hundred milliliters of DeMan–Rogosa–Sharpe (MRS) broth (Merck, Darmstadt, Germany) was used for inoculation of L. plantarum and L. johnsonii strains. The cultures were grown at 37°C in a shaker incubator for 24 h. After this time, selenium dioxide (Merck Schuchardt, Hohenbrunn, Germany) was dissolved in distilled water (289.5 mg/l) and sterilized by a Millipore filter apparatus (Millipore Corporation, Milford, MA, USA). This selenium dioxide solution was added aseptically to each of the Lactobacillus cultures to obtain a final concentration of 200 mg/l of Se. The cultures were further incubated at 37°C for 96 h. Two-milliliter samples were withdrawn at zero time and at intervals (24, 48, and 96 h) under aseptic conditions. The bacterial cells were removed from the cultures by centrifugation at 5,000 × g for 10 min (Hettich Mikro 200, Tuttlingen, Germany). The supernatant at each time interval was used to measure the concentration of Se remaining in the medium by Somer and Kutay’s spectrophotometric method [17]. The cell pellet from a culture of L. plantarum and isolated selenium nanoparticles (SeNPs) were examined at 100 kV by a Philips EM-208 transmission electron microscope (TEM) (FEI Ltd., Eindhoven, The Netherlands) to evaluate the presence and the size of Se NPs deposited inside the L. plantarum cells as previously described [18]. To determine the elemental composition of the nanoparticles (NPs), energy dispersive X-ray spectrum (EDX) microanalysis (Vega Tescan, Brno, Czech Republic) was also performed.
Preparation of Lactobacillus cultures for antifungal activity assays
Four flasks, each containing 100 ml MRS broth, were used for inoculation of two sets each of L. plantarum and L. johnsonii strains. The cultures were aerobically grown at 37°C in a shaker incubator for 24 h and one set of each bacterium was treated with selenium dioxide as previously described [16]. The cultures were incubated at 37°C for another 96 h. At zero time and every 24 h, 1 ml samples from all four sets of cultures were harvested under aseptic conditions. The samples were centrifuged at 5000 × g for 15 min. All collected supernatants were assayed for antifungal activity against C. albicans. Cultures incubated for additional time (96 h) were subjected to further centrifugations to isolate enough culture supernatants for the time-kill assay.
Assay for antifungal activity of L. plantarum and L. johnsonii grown with or without selenium dioxide
Both a conventional hole-plate diffusion method and a time-kill assay were used to detect antimicrobial activity in the samples. The supernatants of L. plantarum and L. johnsonii, grown with or without selenium dioxide, were sterilized by filtration through a 0.22 μm Millipore filter. Sabouraud dextrose agar (SDA) plates were inoculated with C. albicans and used to test anti-Candida effects of the collected supernatants from each culture. 14-mm diameter holes were punched aseptically in each plate and filled with 100 μl of the cell-free supernatants. As a negative control, sterile MRS liquid medium (100 μl) was used. The plates were incubated aerobically for 18 h at 37°C and the diameters of the inhibition zones (mm) were measured.
The effect of cell-free supernatants of 72-h cultures of L. plantarum and L. johnsonii, grown with or without selenium dioxide, on the survival of C. albicans was also evaluated by conventional time-kill assays. To the tubes containing each of the supernatants, C. albicans (3 × 106/ml) was added and incubated at 37°C. The viability of C. albicans was studied by plating samples taken at different intervals (0.5, 4, 8, 12, and 24 h) on SDA medium and counting the CFU of surviving C. albicans.
The antifungal activity of bacterial cells of L. plantarum and L. johnsonii, grown with or without selenium dioxide, was also assayed with C. albicans. Each of the bacterial pellets prepared as previously described was suspended in a normal saline solution containing 3 × 106 CFU/ml of C. albicans. The yeast: bacterial ratio in the suspension was approximately 1/1000 CFU/ml. Samples were withdrawn at different intervals (0.5, 4, 8, 12, and 24 h) for determining the number of surviving C. albicans in each challenge test. The samples were plated on SDA medium and incubated at 37°C overnight. The surviving C. albicans CFU were counted. These experiments were repeated three times.
Results
Selenium dioxide reduction
After 24 h incubation with both L. plantarum and L. johnsonii, the concentrations of selenium in the supernatants were considerably reduced. Figure 1 shows the concentration of selenium remaining in the culture medium taken every 24 h. The amount of Se remaining in the supernatants of Lactobacillus cultures after 72 h was approximately 3 mg/l, indicating that 98.5% of the selenium ions were reduced in each of the Lactobacillus cultures (Figure 1). No appreciable amount of Se was present in the culture supernatant of any of the strains after 96 h of incubation. The selenium-enriched L. plantarum cells were selected for a TEM experiment (Figure 2A). Spherical SeNPs of various sizes had formed inside the L. plantarum cells (Figure 2B). Figure 2C shows a size histogram of the SeNPs inside the cells; the particle sizes ranged from 25 to 250 nm. Furthermore, EDX microanalysis of the separated NPs exhibited Se absorption peaks consisting of SeLα, SeKα and SeKβ at 1.37, 11.22 and 12.49 keV, respectively (Figure 2D) and confirmed the presence of Se in the samples.
Anti-Candida effect of fermented broth of L. plantarum and L. johnsonii
The anti-Candida effects of the supernatants from L. plantarum and L. johnsonii, grown with and without selenium dioxide, were compared by measuring the zone of inhibition formed around each of the samples applied to the plates in the hole-plate diffusion method (Table 1). Negligible inhibition zones were observed on the plates containing culture supernatants of either of the species grown without selenium dioxide or sterile MRS broth supplemented with selenium dioxide (200 mg/l). Culture supernatants from L. plantarum and L. johnsonii grown with selenium dioxide for 48, 72, and 96 h showed potent anti-Candida activity and inhibited growth. The maximum antifungal activity was observed in 72 h cultures (Table 1). The time-kill assay, measuring the effect of culture supernatants of each strain, grown with or without selenium dioxide, on the viability of C. albicans, confirmed the increase in the antifungal activity of the strains grown with selenium dioxide (Figure 3). Whereas incubation of C. albicans with the supernatants of all cultures decreased viability, the number of surviving C. albicans cells substantially decreased when incubated with the supernatants of cultures grown for 72 h with selenium dioxide. The difference in the effect, which was observed even after 0.5 h incubation, increased with time. No viable C. albicans was present after 4 h incubation with culture supernatants of either species grown with selenium dioxide, but some viable C. albicans cells were present even after 24 h incubation with culture supernatants of species grown without selenium dioxide.
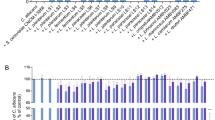
Antifungal effect of L. plantarum and L. johnsonii cells against C. albicans
The anti-Candida effects of the selenium-enriched and non-enriched cells of L. plantarum and L. johnsonii were also evaluated. The viability of C. albicans decreased following co-culture with both bacteria (Figure 4), but cells from selenium-enriched cultures were more effective at killing C. albicans (Figure 4). After 0.5 h incubation of C. albicans with the Lactobacillus strains grown without selenium dioxide, viability of C. albicans had decreased by approximately 10-fold, whereas a 1000-fold decrease was seen when incubated with the SeNPs-enriched species (Figure 4). However, the number of viable cells did not decrease with increased incubation time.
Discussion
Inhibition of C. albicans by some strains of Lactobacillus species is known and results of clinical trials have shown the effectiveness of some strains of Lactobacillus spp. in prevention of C. albicans infections [19]. In this study, we have evaluated the interaction of L. plantarum and L. johnsonii with selenium dioxide on the antifungal activity of these bacteria for C. albicans. Both strains converted selenium dioxide to SeNPs of various sizes, which accumulated inside the cells. Whereas both cells and culture supernatants had anti-C. albicans activity, substantially higher antifungal activity was observed in the culture supernatants of strains grown with selenium dioxide. It appears that selenium dioxide in the cultures enhanced production of soluble metabolites involved in killing C. albicans cells.
The antifungal activity of L. plantarum is related to the production of specific compounds, such as phenyllactic acid and 4-hydrophenyllactic acid [20]. When C. albicans was mixed with the SeNPs enriched Lactobacillus cells or the cell-free fermented broth, clear decreases in viability were observed. The addition of selenium dioxide to the culture medium of either Lactobacillus species led to potent increases in antifungal activity against C. albicans. Other studies indicate that the adverse effect of LAB can result from production of lactic acid, acetic acid, H2O2, CO2, bacteriocins, and uncharacterized compounds [10–13]. Therefore, selenium dioxide may induce the production of these exometabolites or induce the synthesis of novel anti-Candida compounds. At this time, the nature of the exometabolites responsible for the observed antimicrobial activity is not known, and further bioassay-guided fractionation assays should be used to isolate and characterize the active constituent(s).
During the cultivation of Lactobacillus spp. in MRS broth containing selenium dioxide, this compound was reduced to elemental SeNPs, which accumulated in intracellular spaces of Lactobacillus spp. and may have contributed to the increased antifungal activity of the treated cells.
The modification of the microenvironment of LAB is being considered as a means of preventing infections of the urogenital and intestinal tracts [6–8]. The application of SeNPs enriched- Lactobacillus may be a good approach for the design of new strategies in enhancing the activities of probiotics for curing infections caused by urogenital pathogens, such as C. albicans.
Conclusions
The present work assessed the viability of C. albicans following exposure to selenium NPs-enriched Lactobacillus species or their cell-free culture media. A greater decrease in viability of C. albicans was seen for bacteria grown with selenium dioxide than for non-Se-enriched bacteria. A direct antifungal effect was observed when SeNPs-enriched Lactobacillus spp. were co-cultured with C. albicans. In addition, evidence for release of potent exometabolites was indicated by the strong inhibition of growth of C. albicans treated with cell-free culture media from the SeNPs-enriched Lactobacillus species. This is the first time in which antifungal activity of the combination of Lactobacillus spp. and selenium has been studied. This phenomenon should be further evaluated for its practical application.
References
Calderone RA, Fonzi WA: Virulence factors of Candida albicans. Trends Microbiol. 2001, 9: 327-335. 10.1016/S0966-842X(01)02094-7.
Koga-Ito CY, Martins CAP, Jorge AOC: Estudo do gênero Candida. Jorge AOC. 2006, Editora Santos, São Paulo, Brazil: Princípios de Microbiologia e Imunologia, 1
Vieira JDG, Ribeiro EL, Campos CC, Pimenta FC, Toledo OA, Nagato GM, Souza NA, Ferreira WM, Cardoso CG, Dias SMS, Araújo CA, Zatta DT, Santos JS: Candida albicans isoladas da cavidade bucal de crianças com síndrome de Down: ocorrência e inibição do crescimento por Streptomyces sp. Rev Soc Bras Med Trop. 2005, 38: 383-386. 10.1590/S0037-86822005000500003.
Savino F, Cordisco L, Tarasco V, Locatelli E, Di Gioia D, Oggero R, Matteuzzi D: Antagonistic effect of Lactobacillus strains against gas-producing coliforms isolated from colicky infants. BMC Microbiol. 2011, 11: 157-10.1186/1471-2180-11-157.
Jacobsen CN, Nielsen VR, Hayford AE, Moller PL, Michael Sen KF, Paerregaard A, Sandström B, Tvede M, Jacobsen M: Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol. 1999, 11: 4949-4956.
Eschenbach DA, Hillier SL, Critchlow C, Stevens C, De Rouen T, Holmes KK: Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988, 158: 819-828. 10.1016/0002-9378(88)90078-6.
Galask RP: Vaginal colonization by bacteria and yeast. Am J Obstet Gynecol. 1989, 158: 993-995.
Klebanoff SJ, Hillier SL, Eschenbach DA, Waltersdorph AM: Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J Infect Dis. 1991, 164: 94-100. 10.1093/infdis/164.1.94.
McGoarty JA: Probiotic use of lactobacilli in the human female urogenital tract. FEMS Immunol Med Microbiol. 1993, 6: 251-264. 10.1111/j.1574-695X.1993.tb00337.x.
Jay JM: Antimicrobial properties of diacetyl. Appl Environ Microbiol. 1982, 44: 525-532.
Klaenhammer TR: Bacteriocins of lactic acid bacteria. Biochimie. 1988, 70: 337-349. 10.1016/0300-9084(88)90206-4.
Piard JC, Desmazeaud M: Inhibiting factors produced by lactic acid bacteria: 1. Oxygen metabolites and catabolism end products. Lait. 1991, 71: 525-541. 10.1051/lait:1991541.
Pikkemaat MG, Oostra-van Dijk S, Schouten J, Rapallini M, Egmond HJ: A new microbial screening method for the detection of antimicrobial residues in slaughter animals: The Nouws antibiotic test (NAT-screening). Food Cont. 2008, 19: 781-789. 10.1016/j.foodcont.2007.08.002.
Reid G, Jass J, Sebulsky T, Sebulsky MT, McCormick JK: Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003, 16: 658-672. 10.1128/CMR.16.4.658-672.2003.
Yang J, Huang K, Qin S, Wu X, Zhao Z, Chen F: Antibacterial action of selenium-enriched probiotics against pathogenic Escherichia coli. Dig Dis Sci. 2009, 54: 246-254. 10.1007/s10620-008-0361-4.
Yazdi MH, Mahdavi M, Setayesh N, Esfandyar M, Shahverdi AR: Selenium nanoparticle-enriched Lactobacillus brevis causes more efficient immune responses in vivo and reduces the liver metastasis in metastatic form of mouse breast cancer. Daru. 2013, 21: 33-10.1186/2008-2231-21-33. Doi: 10.1186/2008-2231-21-33
Somer G, Kutay N: A new and simple spectrophotometric method for the determination of selenium in the presence of copper and tellurium. Can J Chem. 1993, 71: 834-835. 10.1139/v93-111.
Yazdi MH, Mahdavi M, Kheradmand E, Shahverdi AR: The preventive oral supplementation of a selenium nanoparticle-enriched probiotic increases the immune response and lifespan of 4T1 breast cancer bearing mice. Arzneimittelforschung. 2012, 62: 525-531.
Falagas ME, Betsi GI, Athanasiou S: Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006, 58: 266-272. 10.1093/jac/dkl246.
Lavermicocca P, Valerio F, Evidente A, Lazzaroni S, Corsetti A, Gobetti M: Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl Environ Microbiol. 2000, 66: 4084-4090. 10.1128/AEM.66.9.4084-4090.2000.
Acknowledgment
This work was supported by the Deputy of Research, Tehran University of Medical Sciences, Tehran, Iran. There is no conflict of interest for authors in this work. The views presented in this article do not necessarily reflect those of the U. S. Food and Drug Administration.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EK: Conducted experiments. FR: Supervised antifungal experiments and revision of manuscript. MHY and AAS: Participated in microbiological experiments. ARS: Project design and manuscript preparation. MRO: Participated in discussion on experimental procedures. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kheradmand, E., Rafii, F., Yazdi, M.H. et al. The antimicrobial effects of selenium nanoparticle-enriched probiotics and their fermented broth against Candida albicans. DARU J Pharm Sci 22, 48 (2014). https://doi.org/10.1186/2008-2231-22-48
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2008-2231-22-48