Abstract
Background
The marine environment is a unique source of bioactive natural products, of which Sargassum muticum (Yendo) Fensholt is an important brown algae distributed in Jeju Island, Korea. S. muticum is a traditional Korean food stuff and has pharmacological functions including anti-inflammatory effects. However, the active ingredients from S. muticum have not been characterized.
Methods
Bioguided fractionation of the ethanolic extract of S. muticum, collected from Jeju island, led to the isolation of a norisoprenoid. Its structure was determined by analysis of the spectroscopic data. In vitro anti-inflammatory activity and mechanisms of action of this compound were examined using lipopolysaccharide (LPS)-stimulated RAW 264.7 cells through ELISA assays and Western blot analysis.
Results
Apo-9′-fucoxanthinone, belonging to the norisoprenoid family were identified. Apo-9′-fucoxanthinone effectively suppressed LPS-induced nitric oxide (NO) and prostaglandin E2 (PGE2) production. This compound also exerted their anti-inflammatory actions by down-regulating of NF-κB activation via suppression of IκB-α in macrophages.
Conclusions
This is the first report describing effective anti-inflammatory activity for apo-9’-fucoxanthinone′-fucoxanthnone isolated from S. muticum. Apo-9′-fucoxanthinone may be a good candidate for delaying the progression of human inflammatory diseases and warrants further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Inflammation is the response of an organism to invasion by foreign pathogens such as parasites, bacteria and viruses. The inflammatory response is an important protective reaction to injury, irritation and infection and is characterised by redness, heat, swelling, loss of function and pain [1]. In the inflammatory state, activated immune cells, such as macrophages secrete large amounts of proinflammatory cytokines, nitric oxide (NO), and prostaglandin E2 (PGE2). However, high levels of NO and PGE2 in a chronic inflammation state can result in various pathological conditions [1–4]. For this reason, regulation of the production of NO and PGE2 in macrophages are current research topics for the development of new anti-inflammatory agents. There have been many attempts to derive new anti-inflammatory agents from natural compounds [5–7]. Traditional remedies derived from terrestrial plants and maritime plants such as seaweeds have been considered safe, less toxic, and readily available, even through their modes of action are yet infinite for the most part. Thus, uncovering the molecular mechanism underlying the biological function of natural products might be a good strategy for identifying new therapeutic agents [8, 9].
Sargassum muticum (Yendo) Fensholt, a brown alga, is the most important economic seaweed, and widely distributed on the seashore of southern and eastern Korea. It is commonly consumed as a popular marine vegetable for more than 1000 years in Korea, particularly in Jeju Island. It has various biological activities, including antioxidant, anti-inflammatory, and antibacterial activities [10, 11]. Previously, our research group documented the anti-inflammatory properties of various seaweads [11–14]. During our on-going screening program designed to identify the anti-inflammatory potential of natural compounds, we have isolated apo-9′-fucoxanthinone from S. muticum, using activity-directed fractionation, and characterized apo-9′-fucoxanthinone’s structural identity using spectroscopy (1H and 13NMR) in this study. Also, as a prelude to revealing the anti-inflammatory effects and its mechanisms of apo-9′-fucoxanthinone, the present study focused on whether apo-9′-fucoxanthinone inhibited the production of NO and PGE2 and expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 in LPS-stimulated macrophages.
Methods
Extraction and isolation of apo-9′-fucoxanthinone
S. muticum was collected from the coasts of Jeju Island in March 2009, and verified by Dr. Wook Jae Lee at Jeju Technopark (JTP). A voucher specimen (CSC-002) was deposited at Department of Chemistry, Jeju National University, Jeju, Korea. S. muticum were washed 3 times with water to remove any salt, epiphytes, and sand attached to the surface. They were dried at 60°C for 24 h in an oven, and pulverized in a grinder prior to extraction. The dried powder (800 g) was extracted with 70% aqueous ethanol with stirring for 2 days at room temperature. The filtrate was concentrated under reduced pressure. The extract (105 g) was suspended in water (1.0 L), and successively partitioned into n-hexane, methylene chloride, ethyl acetate, and n-butanol fractions. The fraction of methylene chloride (7 g), being dissolved in solvent, mixed with celite, and evaporated using a rotary vacuum evaporator. After lyophilization, it was chromatographed and eluted by using the solvents 500 mL of into n-hexane, methylene chloride/ethyl acetate (10:1, 5:1, 2:1), methylene chloride, ethyl acetate, and methanol in order. The hexane fraction was chromatographed over a silica gel column using n-hexane:EtOAc (3:1) in order to obtain 10 sub-fractions (F-1 to F-10). All fractions containing the same constituent(s) identified on the TLC plates were combined and the solvents were evaporated using a rotary vacuum evaporator. Structures of fraction 10 of them (F10, 2.3 g) were determined using proton-nuclear magnetic resonance (1H NMR) and 13C NMR. The compound’s structural identity was determined by one-and two-dimensional nuclear magnetic resonance (NMR) spectroscopic analysis (Additional file 1) and comparison to published values. Structures of these compounds are given in Figure 1.
Chemicals and reagents
Dulbecco’s modified Eagle’s medium (DMEM) and foetal bovine serum (FBS) were obtained from Invitrogen-Gibco (Grand Island, NY, USA). Enzyme-linked immunosorbent assay (ELISA) kits for prostaglandin E2 (PGE2) was purchased from R&D Systems, Inc. (St. Louis, MO, USA). Anti-IκB-α, anti-phosphorylated IκB-α (anti-p-IκB-α) were purchased from Cell Signaling Technology (Beverly, MA, USA). Pyrollidine dithiocarbamate (PDTC, a specific inhibitor of NF-κB) was purchased from Calbiochem (San Diego, CA, USA). All other reagents were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, USA).
RAW 264.7 cell culture
RAW 264.7 cells were obtained from the Korean Cell Line Bank (KCLB; Seoul, Korea) and maintained at sub-confluence in a 95% air, 5% CO2 humidified atmosphere at 37°C as described previously [11–14]. Cells at passages 10–20 were used for the experiments and subcultured every 2–3 days. The medium for routine sub-cultivation was DMEM supplemented with FBS (10%), penicillin (100 units/mL), and streptomycin (100 μg/mL). Cells were counted with a haemocytometer, and the number of viable cells was assessed by trypan blue dye exclusion method.
MTT assay for cell viability
Cell viability was measured as described previously [11–14] with slight modification using MTT assay. RAW 264.7 cells were cultured in 96-well plates for 18 h, followed by treatment with LPS (1 μg/mL) in the presence of various concentrations of the sample. After a 24-h incubation, MTT was added to the medium for 4 h. Finally, the supernatant was removed and the formazan crystals were dissolved in DMSO. Absorbance was measured at 540 nm. The percentage of cells showing cytotoxicity relative to the control group was determined.
Nitric oxide determination
RAW 264.7 cells were plated at 1.5 × 105 cells/well in 24-well plates and then incubated with or without LPS (1 μg/mL) in the absence or presence of various concentrations (12.5, 25, 50, and 100 μg/mL) of apo-9-fucoxanthinone for 24 h. Nitrite levels in culture media were determined as described previously [11–14] with slight modification using the Griess reaction and presumed to reflect NO levels. Briefly, the culture supernatant (100 μL) was mixed with the same volume of Griess reagent (1% sulphanilamide and 0.1% N-[1-naphthyl]-ethylenediamine dihydrochloride in 5% phosphoric acid %) for 10 min. Absorbance was the measured at 540 nm using spectrophotometer. Fresh culture media were used as blanks in all experiments. NO levels in samples were read off a standard sodium nitrite curve.
Detection of PGE2 in supernatant
Sandwich ELISA was used to determine the inhibitory effects of various concentrations (12.5, 25, 50, and 100 μg/mL) of apo-9-fucoxanthinone on the production of cytokines PGE2 in LPS-treated RAW 264.7 cells. RAW 264.7 cells were stimulated for 24 h before the supernatant was harvested and assayed according to the manufacturer’s protocol for the relevant ELISA kit. Results from 3 independent experiments were used for statistical analysis.
Western blot analysis
Western blotting was performed with a SDS-PAGE Electrophoresis System as described previously [11–14]. Briefly, the RAW 264.7 cells (5.0 × 105 cells/mL) were pre-incubated for 18 h and then treated with LPS (1 μg/mL) plus aliquots sample for 24 h. After incubation, the cells were washed twice with cold PBS. Whole-cell lysates (25 μg) were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and electro-transferred to a polyvinylidene fluoride (PVDF) membrane (BIO-RAD, HC, USA). The membrane was incubated for 24 h with 5% skim milk and then incubated with iNOS (1:2500), COX-2 (1:2500), IκB-α (1:1000), phosphorylated IκB-α, antibodies (1:1000) at room temperature for 2 h. The membrane was washed 4 times with TTBS and incubated for 30 min with a peroxidase-conjugated secondary antibody (1:5000) at room temperature. Finally, The immunoactive proteins were detected using an enhanced chemiluminescence (ECL) Western blotting detection kit (Amersharm Pharmacia Biotech., NY, USA).
Statistical analysis
Results are presented as the means ± standard deviation of at least three replicates. The Student t-test was used for statistical analyses of the difference noted. P values of 0.05 or less were considered statistically significant.
Results and discussion
Brown algae have proven to be rich sources of structurally novel and biologically active natural compounds in recent study. These compounds have served as important chemical prototypes for the discovery of new drugs for use in the treatment of various human diseases [15]. Brown algae are also very popular sea vegetables, and many people consider this vegetable as a food of health benefit in East Asia such as Korea, China, and Japan. Jeju Island, the largest island in Korea, is located in the southwest of the Korean Strait, and is well known for its distinctive environment. In particular, the sea levels around this island are known to fluctuate rapidly as a result of global warming. Therefore, in response to this unusual environment, the brown algae that are present on Jeju Island may possess substantial endogenous protective mechanisms [12]. Some studies on brown algae-derived anti-inflammatory compounds have investigated potential inhibitory effects by using the LPS-stimulated murine macrophages [16–18]. Previously, we found that the a S. muticum extract displayed an appreciable anti-inflammatory effect in mouse macrophage RAW264.7 cells [11]. In the present study, we isolated the active substance, in an attempt to understand the possible anti-inflammatory mechanism of S. muticum. To identify its active components, the ethanol extract was suspended in H2O and extracted successively with n-hexane, methylene chloride, ethyl acetate, and n-butanol. The methylene chloride fraction was subjected repeatedly to column chromatography over celite and silica gel in various solvent systems, to yield the active ingradent. It was identified as apo-9′fucoxanthinone (Figure 1) by comparison of physical and spectroscopic data with published values.
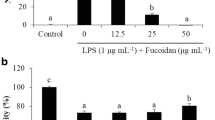
In murine macrophage RAW264.7 cells, LPS alone induces the transcription and protein synthesis of iNOS and COX-2, which produce large amounts of NO and PGE2, respectively. Excess production of NO by iNOS has been implicated in a wide spectrum of diseases including septic shock, rheumatoid arthritis, cerebral ischemia, multiple sclerosis, and diabetes [19]. For this reason, NO production induced by LPS through iNOS can reflect the degree of inflammation, and a change in NO level through inhibition of iNOS enzyme activity or iNOS induction provides a means of assessing the effect of agents on the inflammatory process. Therefore, the modulation of macrophage-mediated inflammatory responses is emerging as a promising new therapeutic approach against inflammatory diseases [12–14, 20, 21]. In an effort to characterize the anti-inflammatory activities of apo-9′fucoxanthinone, we firstly assessed the effects of apo-9′fucoxanthinone on LPS induced NO production in RAW 264.7 cells. Since the half-life of NO is very short, we used nitrite production as an indicator of NO released by LPS-activated macrophages. As shown in Figure 2A, compared to in normal macrophages, NO production increased >15 fold in LPS-activated macrophages. apo-9′-fucoxanthinone reduced LPS-induced NO production in a dose-dependent manner: At apo-9′-fucoxanthinone concentrations of 12.5 μg/mL, 25 μg/mL, 50 μg/mL, and 100 μg/mL, the production of NO by LPS-treated macrophages decreased, as compared with LPS-treated macrophages not treated with apo-9′-fucoxanthinone (Figure 2A). DMSO, the vehicle control, had no effect on NO production (data not shown), reconfirming its immunological inertness. In parallel, the potential cytotoxicity of apo-9′-fucoxanthinone was evaluated by an MTT assay after incubating cells for 24 h in the absence and presence of LPS. However, cell viability was negligibly affected at the concentrations used (12.5 μg/mL, 25 μg/mL, 50 μg/mL, and 100 μg/mL) to inhibit NO (Figure 2A). Thus, the inhibitory effects of apo-9′-fucoxanthinone were not attributable to cytotoxicity. To further elucidate the mechanisms by which apo-9′-fucoxanthinone inhibited NO production in LPS-activated macrophages, we analyzed apo-9′-fucoxanthinone’s effect on LPS-induced iNOS gene expression in macrophages. Under normal conditions, RAW 264.7 cells expressed non-detectable levels of iNOS mRNA, but iNOS mRNA levels increased markedly after 24 h of LPS stimulation (Figure 3A). With the addition of apo-9′-fucoxanthinone (12.5 μg/mL - 100 μg/mL), dose-dependent inhibition of iNOS expression was observed, indicating that apo-9′-fucoxanthinone modulates iNOS expression.
Effect of Apo-9′-fucoxanthinone on nitric oxide and PGE 2 production in LPS-stimulated RAW264.7 cells. The cells were stimulated with 1 μg/mL of LPS only or with LPS plus various concentrations (12.5, 25, 50, and 100 μg/mL) of Apo-9′ for 24 hr. Nitric oxide production was determined by the Griess reagent method. After a 24-h incubation, PGE2 in the culture supernatants was measured by an enzyme-linked immunosorbent assay (ELISA) kit. Cell viability was determined from the 24 hr culture of cells stimulated with LPS (1 μg/mL) in the presence of Apo-9′. The data represent the mean ± SD of triplicate experiments.*P < 0.05, **P < 0.01 versus LPS alone.
Effect of Apo-9′-fucoxanthinone on the activation of iNOS and COX-2 in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells (5.0 × 105 cells/mL) were stimulated with LPS (1 μg/mL) in the Apo-9′ (12.5, 25, 50, and 100 μg/mL) for 24 hr. Whole-cell lysate (25 μg) were prepared and the protein level was subjected to 10% SDS-PAGE, and expression of iNOS, COX-2, and β-actin were determined by Western blotting.
PGE2 is an inflammatory mediator that is produced from the conversion of arachidonic acid by cyclooxygenase. In a variety of inflammatory cells, including macrophages, COX-2 is induced by cytokines and other activators, such as LPS, resulting in the release of a large amount of PGE2 at inflammatory sites. Numerous studies have reported that prostaglandin (PGE)2 participate in inflammatory and nociceptive events [22–24]. Therefore its ubiquitous role in the pathogenesis of inflammatory gene expression, PGE2 is a current target for treating various diseases. For this reason, we next examined the effects of apo-9′-fucoxanthinone on PGE2 production in LPS-stimulated RAW 264.7 macrophages. Cells were pre-incubated with apo-9′-fucoxanthinone for 1 h, following which they were stimulated with 1 μg/mL LPS for 24 h. As shown in Figure 2B, Compared to unstimulated macrophages, the PGE2 level increased dramatically by 15-fold in LPS-stimulated macrophages. With the addition of apo-9′-fucoxanthinone (12.5 μg/mL, 25 μg/mL, 50 μg/mL, and 100 μg/mL) a dose-dependent reduction in PGE2 was observed (Figure 2B). In order to determine the mechanism by which apo-9′-fucoxanthinone reduces LPS-induced PGE2 production, we studied the ability of apo-9′-fucoxanthinone to influence the LPS-induced expression of COX-2. The addition of LPS resulted in a clearly defined increase in COX-2 expression that was markedly attenuated in a dose-dependent fashion when treated with apo-9′-fucoxanthinone (Figure 3B), corroborating that apo-9′-fucoxanthinone induces a decrease in COX-2, which translates into a dramatic decrease in PGE2.
NF-κB activation, in response to pro-inflammatory stimuli, involves the rapid phosphorylation of IκBs by the IKK signalosome complex. Free NF-κB produced by this process translocates to the nucleus, where it binds to κB-binding sites in the promoter regions of target genes. It then induces the transcription of pro-inflammatory mediators such as iNOS and COX-2. Actually, several studies have shown that anti-inflammatory agents inhibit the activation of NF-κB by preventing IκB degradation [25–27]. Thus, we attempted in this study to determine whether or not apo-9′-fucoxanthinone inhibits the phosphorylation and degradation of IκB. Accordingly, RAW 264.7 cells were pretreated for 30 min with 9′fucoxanthinone, and IκB-α protein levels were determined after 15 min of further LPS exposure (1 μg/mL). As shown in Figure 4, apo-9′-fucoxanthinone was shown to significantly suppress the LPS-induced IκB-α degradation. As expected, the reference compounds 2-amino-4-methyl pyridine (iNOS inhibitor) also potently inhibited the LPS-induced IκB-α degradation at 40 μM. These results show that apo-9′-fucoxanthinone inhibits LPS induced NF-κB activation by preventing the IκB-α degradation.
Effects of Apo-9′-fucoxanthinone on the degradation of IκB-α in LPS stimulated RAW 264.7 cells. RAW 264.7 cells (1.0 × 106 cells/mL) were stimulated with LPS (1 μg/mL) in the presence of apo-9′-fucoxanthinone (12.5, 25, 50, and 100 μg/mL) or PDTC (40 μM) for 15 min. Whole cell lysates (30 ug) were prepared and the protein level was subjected to 12% SDS-PAGE, and expression of IkB-α and β-actin were determined by Western blotting. The β-actin antibody as a loading control.
Conclusions
The results of this study reveal, for the first time, that the apo-9′-fucoxanthinone isolated from S. muticum exhibit anti-inflammatory properties through suppressing NO and PGE2 production in LPS-stimulated RAW 264.7 cells by attenuation of NF-κB-mediated iNOS and COX-2 expression. It is proposed that that apo-9′-fucoxanthinone is a potential anti-inflammatory agent and may be used in the future to treat inflammation-associated human health. To our knowledge, this is the first report concerning the evaluation of the anti-inflammatory properties of apo-9′-fucoxanthinone.
References
Liu SX, Jin HZ, Shan L, Zeng HW, Chen BY, Sun QY, Zhang WD: Inhibitory effect of 4,4′-dihydroxy-α-truxillic acid derivatives on NO production in lipopolysaccharide-induced RAW 264.7 macrophages and exploration of structure-activity relationships. Bioorg Med Chem Lett. 2013, 23: 2207-22311. 10.1016/j.bmcl.2013.01.091.
Medeiros A, Peres-Buzalaf C, Fortino Verdan F, Serezani CH: Prostaglandin E2 and the suppression of phagocyte innate immune responses in different organs. Mediators Inflamm. 2012, 2012: 327568-
Aoki T, Narumiya S: Prostaglandins and chronic inflammation. Trends Pharmacol Sci. 2012, 33: 304-311. 10.1016/j.tips.2012.02.004.
Wang S, Xu Y, Jiang W, Zhang Y: Isolation and identification of constituents with activity of inhibiting nitric oxide production in RAW 264.7 macrophages from Gentiana triflora. Planta Med. 2013, 79: 680-686.
Yan M, Zhu Y, Zhang HJ, Jiao WH, Han BN, Liu ZX, Qiu F, Chen WS, Lin HW: Anti-inflammatory secondary metabolites from the leaves of Rosa laevigata. Bioorg Med Chem. 2013, 21: 3290-3297. 10.1016/j.bmc.2013.03.018.
Lee J, Yang G, Lee K, Lee MH, Eom JW, Ham I, Choi HY: Anti-inflammatory effect of Prunus yedoensis through inhibition of nuclear factor-kappaB in macrophages. BMC Complement Altern Med. 2013, 13: 92-10.1186/1472-6882-13-92.
Chen TY, Sun HL, Yao HT, Lii CK, Chen HW, Chen PY, Li CC, Liu KL: Suppressive effects of Indigofera suffruticosa Mill extracts on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 macrophages. Food Chem Toxicol. 2013, 55: 257-264.
Hwang PA, Chien SY, Chan YL, Lu MK, Wu CH, Kong ZL, Wu CJ: Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J Agric Food Chem. 2011, 59: 2062-2068. 10.1021/jf1043647.
Chen JH, Lim JD, Sohn EH, Choi YS, Han ET: Growth-inhibitory effect of a fucoidan from brown seaweed Undaria pinnatifida on Plasmodium parasites. Parasitol Res. 2009, 104: 245-250. 10.1007/s00436-008-1182-2.
Kim JY, Lee JA, Kim KN, Yoon WJ, Lee WJ, Park SY: Antioxidative and antimicrobial activities of Sargassum muticum extracts. J Korean Soc Food Sci Nutr. 2007, 36: 663-669. 10.3746/jkfn.2007.36.6.663.
Yoon WJ, Ham YM, Lee WJ, Lee NH, Hyun CG: Brown alga Sargassum muticum inhibits proinflammatory cytokines, iNOS, and COX-2 expression in macrophage RAW 264.7 cells. Turk J Biol. 2010, 34: 25-34.
Yang EJ, Moon JY, Kim MJ, Kim DS, Kim CS, Lee WJ, Lee NH, Hyun CG: Inhibitory effect of Jeju endemic seaweeds on the production of pro-inflammatory mediators in mouse macrophage cell line RAW 264.7. J Zhejiang Univ Sci B. 2010, 11: 315-322.
Yang EJ, Moon JY, Kim MJ, Kim DS, Lee WJ, Lee NH, Hyun CG: Anti-inflammatory effect of Petalonia binghamiae in LPS-induced macrophages is mediated by suppression of iNOS and COX-2. Int J Agri Biol. 2010, 12: 754-758.
Yang EJ, Ham YM, Kim DS, Kim JY, Hong JP, Kim MJ, Moon JY, Lee WJ, Lee NH, Hyun CG: Ecklonia stolonifera inhibits lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and proinflammatory cytokines in RAW264.7 macrophages. Biol. 2010, 65: 362-371.
Ham YM, Kim KN, Lee WJ, Lee NH, Hyun CG: Chemical constituents from Sargassum micracanthum and antioxidant activity. Int J Pharmacol. 2010, 6: 147-151. 10.3923/ijp.2010.147.151.
Yoon WJ, Heo SJ, Han SC, Lee HJ, Kang GJ, Kang HK, Hyun JW, Koh YS, Yoo ES: Anti-inflammatory effect of sargachromanol G isolated from Sargassum siliquastrum in RAW 264.7 cells. Arch Pharm Res. 2012, 35: 1421-1430. 10.1007/s12272-012-0812-5.
Heo SJ, Yoon WJ, Kim KN, Oh C, Choi YU, Yoon KT, Kang DH, Qian ZJ, Choi IW, Jung WK: Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chem Toxicol. 2012, 50: 3336-3342. 10.1016/j.fct.2012.06.025.
Dutot M, Fagon R, Hemon M, Rat P: Antioxidant, anti-inflammatory, and anti-senescence activities of a phlorotannin-rich natural extract from brown seaweed Ascophyllum nodosum. Appl Biochem Biotechnol. 2012, 167: 2234-2240. 10.1007/s12010-012-9761-1.
Galea E, Feinstein DL: Regulation of the expression of the inflammatory nitric oxide synthase (NOS2) by cyclic AMP. FASEB J. 1999, 13: 2125-2137.
Kanwar JR, Kanwar RK, Burrow H, Baratchi S: Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Cur Med Chem. 2009, 16: 2373-2394. 10.2174/092986709788682155.
Murakami A: Chemoprevention with phytochemicals targeting inducible nitric oxide synthase. Forum Nutr. 2009, 61: 193-203.
Scher JU, Pillinger MH: The anti-inflammatory effects of prostaglandins. J Invest Med. 2009, 57: 703-708.
Iyer JP, Srivastava PK, Dev R, Dastidar SG, Ray A: Prostaglandin E(2) synthase inhibition as a therapeutic target. Expert Opin Ther Targets. 2009, 13: 849-865. 10.1517/14728220903018932.
Rao P, Knaus EE: Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharmaceut Sci. 2008, 11: 81s-110s.
Kanarek N, Ben-Neriah Y: Regulation of NF-κB by ubiquitination and degradation of the IκBs. Immunol Rev. 2012, 246: 77-94. 10.1111/j.1600-065X.2012.01098.x.
Kwak JH, Jung JK, Lee H: Nuclear factor-kappa B inhibitors; a patent review (2006–2010). Expert Opin Ther Pat. 2011, 21: 1897-1910. 10.1517/13543776.2011.638285.
Skaug B, Jiang X, Chen ZJ: The role of ubiquitin in NF-kappaB regulatory pathways. Annu Rev Biochem. 2009, 78: 769-796. 10.1146/annurev.biochem.78.070907.102750.
Acknowledgements
This research was supported financially by the Ministry of Trade, Industry & Energy, Korea Institute for Advancement of Technology through the Inter-ER Cooperation Projects (R0002016). We are grateful to Jeju Technopark for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EJY carried out the anti-inflammatory evaluation. YMH carried out the isolation and purification apo-9’-fucoxanthinone. WJL carried out the preparation and identification of alga material. NHL carried out the interpretation of the NMR data and identification of the compounds. CGH conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Eun-Jin Yang, Young Min Ham contributed equally to this work.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yang, EJ., Ham, Y.M., Lee, W.J. et al. Anti-inflammatory effects of apo-9′-fucoxanthinone from the brown alga, Sargassum muticum. DARU J Pharm Sci 21, 62 (2013). https://doi.org/10.1186/2008-2231-21-62
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2008-2231-21-62