Abstract
Background and the purpose of the study
Blood contact with artificial surfaces of the extracorporeal circuit and ischemia-reperfusion injury in CABG with CPB, may lead to a systemic inflammatory response. Hypertonic saline have been recently investigated as a fluid in order to decrease inflammatory response and cytokines generation in patients undergo cardiac operations. Our purpose is to study the prophylactic effect of HS 5% infusion versus NS on serum IL-6 as an inflammatory & IL-10 as an anti-inflammatory biomarker in CABG patients.
Methods
The present study is a randomized double-blinded clinical trial. 40 patients undergoing CABG were randomized to receive HS 5% or NS before operation. Blood samples were obtained after receiving HS or NS, just before operation, 24 and 48 hours post-operatively. Plasma levels of IL-6 and IL-10 were measured by ELISA.
Results and major conclusion
Patients received HS had lower levels of IL-6 and higher level of IL-10 compared with NS group, however these differences were not statistically significant. Results of this study suggest that pre-treatment with small volume hypertonic saline 5% may have beneficial effects on inflammatory response following CABG operation.
Similar content being viewed by others
Introduction
Infusion of Hypertonic saline (HS) solution increases serum osmolarity and markedly intravascular and interstitial fluid volume expansion, which causes improving hemodynamic status[1]. Fluid resuscitation with various concentrations of HS solutions (1.8% - 7.5%) has been investigated in different types of hypovolemic shock[1]; pre-operative, intra-operative and post-operative fluid therapy[2], burn injury and also septic shock[1]. HS is inexpensive and has no risk of anaphylactoid reactions compared with other artificial plasma volume expanders. There is no risk of transmission of infectious agents compared with human plasma[3]. Rapid correction of intravascular volume is achieved with a small infused volume (4 ml/kg)[1].
Recent studies demonstrated immunomodulatory effects of hypertonic saline by blunting neutrophil activation and reducing cytokine production[4, 5].
Cardiac surgery with cardiopulmonary bypass (CPB) leads to acute changes in the composition and volume of body fluid compartments. CPB dilutes serum proteins, decreases the plasma colloid osmotic pressure and reduces endothelial integrity[6]. This causes fluid shifts from the intravascular to extravascular space and leads to a 33% increase in extravascular fluid space and tissue edema[7]. Complement activation following with systemic inflammatory response syndrome (SIRS) occurs during extracorporeal circulation[6]. Depending on the severity, inflammatory response can cause cerebral, myocardial, pulmonary and renal dysfunction[6].
In this study it was hypothesized that administration of HS just before coronary artery bypass surgery (CABG) was be helpful in decreasing of generated inflammatory cytokines because of increasing in intravascular volume and tissue perfusion.
Material & methods
Study design & Patient population: The present study is a randomized double-blinded clinical trial. 40 patients <70 years old, undergoing elective CABG surgery admitted in Tehran Heart Center, Tehran University of Medical Sciences, between December 2010 & March 2011, entered into the study. Patients were randomly assigned to control (A) and intervention (B) groups, based on a computerized randomization sequence form. All patients had undergo necessary clinical and paraclinical examinations including: CBC, Diff, Electrolytes, PFT, LFT, renal function tests, chest X ray, ECG, Cardiac Enzyme Assay, PT, PTT, INR, thyroid tests, blood group and matching, Carotid Doppler,…, patient educations, consultations, and preparing processes before surgery.
All the perioperative cares and also surgeries were carried out by the same (single) surgeon and intensivist who were blinded to the treatment groups. Patients also were blinded to the choice of fluids.
All patients had ejection fraction (EF) >35% and their serum creatinine were between 0.5-1.5 mg/dl pre-operatively. Our research was approved by Pharmaceutical Sciences Research Center ethics committee by ethical code of 89-7-7:15–1 according to the declaration of Helsinki and written informed consent was obtained from the legal guardian of each patient before enrollment. Exclusion criteria included any concomitant operation, recent myocardial infarction (during 6 months), emergent surgery, urgent or elective intubation before surgery, unstable hemodynamic status, recent cerebral vascular accident, neurological complications or consciousness disorder or significant neuralgic defect, head trauma, uncontrolled diabetes mellitus, congenital heart disorder, blood transfusion before operation, pre-operative infection, serum creatinine >1.5 mg/dl or GFR < 50, Na > 150 mmole/dl, BMI > 40 and Chronic Obstructive Pulmonary Disease (COPD) or abnormal pulmonary function test [respiratory distress, Carbon Dioxide Pressure (PCO2) > 45 mmHg and Oxygen Pressure (PO2) < 60 mmHg, Forced Expiratory Volume in 1 second (FEV1) < 60% or Forced Vital Capacity (FEV1/ FVC) < 60% and Vital Capacity (VC) <50%].
Patients were randomized to receive 100 ml hypertonic saline (5%) + 400 ml normal saline (0.9%) equivalent to 155 mmole NaCl (group B) or 500 ml normal saline (0.9%) equivalent to 77 mmole NaCl (group A); in a uniform packages from a peripheral venous line in upper arm during 6 hour before surgery.
All preparation and treatment measurements were performed in a unique manner based on the attending hospital protocols. Patients received oxazepam 10 mg/po at the night before surgery, promethazine 25 mg/ po 1 hour before surgery, and clonidine 0.1 mg/po before surgery as premedication. Patients were NPO (except medication) for 8 hour before surgery.
During surgery all patients had standard continuously monitoring of ECG, pulse oximetery, invasive blood pressure monitoring, non- invasive blood pressure monitoring, central venous pressure, end tidal capnometery, central and peripheral temperature monitoring, bipectoral index, and also intermittent monitoring of arterial blood gas, Na, K, BS, CBC, ACT test for heparin loading & reversal. All patients received the same anesthetic regimen and routine CPB management. Anesthesia was induced by midazolam (0.05 mg/kg), fentanyl (5 mcg/kg), propofol 2 mg/kg and pancuronium (0.1 mg/kg), and was maintained with propofol infusion (10 mg/kg/h) and additional doses of fentanyl & pancuronium.
Just after ICU admission, cuff pressure was controlled and it has monitored every 8 hrs. The ventilator machine was set on SIMV-PSV mode (SIMV: Synchronized Intermittent Mechanical Ventilation, PSV: Pressure Support Ventilation), based on tidal volume = 8 cc/kg of ideal body weight, F (IMV) = 10/min, F (IMV + PSV) = 15/min, I/E (Inspiratory/Expiratory) = 1/2, PEEP (Positive End-Expiratory Pressure) = 5 cmH2O, PSV = 5 cmH2O above PEEP (total inspiratory pressure support = 10 cmH2O), pressure support = 10 cmH2O, oxygen flow = 60 lit/min and FiO2 =50% at first, then adjusted based on PaO2. Subsequently ventilation was adjusted based on ABG, and other laboratory and clinical parameters. Furthermore, RSBI (rapid shallow breathing index), which is the proportion of respiratory rate/ tidal volume, has been considered as a criteria for the extubation; if this proportion was >105, patients could not stand the extubation. The recruitment maneuver was done for all of patients before extubation.
Specimen collection: A 5 ml sample of blood was obtained from each patient after receiving HS or NS, just before operation and 24 and 48 hrs post-operatively, and anonymously referred to local laboratory to be centrifuged and the serum stored at −70°C. Plasma IL-6 & IL-10 levels were measured. We also monitored heart rate, systolic and diastolic blood pressure, central venous pressure, arterial pH, PaO2, FIO2, blood sugar, Na, K, Mg, hemoglobin, white blood cell, hematocrit and platelet for each patient before and after surgery.
Marker measurements: Enzyme-Linked Immunosorbent Assay (ELISA) technique was used to measure IL-6 (BMS213/2CE) and IL-10 (BMS215/2CE) (eBioscience®, Austria) samples plasma level according to manufacturer’s instructions.
Statistical Analysis: Data were analyzed using the statistical software SPSS version 15.0 for windows (SPSS Inc, Chicago, IL). Continuous variables are presented as mean ± standard deviation (SD) or mean ± standard error of mean (SEM), while categorical variables are summarized by absolute frequencies and percentages. Continuous variables were compared using the Student's t-test or nonparametric Mann–Whitney U test, whenever the data did not appear to have normal distributions; and categorical variables were compared using Chi-Square test, as required. Repeated measure ANOVA was used to evaluate inter-group differences and intra-group changes. All p values <0.05 were considered statistically significant.
Result
An equal number of patients received NS or HS. There were no differences in demographic data (Table 1). Detailed clinical information of HS and control group has been summarized in Table 2.
Of the 40 patients included, 4 in HS group versus 2 in NS group had received inotropic drug within the first 24 hrs after surgery, however the different was not statistically significant (0.336) (Table 2). Plasma concentration of sodium was nearly similar in two groups which indicate that hypernatremia was not happened as a side effect of HS use. Also serum concentration of other electrolytes (potassium & magnesium) were not different between two groups (Table 2).
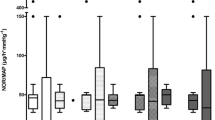
The plasma levels of pro-inflammatory (IL-6) and anti-inflammatory (IL-10) cytokines were significantly changed during 48 hrs post-operatively in two groups (p < 0.001 & p < 0.005 respectively) (Figure 1). The mean of IL-6 levels at 24 hrs and 48 hrs post-operatively in HS group were lower than NS group, but this differences were not statistically significant (p = 0.437). The mean of IL-10 levels pre-operatively and at 24 hrs and 48 hrs post-operatively in HS group were higher than NS group, but the mean levels between two groups were not statistically significant (p = 0.276).
IL-6 levels rose significantly during 24 hrs in both groups but its changes were more considerable in NS group in comparison to HS group, while the mean level was 145.53 pg/ml 24 hrs post-operatively in HS group versus 174.41 pg/ml in NS group. We had a decline in IL-6 levels between 24–48 h after surgery in two groups; the mean level after 48 h was 113.13 pg/ml for HS group and 146.97 pg/ml for NS group (Table 3, Figure 1). During the first 24 h after surgery IL-10 levels in two groups rose from baseline and its level in HS group (mean value: 12.55) was higher than NS group (mean value: 10.04). IL-10 levels manifested a decline 48 h after operation and mean IL-10 level was 8.35 in HS group and 6.24 in NS group. (Table 3, Figure 1).
Discussion
Patients undergoing CABG surgery with CPB, experience many aggressive factors, including operative trauma, cardioplegia, ischemia-reperfusion injury and the contact of blood with bioactive surfaces that potentially related to a whole body inflammatory response[8]. Although this inflammatory reaction to CPB often remains at subclinical levels, it can also lead to important clinical implications. In 1999 the Society of Thoracic Surgeons National Database, reported that 20% of “low-risk” patients developed post-operative complications[9]. Another work in these patients had shown the incidence of multiple organ dysfunction syndrome (MODS) following CPB was 11%, with a mortality rate of 41%[6]. Various studies have investigated different methods to reduce this vigorous inflammatory response following cardiac surgery with CPB; include perioperative administration of corticosteroids[10], aprotinin[11], statins[12], pentoxifylline[13], milrinone[14], ketamine[15], and bovine intestinal alkaline phosphatase[16].
A review article by Pasnik J[17] indicates that mechanism of inflammatory response to CPB is due to neutrophil activation, degranulation and endothelial dysfunction.
Ischemia-reperfusion injury that often occur following aortic declamping, cause a significant elevation in plasma cytokine levels during and after cardiac surgery under CPB. Consequences of reperfusion injury in major organs such as heart, lung, and central nervous system, include myocardial stunning and alteration in beta-receptor function (that related to transient and chronic myocardial ischemia respectively)[18]; acute respiratory distress syndrome (ARDS) and prolonged mechanical ventilation[19]; delirium, encephalopathy, stroke and cognitive brain dysfunction[20]; might infringe the clinical benefit of interventions employing extracorporeal circulation and clamping of the aorta.
HS solutions have been examined in numerous studies as plasma volume expanders for resuscitation in various hypovolemic situations but there is little attention to their immunomodulatory effects. Basic science studies have demonstrated that HS has marked effects on the immune system. More investigations have confirmed that HS blunted neutrophil (PMNs) activation and diminished the monocytes production profile in patients with traumatic hemorrhagic shock[1]. HS solution also reduced pro-inflammatory tumor necrosis factor (TNF)-alpha production significantly, while increasing anti-inflammatory IL-10, thus altering the balance between pro-inflammatory and anti-inflammatory cytokines[4, 5]. Coelho and collageous[21], demonstrated that expression of cyclooxygenase (COX)-2 and inducible nitric oxide synthase (iNOS) was markedly increased in the pancreas of the acute pancreatitis patients and was reduced by treatment with HS. HS also reduced the levels of TNF-alpha and IL-6 but not of IL-10 in the pancreatic tissue. In another study, Coimbra and collageous[22] demonstrated that HS reduce traumatic shock induced T-cell dysfunction.
IL-6 is produced by monocytes, lymphocytes, and endothelial cells. IL-6 induces the adhesive neutrophil-cardiac myocyte interaction and myocardial damage following CPB surgery. Highest plasma level of IL-6 significantly correlated with the duration of SIRS[23] and also outcome in other setting of SIRS positive patients like[24–27]. Results of this study indicated anti-inflammatory effect of pre-operative administration of HS in patients undergoing CABG (Figure 1). IL-6 plasma levels were lower (but not statistically significant) in HS group during 48 h after surgery. This effect may be justified by HS osmotic effect. Osmotic effects of HS solution resulted in fluid shift from intracellular to the interstitial and intravascular space[1].
In the state of ischemia (duration of aortic cross clamp) endothelial surface layer thickness and glycocalyx structure have impaired, and dimension of glycocalyx degradation was proportional to the duration of ischemia[28], therefore endothelial cell membrane ion exchange has disturbed and ATP loss has occurred[1]. Destruction of the glycocalyx could be a trigger for increased trans-endothelial permeability leading to the development of tissue edema. Pre-operative use of HS induces normalization of endothelial cell volume and edema that increase capillary diameter and reduce resistance to flow. Therefore plasma viscosity is reduced as a result of the plasma volume expansion[1]. Hypertonicity has a direct relaxant effect on vascular smooth muscle with resultant arteriolar vasodilatation[1]. Overally, these physiological effects result in increased capillary blood flow and this improvement observed in all areas of microcirculation by HS[1].
After declamping aorta and reestablishing the microvascular perfusion, the ischemic process has been stopped by supplying the oxygen and nutrients, but at the same time a cascade of events has similar characteristics to inflammatory response is rapidly initiated. This inflammatory-like response to ischemia-reperfusion, mediated largely by neutrophils[29]. Stimulated polymorphonuclears (PMNs) via the expression of adhesion molecules leading to interaction between endothelial cells therefore play a critical role in extending the tissue damage[30].
IL-10 is a prototypical endogenous anti-inflammatory and immunosuppressive cytokine[31], which inhibits monocyte/macrophage activation and down-regulates the biosynthesis of TNF-α and IL-1β, while preventing their biologic actions via up-regulation of IL-1ra[32]. IL-10 also decreases leukocyte adhesion and its recruitment to sites of inflammation[31]. Together these mediators serve to limit the potentially injurious effects of excessive inflammatory reactions[32]. Several recent investigations have shown that early therapeutic administration of IL-10 is effective in preventing the initial surge in TNF-α observed after traumatic hemorrhagic shock[33], and also in reducing the systemic inflammatory response and lethality in murine models of sepsis and reperfusion injury[34].
Higher levels of IL-10 in HS group of our study may indicate its ability to evoke host anti-inflammatory cytokines to reduce reperfusion damage.
HS solutions have benefits on cardiac outputs, because of the increasing preload (venous return and volume expanding effect) and decreasing afterload (vasodilation and reduction in pulmonary and systemic vascular resistance)[1, 35]. Whether cardiac output improving, because of the direct effect of HS on increasing cardiac contractility and having inotropic effect or due to preload effect must be further investigated[1, 35]. In our study we have found that patients receiving HS required inotropic support more frequently comparing to normal saline group (4 vs. 2 p = 0.366) in the first 24 h after surgery, that may refute cardiac benefits of HS, but not statistically significant. We can explain this phenomenon, that myocardial stunning or altering in beta-receptor function as a result of ischemia-reperfusion injury occurred in some of them. Moreover patients receiving HS had lower systolic and diastolic pressure in comparison with NS receiving group which can due to possible transient hypotensive effect after administration of hypertonic saline, which was noted by Boldt J and collageous's[36]. We can suggest designing another study using HS during CPB, because in this period dropping of blood pressure is more considerable. Looking at the positive clinical trend of our patient’s treatment groups challenged with HS, higher dose of HS could be considered in a larger sample size study following CABG to reach improved statistical and clinical results.
Limited number of eligible candidate for intervention with hypertonic saline before CABG, uni-center nature of the study, high cost of cytokine evaluation and ethical concern regarding the safety of hyperoncotic fluids on subject with venerable hemodynamic profiles were mean parts of our study’s limitations.
Conclusion
Pre-treatment with small volume hypertonic saline may have beneficial effects on inflammatory response following CABG operation.
Authors contribution
MM was responsible for data gathering and manuscript preparation. FY was responsible for study design, patient selection, data gathering, manuscript preparation, MA in-charged for statistical analysis, cytokine bio-assays. HH was responsible for manuscript preparation. KB was responsible for patients’ selection. MAB was responsible for sample preparation and Laboratory tests. AJ was responsible for statistical analysis. AA was in-charged for study design and proposal preparation. SM was involved in study design. MB was involved in data gathering. MM was mean main scientific manager of the study, and was involved in design of the study and proposal preparation, responsible for mean main idea, and discussion for finding of study. All authors read and approved the final manuscript.
References
Strandvik GF: Hypertonic saline in critical care: a review of the literature and guidelines for use in hypotensive states and raised intracranial pressure. Anaesthesia. 2009, 64: 990-1003. 10.1111/j.1365-2044.2009.05986.x.
Azoubel G, Nascimento B, Ferri M, Rizoli S: Operating room use of hypertonic solutions: a clinical review. Clinics. 2008, 63: 833-840.
Vassar MJ, Perry CA, Holcroft JW: Analysis of potential risks associated with 7.5% sodium chloride resuscitation of traumatic shock. Arch Surg. 1990, 125: 1309-1315. 10.1001/archsurg.1990.01410220093013.
Oliveira RP, Velasco I, Soriano F, Friedman G: Clinical review: hypertonic saline resuscitation in sepsis. Crit Care. 2002, 6: 418-423. 10.1186/cc1541.
Rizoli SB, Rhind SG, Shek PN, Inaba K, Filips D, Tien H, Brenneman F, Rotstein O: The immunomodulatory effects of hypertonic saline resuscitation in patients sustaining traumatic hemorrhagic shock: a randomized, controlled, double-blinded trial. Ann Surg. 2006, 243: 47-57. 10.1097/01.sla.0000193608.93127.b1.
Laffey JG, Boylan JF, Cheng DCH: The systemic inflammatory response to cardiac surgery. implications for the anesthesiologist. Anesthesiology. 2002, 97: 215-222. 10.1097/00000542-200207000-00030.
Bueno R, Resende AC, Melo R, Neto VA, Stolf NAG: Effects of hypertonic saline-dextran solution in cardiac valve surgery with cardiopulmonary bypass. Ann Thorac Surg. 2004, 77: 604-611. 10.1016/S0003-4975(03)01486-3.
Butler J, Rocker GM, Westaby S: Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1993, 55: 552-559. 10.1016/0003-4975(93)91048-R.
Grover FL: The Society of Thoracic Surgeons National Database: Current status and future directions. Ann Thorac Surg. 1999, 68: 367-373. 10.1016/S0003-4975(99)00599-8.
Sobieski MA, Graham JD, Pappas PS, Tatooles AJ, Slaughter MS: Reducing the effects of the systemic inflammatory response to cardiopulmonary bypass: can single dose steroids blunt systemic inflammatory response syndrome?. ASAIO J. 2008, 54: 203-206. 10.1097/MAT.0b013e3181640331.
Ferreira CA, Andrade Vicente WV, Barbosa Evora PR, Rogrigues AJ, Klamt JG, Carvalho Panzeli Carlotti AP, Carmona F, Manso PH: Assessment of aprotinin in the reduction of inflammatory systemic response in children undergoing surgery with cardiopulmonary bypass. Rev Bras Cir Cardiovasc. 2010, 25: 85-98. 10.1590/S0102-76382010000100018.
Morgan C, Zappitelli M, Gill P: Statin prophylaxis and inflammatory mediators following cardiopulmonary bypass: a systematic review. Crit Care. 2009, 13: 10.1186/cc8135.
Cagli K, Ulas MM, Ozisik K, Kale A, Bakuy V, Emir M, Balci M, Topbas M, Sener E, Tasdemir O: The intraoperative effect of pentoxifylline on the inflammatory process and leukocytes in cardiac surgery patients undergoing cardiopulmonary bypass. Perfusion. 2005, 20: 45-51. 10.1191/0267659105pf779oa.
Mollhoff T, Loick HM, Van Aken H, Schmidt C, Rolf N, Tjan TD, Asfour B, Berendes E: Milrinone modulates endotoxemia, systemic inflammation, and subsequent acute phase response after cardiopulmonary bypass (CPB). Anesthesiology. 1999, 90: 72-80. 10.1097/00000542-199901000-00012.
Ziberestein G, Levy R, Rachinsky M, Fisher A, Greemberg L, Shapira Y, Appelbaum A, Roytblat L: Ketamine attenuates neutrophil activation after cardiopulmonary bypass. Anesth Analg. 2002, 95: 531-536.
Kats S, Brands R, Seinen W, Jager W, Bekker MW, Hamad MA, Tan ME, Schonberger JP: Anti-inflammatory effects of alkaline phosphatase in coronary artery bypass surgery with cardiopulmonary bypass. Recent Pat Inflamm Allergy Drug Discov. 2009, 3: 214-220. 10.2174/187221309789257388.
Pasnik J: The significance of neutrophil in inflammatory response after cardiac surgery with cardiopulmonary bypass. Wiad Lek. 2007, 60: 171-177.
Leone BJ, Huggins CP, Johns J, McRae RL, Smith B, White W: Acute regional myocardial ischemia and recovery after cardiopulmonary bypass: effects of intensity of antecedent ischemia. J Card Surg. 1995, 10: 396-399. 10.1111/j.1540-8191.1995.tb00668.x.
Asimakopoulos G, Smith PL, Ratnatunga CP, Taylor KM: Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg. 1999, 68: 1107-1115. 10.1016/S0003-4975(99)00781-X.
Royter V, Bornstein NM, Russell D: Coronary artery bypass grafting (CABG) and cognitive decline: a review. J Neurol Sci. 2005, 229–230: 65-67.
Coelho AM, Jukemura J, Sampietre SN, Martins JO, Molan NA, Patzina RA, Lindkvist B, Jancar S, Cunha JE, D'Albuquerque LA, Machado MC: Mechanisms of the beneficial effect of hypertonic saline solution in acute pancreatitis. Shock. 2010, 34: 502-507. 10.1097/SHK.0b013e3181defaa1.
Coimbra R, Junger WG, Liu FC, Loomis WH, Hoyt DB: Hypertonic/hyperoncotic fluids reverse prostaglandin E2 (PGE2)-induced T-cell suppression. Shock. 1995, 4: 45-49.
Hirani S: Systemic inflammatory response syndrome after cardiac surgery under cardiopulmonary bypass. Ann Thorac Cardiovasc Surg. 2003, 9: 365-370.
Hamishehkar H, Beigmohammadi MT, Abdollahi M, Mahmoodpour A, Mirjalili MR, Kanani M, Abrishami R, Baeeri M, Ahmadi A, Khoshayand MR, Mojtahedzadeh M, Eslami K: Identification of enhanced cytokine generation following sepsis. Dream of magic bullet for mortality prediction and therapeutic evaluation. Daru. 2010, 18: 155-162.
Moosivand A, Peivandi Yazdi A, Hamishehkar H, Khalili H, Abdollahi H, Mojtahedzadeh M, Abrishami R: Comparison the inflammatory effects of early supplemental parenteral nutrition plus enteral nutrition versus enteral nutrition alone in critically ill patients. Daru. 2010, 18: 1-2.
Mousavi S, Abdollahi M, Ahmadi A, Najafi A, Pazouki M, Hajibabaie M, Ziaee S, Hamishehkar H, Kebriaeezadeh A, Mojtahedzadeh M: The dilemma of hyperoxia following positive pressure mechanical ventilation: role of iron and the benefit of iron chelation with deferasirox. Eur Rev Med Pharmacol Sci. 2011, 15: 1141-
Ansari G, Mojtahedzadeh M, Kajbaf F, Najafi A, Khajavi MR, Khalili H, Rouini MR, Ahmadi H, Abdollahi M: How does blood glucose control with metformin influence intensive insulin protocols? Evidence for involvement of oxidative stress and inflammatory cytokines. Adv Ther. 2008, 25: 681-702. 10.1007/s12325-008-0075-1.
Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Reichart B, Peter K, Becker BF: Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007, 116: 1896-1906. 10.1161/CIRCULATIONAHA.106.684852.
Jordan JE, Zhao ZQ, Johansen JV: The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res. 1999, 43: 860-878. 10.1016/S0008-6363(99)00187-X.
Schwartz JD, Shamamian P, Schwartz DS, Grossi EA, Jacobs CE, Steiner F, Minneci PC, Baumann FG, Colvin SB, Galloway AC: Cardiopulmonary bypass primes polymorphonuclear leukocytes. J Surg Res. 1998, 75: 177-182. 10.1006/jsre.1997.5287.
Moore KW, Waal Malefyt R, Coffman RL, O'Garra A: Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001, 19: 683-765. 10.1146/annurev.immunol.19.1.683.
Standiford TJ: Anti-inflammatory cytokine and cytokine antagonists. Curr Pharma Des. 2000, 6: 633-649. 10.2174/1381612003400533.
Karakozis S, Hinds M, Cook JW, Kim D, Provido H, Kirkpatrick JR: The effects of interleukin-10 in hemorrhagic shock. J Surg Res. 2000, 90: 109-112. 10.1006/jsre.2000.5860.
Kahlke V, Dohm C, Mees T, Brotzmann K, Schreiber S, Schroder J: Early interleukin-10 treatment improves survival and enhances immune function only in males after hemorrhage and subsequent sepsis. Shock. 2002, 18: 24-28.
Kramer GC: Hypertonic resuscitation: physiologic mechanisms and recommendations for trauma care. J Trauma. 2003, 54: 89-99.
Boldt J, Zickmann B, Ballesteros M, Herold C, Dapper F, Hempelmann G: Cardiorespiratory responses to hypertonic saline solution in cardiac operations. Ann Thorac Surg. 1991, 51: 610-615. 10.1016/0003-4975(91)90320-P.
Acknowledgment
This study was granted by Pharmaceutical Sciences Research Center, Tehran University of Medical Sciences. The authors would like to express their deep appreciation to the stuff of Tehran Heart Center and Gholhak laboratory who accompanied us sincerely with this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Mahnaz Mazandarani, Fardin Yousefshahi, Mohammad Abdollahi, Hadi Hamishehkar, Khosro Barkhordari, Mohammad Ali Boroomand, Arezoo Ahmadi, Reza Shariat Moharari, Mona Bashirzadeh and Mojtaba Mojtahedzadeh contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mazandarani, M., Yousefshahi, F., Abdollahi, M. et al. Comparison of hypertonic saline versus normal saline on cytokine profile during CABG. DARU J Pharm Sci 20, 49 (2012). https://doi.org/10.1186/2008-2231-20-49
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2008-2231-20-49