Abstract
Background
Primulina cardaminifolia Yan Liu & W.B. Xu (Gesneriaceae), a distinct new species with imparipinnate leaves, is described and illustrated from a limestone valley in Guangxi Zhuangzu Autonomous Region, China. To assure its generic placement and phylogenetic affinity, phylogenetic analyses were performed using DNA sequences of nuclear ITS and chloroplast trnL-F intron spacer region. Additionally, somatic chromosome number was counted and pollen stainability was tested.
Results
Phylogenetic analyses support its placement in Primulina; however, two phylogenetically distinct ITS sequence types were detected, suggesting a probable hybrid origin. Its pollen stainability is 100% and its chromosome number, 2n = 36, is congruent with all known counts of diploid species of the genus.
Conclusion
All available data support the recognition of the new species Primulina cardaminifolia and suggest that it could have derived from homoploid hybrid speciation. Color plates, line drawings and a distribution map are provided to aid in identification.
Similar content being viewed by others
Background
Recent progress in molecular phylogenetic studies of the Old World Gesneriaceae has foreseen the restructuring of the highly heterogeneous Chirita D. Don (Li and Wang 2007; Möller et al. 2009; Möller et al. 2011). However it was unexpected that Chirita has been forsaken (Wang et al. 2011; Weber et al. 2011) and the formerly monotypic Primulina Hance recircumscribed and expanded to include Chirita sect. Gibbosaccus C.B. Clarke, Chiritopsis W.T. Wang, and two species of Wentsaiboea D. Fang & D.H. Qin (Wang et al. 2011; Weber et al. 2011). With over 130 species transferred to (Wang et al. 2011; Weber et al. 2011; Xu et al. 2012b) and more than ten species newly described (Liu et al. 2011; Hong et al. 2012; Huang et al. 2012; Li et al. 2012; Wen et al. 2012abc; Wu et al. 2012a2012b; Xu et al. 2012a; Chung et al. 2013), Primulina has now become one of the largest genera of the Old World Didymocarpoid Gesneriaceae. Under this new delimitation, Primulina is essentially a calciphilous genus distributed in southern China and adjacent northern Vietnam, with the major center of diversity in the limestone karsts of Guangxi Zhuangzu Autonomous Region (Wang et al. 1998; Li and Wang 2004; Hou et al. 2010; Wei 2010; Weber et al. 2011; Xu et al. 2012ab).
Although the new generic circumscription reflects better the evolutionary relationships and ecological preferences of the genus, diagnostic characters of Primulina, such as perennial habit and acaulescent rosette (2011) and the basic chromosome number, x = 18 (Christie et al. 2012; Liu et al. 2012), are not exclusive to the genus. The lack of strong morphological synapomorphies to distinguish it from related genera in the region necessitates molecular data in ascertaining generic placements (Xu et al. 2012ab). For example, the transfer of Chirita tamiana B.L. Burtt to Primulina [i.e., P. tamiana (B.L. Burtt) Mich. Möller & A. Weber] made without support from molecular data by Weber et al. (2011) was recently challenged by chromosomal cytology and molecular data (Christie et al. 2012). Similarly, our ongoing molecular phylogenetic studies have also identified a number of misplacments in Primulina (Chung, unpubl. data).
In the course of a floristic survey in central Guangxi in 2007, a distinct species of Gesneriaceae with imparipinnate leaves was collected by the authors. After consulting national and local floras and the relevant literature (Wang et al. 1998; Li and Wang 2004; Wei 2010; Liu et al. 2011; Wang et al. 2011; Wu et al. 2012a; Xu et al. 2012ab) as well as herbarium specimens, we conclude that it represents a new species of Primulina, which is described and illustrated here. Chromosome count of the new species is in agreement with those reported for Primulina in Christie et al. (2012). Its generic placement is further confirmed by molecular and chromosome data.
Methods
Chromosome preparations
The plant for chromosome studies was collected from the type locality and cultivated in the experimental greenhouse of Academia Sinica, Taipei. A voucher specimen (Ku et al. 2035) has been deposited in HAST. Root tips were gathered and pretreated in 2 mM 8-hydroxyquinoline at 15–18°C for about 6 h and fixed overnight in an ethanol-acetic acid solution (3:1) below 4°C. The chromosomes were stained and macerated in 2% acetic orcein with 1 N hydrochloric acid (10:1). Classification of chromosome complement based on centromere position at mitotic metaphase followed Levan et al. (1964).
Molecular methods
DNA sequences of the nuclear ribosomal internal transcribed spacers (ITS) and the chloroplast trnL-F intron spacer region were gathered using protocols outlined in Xu et al. (2012a). Because direct sequencing of the ITS PCR products of the new species resulted in DNA sequences with overlapping signals, molecular cloning was performed. Following the manufacturer’s protocol, the purified ITS templates were ligated to the pGEMT-T vector system (Promega, Madison, Wisconsin, USA) and subsequently transformed into competent cells (DH5α) to perform molecular cloning. After overnight culture at 37°C on the LB ampicillin/IPTG/X-gal selective plate, colonies carrying the ITS insert were identified by color (white) and further verified by PCR using the T7 and SP6 promoter primer pairs (Promega, Madison, Wisconsin, USA). Ten colonies with positive ITS insert were then transferred and grown in 2 μl LB medium at 37°C for 15 h. Plasmids were extracted using the Mini Plus™ Plasmid DNA Extraction System (Viogene, Taipei, Taiwan) and cycle-sequenced using the T7 and SP6 promoter primer pairs.
For phylogenetic analyses, matrices of Xu et al. (2012a) were adopted, keeping one accession for each species (Appendix 1) and ITS and trnL-F regions as separated matrices. Primulina pinnata (W.T. Wang) YinZ. Wang, which was not included in Xu et al. (2012a) because of the presence of an access of ambiguous sites in its ITS sequence in the GenBank (i.e., FJ501349), were added in current analyses to test for its putative relationships with the new species. The final matrix contained 24 species of Primulina with Petrocodon dealbatus Hance, Pet. scopulorum (Chun) YinZ. Wang, and Didymocarpus podocarpus C.B. Clarke chosen as outgroups based on recent phylogenetic analyses (Möller et al. 2011; Weber et al. 2011). The DNA sequences were aligned using the program MUSCLE implemented in the software MEGA5 (Tamura et al. 2011) with minor manual adjustments. Phylogenetic trees were reconstructed separately for ITS and trnL-F based on maximum parsimony (MP) and maximum likelihood (ML) criteria implemented in MEGA5. MP trees were searched using the Tree-Bisection-Reconnection (TBR) search option with the initial trees setting at 50, MP search level setting at 5, and maximum number of trees setting at 2000. Clade supports were calculated based on 100 bootstrap resamplings (parsimony bootstrap; PB). ML trees were reconstructed using the nearest-neighbor-interchange (NNI) method with all site used and the initial tree automatically selected under the model(s) selected by MEGA5. Clade supports of ML analysis were evaluated based on 100 bootstrap resamplings (likelihood bootstrap; LB).
Results and discussion
Taxonomic treatment
Primulina cardaminifolia
Yan Liu & W.B. Xu, sp. nov.—TYPE: CHINA. Guangxi Zhuangzu Autonomous Region, Laibin Shi (City), Fenghuang Zhen (Township), alt. 280 m, on moist limestone rock face in a valley, 14 July 2008, Wei-Bin Xu & Yan Liu 08050 (holotype: IBK; isotypes: HAST and PE). 碎米薺葉報春苣苔 Figures 1, 2.
Diagnosis
Primulina cardaminifolia Yan Liu & W.B. Xu resembles Primulina pinnata (W.T. Wang) YinZ. Wang in having imparipinnate leaves, but is clearly distinct from this species by the ovate-cordate terminal leaflet of 3–7 × 3–6.5 cm, 1 or 2 pairs of broadly ovate to sub-ovate lateral leaflets, 1–3-branched cymes with 3 to 10-flowers, and the entire-margined calyx lobes with acuminate apex.
Description
Herbs perennial. Rhizome subterete, 3–5 mm across. Leaves 5–7, in basal rosette, imparipinnate, 10–20 cm long, papery when dry; petiole subterete, 6–12.5 cm long, densely pubescent; terminal leaflet ovate-cordate, 3–7 × 3–6.5 cm, apex obtuse, base cordate, margin repand to irregularly pinnately lobed, densely pubescent on both surfaces; lateral leaflets 1 or 2 pairs, opposite or alternate, broadly ovate to rotund, 1–3 × 1–3 cm, margin repand to irregularly pinnately lobed, densely pubescent on both surfaces, petiolules short, 2–10 mm long. Cymes 2–4, axillary, 1–3-branched, 3–10-flowered; peduncle 4–8 cm long, 1–2 mm in diam., densely pubescent; bracts 2, opposite, linear-lanceolate, 7–8 × 2–3 mm, margin entire, pubescent; pedicel 4–7 mm long, densely pubescent. Calyx 5-parted to base, lobes lanceolate-linear, 8–12 × 2–3 mm, apex acuminate, outside pubescent, inside glabrous, margins entire. Corolla white to pale purple, 3.2–3.5 cm long, outside glandular-pubescent, inside sparsely puberulent, with 2 pale-yellow stripes; corolla tube 2.1–2.3 cm long, 8–12 mm in diam. at the mouth, 2.5–3 mm in diam. at the base; limb pale purple, distinctly 2-lipped; adaxial lip 2-parted to over the middle, lobes oblong, 8–10 × 6–7 mm; abaxial lip 3-lobed to over the middle, lobes oblong, 12–13 × 4–5 mm; stamens 2, adnate to 1.5 cm above the corolla tube base; filaments linear, ca. 1.2 cm long, geniculate above the base, sparsely glandular-puberulent; anthers ca. 3 mm long, ca. 1.5 mm wide, dorsifixed, glabrous; staminodes 2, ca. 5 mm long, apex capitate, glabrous, adnate to ca. 6 mm above the base of corolla tube. Disc ring-like, ca. 1 mm in height, margin repand, glabrous. Pistil 2.5–2.8 cm long, ovary 7–8 mm long, ca. 1.5 mm across, puberulent; style 1.5–1.8 cm long, ca. 0.6 mm across, puberulent; stigma obtrapeziform, ca. 2 mm long, apex 2-lobed. Capsules not seen.
Additional specimens examined
CHINA. Guangxi Zhuangzu Autonomous Region, Laibin Shi, Fenghuang Zhen, 3 July 2008, Wei-Bin Xu & Yan Liu 08040 (IBK); same locality, 8 Sep 2008, Wei-Bin Xu & Kuo-Fang Chung 08472 (IBK), Shin-Ming Ku et al. 2035 (HAST); same locality, 28 June 2007, Hong-Jin Wei & Wei-Bin Xu 07244 (IBK).
Ecology and distribution
Primulina cardaminifolia is extremely rare, currently known only from the type locality in Laibin Shi, Guangxi Zhuangzu Autonomous Region, China (Figure 3). It grows on a moist limestone rock face in a valley.
Phenology
Flowering from June to July; fruits not observed.
Etymology
The specific epithet is derived from the leaves resembling those of the genus Cardamine L. (Brassicaceae).
Notes
Primulina cardaminifolia resembles Primulina pinnata (W.T. Wang) YinZ. Wang (Figure 4), differing by the terminal leaflet being ovate-cordate, 3–7 × 3–6.5 cm (vs. oblong, 1.6–4.5 × 0.7–2.5 cm); the lateral leaflets 1 or 2 pairs, broadly ovate to rotund, 1–3 × 1–3 cm (vs. 3–5 pairs, oblong to oblong-lanceolate, 0.5–2.5 × 0.4–1.5 cm); cymes 1–3-branched, 3–10-flowered (vs. 1-branched, 1–3-flowered); calyx lobe with acuminate apex and entire margins (vs. acute to obtuse at apex and margins denticulate).
Chromosome cytology
Somatic chromosomes at metaphase of Primulina cardaminifolia were determined to be 2n = 36 (Figure 5). The 36 chromosomes were small and gradually varied from ca. 0.6 μm to 1.3 μm in length. Most chromosomes had centromeres at median positions, while those of some of the shorter chromosomes could not be determined. Satellites were not observed.
In Primulina, chromosome numbers are uniformly diploid with 2n = 36 except for 2n = 32 in P. tamiana that was misplaced in the genus (Christie et al. 2012) and a polyploid with 2n = 72 in P. longgangensis (W.T. Wang) YinZ. Wang (Christie et al. 2012; Liu et al. 2012; Yang et al. 2012). Chromosomes of Primulina at somatic metaphase are generally small, ranging from 0.7 to 1.6 μm (Christie et al. 2012; Liu et al. 2012), to which P. cardaminifolia agrees (Figure 5). Our chromosome count of 2n = 36 in P. cardaminifolia agrees with basic chromosome number, x = 18, and supports its generic placement in the genus.
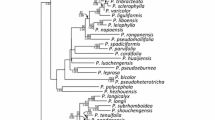
Phylogenetic analyses
Results of molecular cloning of the ITS PCR product revealed two phylogenetically distinct ITS sequences (Figure 6A) with the length of 643 (P. cardaminifolia-A) and 634 (P. cardaminifolia-B) bp, respectively, while only one cpDNA sequence type was detected in the species. With the addition of these two ITS sequences, the ITS matrix contained 28 accessions of 675 aligned positions, of which 182 (26.96%) were parsimoniously informative. Based on the Kimura 2-parameter model using a discrete Gamma distribution (K2 + G) with 5 rate categories selected by the corrected Akaike Information Criterion (AICc) implemented in MEGA5, a single ML tree (log likelihood = −4114.9869) was recovered (Figure 6A). The MP analysis resulted in 6 equally parsimonious trees with 642 steps (CI = 0.70, RI = 0.64, RCI = 0.44). The topology of the strict consensus tree of the 6 equally parsimonious trees was congruent with the ML tree (not shown). The cpDNA matrix contained 27 accessions of 694 aligned positions, of which only 19 (1.3%) were parsimony informative. Based on the Hasegawa-Kishino-Yano (HKY) selected by the AICc implemented in MEGA5, a single ML tree (log likelihood = −1483.76) was obtained (Figure 6B). The MP analysis uncovered 23 equally parsimonious trees with 77 steps (CI = 0.97, RI = 0.97, RCI = 0.94). The topology of the strict consensus tree of the 270 equally parsimonious trees was largely consistent with the ML tree (not shown).
Both ITS and cpDNA dataset placed P. cardaminifolia in Primulina with high (LB = 98, PB = 97) and low (LB = 64, PB = 70) supports in ITS and cpDNA datasets, respectively (Figure 6), confirming its generic placement. Although the phylogenetic trees of the two datasets were not perfectly congruent with each other, especially in the deeper nodes, several well supported subclades were highly consistent between the two trees (Figure 6). Specifically, both datasets identified the clade (Clade A) composed of P. glandulosa, P. repanda var. guilinensis, P. bipinnatifida, P. dryas, P. pinnatifida, and P. multifida. In the ITS tree, the P. cardaminifolia-A sequence was also included in Clade A (Figure 6A). Other congruent clades included the clade consisting of P. spinulosa, P. wentsaii, P. ophiopogoides, and P. minutimaculata, the clade of P. longgangensis and P. linearifolia, and the clade of P. heterotricha and P. pteropoda.
The occurrence of intraindividual ITS polymorphism, or failure of concerted evolution among reiterated loci of ribosomal DNA arrays to nullify various rDNA repeats, could have resulted from hybridization, polyploidization, multiple nucelolar organizing regions on non-homologous chromosomes, rDNA pseudogenization, long generation time, loss of sexual recombination, or extensive introgression during domestication (Denduangboripant and Cronk 2000). The diploid chromosome number 2n = 36 and the presence of two phylogenetically distinct ITS sequence types raised the concern that the distinctive P. cardaminifolia might have been a hybrid (e.g., Peng and Chiang 2000), which has frequently been reported in Gesneriaceae (e.g., Puglisi et al. 2011). Assuming a maternal inheritance of its chloroplast genome, species in Clade B of the cpDNA tree would be the maternal parent, while Clade A could have been its paternal parent (Figure 6). Interestingly, most species in Clade A, including P. glandulosa, P. bipinnatifida, P. pinnatifidai, and P. multifida, are characterized by deeply lobed or pinnatified leaves that are otherwise unknown in Primulina and may have contributed to the unique imparipinnate leaves of P. cardaminifolia (Figures 1 & 2). Nevertheless, the species status of P. cardaminifolia was supported by a 100% pollen fertility suggested by the high level of stainable pollen (Figure 7) using the malachite green-acid fuchsin-orange G stain (Alexander 1969). Given its perfectly developed pollen, the cytological and molecular data instead could have suggested that P. cardaminifolia might be of homoploid hybrid origin, which was formed without changes in chromosome number (cf. Howarth and Baum 2005; Rieseberg and Willis 2007; Abbott et al. 2010). Further studies will be needed to test the probable hybrid and homoploid origin of the new species.
Conclusion
All available data support the recognition of the new species Primulina cardaminifolia, which is described herein. The new species may have arisen via homoploid hybrid speciation. Its generic placement is confirmed by morphological, chromosomal, and molecular analyses.
Appendix 1
GenBank accession numbers: Species: (ITS/trnL-F). Primulina cardaminifolia was collected from the type locality [S.M. Ku 2035(HAST)].
Didymocarpus podocarpus C.B. Clarke: (DQ912688/FJ501514); Petrocodon dealbatus Hance: (FJ501358/FJ501537); Petrocodon scopulorum (Chun) YinZ. Wang [=Tengia scopulorum Chun]: (GU350637/GU350669); Primulina bipinnatifida (W.T. Wang) Yin Z. Wang [=Chiritopsis bipinnatifida W.T. Wang]: (DQ872842/DQ872806); Primulina cardaminifolia Yan Liu & W.B. Xu: (ITS-A: JX506738, ITS-B: JX506739/JX506740); Primulina cordifolia (D. Fang & W.T. Wang) YinZ. Wang [=Chiritopsis cordifolia D. Fang & W.T. Wang]: (DQ872845/DQ872803); Primulina dryas (Dunn) Mich. Möller & A. Weber [=Chirita sinensis Lindl.]: (FJ501348/FJ501524); Primulina gemella (D. Wood) YinZ. Wang [=Chirita gemella D. Wood]: (FJ501345/FJ501523); Primulina glandulosa (D. Fang, L. Zeng & D.H. Qin) Yin Z. Wang [=Chiritopsis glandulosa D. Fang, L. Zeng & D.H. Qin]: (DQ872841/DQ872804); Primulina heterotricha (Merr.) YinZ. Wang [=Chirita heterotricha Merr.]: (DQ872826/DQ872816); Primulina linearifolia (W.T. Wang) YinZ. Wang [=Chirita linearifolia W.T. Wang]: (DQ872834/DQ872810); Primulina longgangensis (W.T. Wang) YinZ. Wang [=Chirita longgangensis W.T. Wang]: (FJ501347/AJ492290); Primulina luochengensis (Yan Liu & W.B. Xu) Mich. Möller & A. Weber [=Wentsaiboea luochengensis Yan Liu & W.B. Xu]: (HQ633046/HQ632949); Primulina minutimaculata (D. Fang & W.T. Wang) YinZ. Wang [=Chirita minutimaculata D. Fang & W.T. Wang]: (DQ872828/DQ872815); Primulina multifida B. Pan & K.F. Chung: (JX507031/JX506756); Primulina ophiopogoides (D. Fang & W.T. Wang) YinZ. Wang [=Chirita ophiopogoides D. Fang & W.T. Wang]: (DQ872829/DQ872814); Primulina pinnata (W.T. Wang) YinZ. Wang [Chirita pinnata W.T. Wang]: (FJ501349/FJ501526); Primulina pinnatifida (Hand.-Mazz.) Yin Z. Wang [=Chirita pinnatifida (Hand.-Mazz.) B.L. Burtt]: (FJ501350/FJ501527); Primulina pseudomollifolia W.B. Xu & Yan Liu: (JX506869/JX506759); Primulina pteropoda (W. T. Wang) Yan Liu [=Chirita pteropoda W.T. Wang]: (DQ872827/DQ872817); Primulina repanda var. guilinensis (W.T. Wang) Mich. Möller & A. Weber [=Chiritopsis repanda var. guilinensis W.T. Wang]: (DQ872846/DQ872808); Primulina spadiciformis (W.T. Wang) Mich. Möller & A. Weber [=Chirita spadiciformis W.T. Wang]: (FJ501346/AJ492291); Primulina spinulosa (D. Fang & W.T. Wang) YinZ. Wang [=Chirita spinulosa D. Fang & W.T. Wang]: (DQ872830/DQ872813); Primulina tabacum Hance: (FJ501352/AJ492300); Primulina weii Mich. Möller & A. Weber [=Chirita mollifolia D. Fang, Y.G. Wei & J. Murata]: (DQ872832/DQ872811); Primulina wentsaii (D. Fang & L. Zeng) YinZ. Wang [=Chirita wentsaii D. Fang & L. Zeng]: (DQ872831/DQ872812).
References
Abbott RJ, Hegarty MJ, Hiscock SJ, Brennan AC: Homoploid hybrid speciation in action. Taxon 2010, 59: 1375–1386.
Alexander MP: Differential staining of aborted and nonaborted pollen. Biotech Histochem 1969, 44: 117–122. 10.3109/10520296909063335
Christie F, Barber S, Möller M: New chromosome counts in Old World Gesneriaceae: numbers for species hitherto regarded as Chirita , and their systematic and evolutionary significance. Edinburgh J Bot 2012, 69: 323–345. 10.1017/S0960428612000169
Chung K-F, Huang H-Y, Peng C-I, Xu W-B: Primulina mabaensis (Gesneriaceae), a new species from a limestone cave of northern Guangdong, China. Phytotaxa 2013, 92: 40–48. 10.11646/phytotaxa.92.2.2
Denduangboripant J, Cronk QCB: High intraindividual variation in internal transcibed spacer sequences in Aeschynanthus (Gesneriaceae): implications for phylogenetics. Proc Roy Soc Biol Sci Ser B 2000, 267: 1407–1415. 10.1098/rspb.2000.1157
Hong X, Zhou S-B, Wen F: Primulina chizhouensis sp. nov. (Gesneriaceae), a new species from a limestone cave in Anhui, China. Phytotaxa 2012, 50: 13–18.
Hou M-F, López-Pujol J, Qin H-N, Wang L-S, Liu Y: Distribution pattern and conservation priorities for vascular plants in Southern China: Guangxi Province as a case study. Bot Stud 2010, 51: 377–386.
Howarth DG, Baum DA: Genealogical evidence of homoploid hybrid speciation in an adaptive radiation of Scaevola (Goodeniaceae) in the Hawaiian Islands. Evolution 2005, 59: 948–961. 10.1111/j.0014-3820.2005.tb01034.x
Huang Y-S, Xu W-B, Wu L, Liu Y: Primulina gongchengensis (Gesneriaceae), a new species from Guangxi, China. Ann Bot Fenn 2012, 49: 107–110. 10.5735/085.049.0118
Levan A, Fredga K, Sandberg AA: Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52: 201–220. 10.1111/j.1601-5223.1964.tb01953.x
Li J, Wang Y, Hua G-J, Wen F: Primulina xiziae sp. nov. (Gesneriaceae) from Zhejiang Province, China. Nord J Bot 2012, 30: 77–81. 10.1111/j.1756-1051.2011.01185.x
Li J-M, Wang Y-Z: Phylogenetic reconstruction among species of Chiritopsis and Chirita sect. Gibbosaccus (Gesneriaceae) based on nrDNA ITS and cpDNA trnL-F sequences. Syst Bot 2007, 32: 888–898. http://www.bioone.org/doi/abs/10.1043/06–99.1?journalCode=sbot 10.1600/036364407783390764
Li Z-Y, Wang Y-Z: Plants of Gesneriaceae in China. Henan Science and Technology Publishing House. Henan: Zhengzhou; 2004.
Liu R-R, Pan B, Zhou T-J, Liao J-P: Cytological studies on Primulina taxa (Gesneriaceae) from limestone karsts in Guangxi province, China. Caryologia 2012, 65: 295–303. 10.1080/00087114.2012.752920
Liu Y, Xu W-B, Huang Y-S: Primulina guangxiensis sp. nov. (Gesneriaceae) from a karst cave in Guangxi, China. Nord J Bot 2011, 29: 682–686. 10.1111/j.1756-1051.2011.01089.x
Möller M, Forrest A, Wei Y-G, Weber A: A molecular phylogenetic assessment of the advanced Asiatic and Malesian didymocarpoid Gesneriaceae with focus on non-monophyletic and monotypic genera. Pl Syst Evol 2011, 292: 223–248. 10.1007/s00606-010-0413-z
Möller M, Pfosser M, Jang C-G, Mayer V, Clark A, Hollingsworth ML, Barfuss MHJ, Wang Y-Z, Kiehn M, Weber A: A preliminary phylogeny of the 'Didymocarpoid Gesneriaceae' based on three molecular data sets: incongruence with available tribal classifications. Am J Bot 2009, 96: 989–1010. 10.3732/ajb.0800291
Peng C-I, Chiang T-Y: Molecular confirmation of unidirectional hybridization in Begonia × taipeiensis Peng (Begoniaceae) from Taiwan. Ann Missouri Bot Gard 2000, 87: 273–285. 10.2307/2666164
Puglisi C, Wei Y-G, Nishii K, Möller M: Oreocharis × heterandra (Gesneriaceae): a natural hybrid from the Shengtangshan Mountains, Guangxi, China. Phytotaxa 2011, 38: 1–18.
Rieseberg LH, Willis JH: Plant speciation. Science 2007, 317: 910–914. 10.1126/science.1137729
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S: MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011, 28: 2731–2739. 10.1093/molbev/msr121
Wang W-T, Pan K-Y, Li Z-Y, Weitzman AL, Skog LE: Gesneriaceae. In Flora of China, vol 18. Edited by: Wu Z-Y, Raven PH. Louis: Science Press and Missouri Botanical Garden Press, Beijing and St; 1998:244–401.
Wang Y-Z, Mao R-B, Liu Y, Li J-M, Dong Y, Li Z-Y, Smith JF: Phylogenetic reconstruction of Chirita and allies (Gesneriaceae) with taxonomic treatments. J Syst Evol 2011, 49: 50–64. 10.1111/j.1759-6831.2010.00113.x
Weber A, Middleton DJ, Forrest A, Kiew R, Lim CL, Rafidah AR, Sontag S, Triboun P, Wei Y-G, Yao TL, Möller M: Molecular systematics and remodelling of Chirita and associated genera (Gesneriaceae). Taxon 2011, 60: 767–790.
Wei Y-G (Ed): Gesneriaceae of South China. Guilin, Guangxi: Guangxi Science and Technology Publishing House; 2010. 777 pp 777 pp
Wen F, Wang F, Wei Y-G: Primulina yangshuoensis , a new species of Gesneriaceae from Guangxi, China. Taiwania 2012, 57: 55–61.
Wen F, Qin G-L, Wei Y-G, Liang G-Y, Gao B: Primulina hochiensis var. rosulata (Gesneriaceae)―a new variety at an entrance of a limestone cave from Guangxi, China. Phytotaxa 2012, 54: 37–42.
Wen F, Xi S-L, Wang Y, Xiang M-S, Fu L-F: Primulina fengshanensis (Gesneriaceae), a new species from Guangxi, China. Ann Bot Fenn 2012, 49: 103–106. 10.5735/085.049.0117
Wu L, Zhang Q, Xu W-B, Mo S-S: Primulina guigangensis (Gesneriaceae): a new species from limestone area in Guangxi, China. Phytotaxa 2012, 38: 19–23.
Wu W-H, Meng T, Xu W-B, Liu S-Y, Zhang Q: Primulina sinovietnamica (Gesneriaceae), a new species identified by both morphological and molecular characters from the limestone area in Guangxi, China. Phytotaxa 2012, 60: 32–40.
Xu W-B, Pan B, Liu Y, Peng C-I, Chung K-F: Two new species, Primulina multifida and P. pseudomollifolia (Gesneriaceae), from karst caves in Guangxi, China. Bot Stud 2012, 53: 165–175.
Xu W-B, Zhang Q, Wen F, Liao W-B, Pan B, Chang H, Chung K-F: Nine new combinations and one new name of Primulina (Gesneriaceae) from South China. Phytotaxa 2012, 64: 1–8.
Yang X-Y, Liang K-M, Zhang X-H, Ma G-H: Karyotype analysis of an endemic species Primulina tabacum (Gesneriaceae). Pl Diversity Resources 2012, 34: 25–27.
Acknowledgments
The authors are grateful to Mr. Hong-Jin Wei for field assistance, Mr. Wen-Hong Lin (IBK) and Mr. Yun-Xi Zhu (IBK) for the high quality illustrations, Miss Hsun-An Yang for testing pollen stainability, and Dr. Michael Möller for critical review on the manuscript. This study was supported by the National Natural Science Foundation of China (Grant no. 41161011) and the Foundation of Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences to Yan Liu (IBK), and the National Geographic Society Grant # 8358–07 to Ching-I Peng (HAST), Yan Liu (IBK) and Kuo-Fang Chung (NTUF).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WBX and YL discovered the new species, WBX, YL, CIP, and KFC designed the project, YK collected the cytological data, and HC collected the molecular data. KFC performed the analyses and wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Xu, WB., Liu, Y., Kono, Y. et al. Primulina cardaminifolia (Gesneriaceae), a rare new species from limestone areas in Guangxi, China. Bot Stud 54, 19 (2013). https://doi.org/10.1186/1999-3110-54-19
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1999-3110-54-19