Abstract
Background
Conventionally, a nebulized short-acting β-2 agonist like salbutamol is often used as the reliever in acute exacerbations of asthma. However, recent worldwide respiratory outbreaks discourage routine use of nebulization. Previous studies have shown that combined budesonide/formoterol (Symbicort®, AstraZeneca) is effective as both a maintenance and reliever anti-asthmatic medication.
Methods
We performed a randomized, open-label study from March until August 2011 to compare the bronchodilatory effects of Symbicort® vs. nebulized salbutamol in acute exacerbation of mild to moderate asthmatic attack in an emergency department. Initial objective parameters measured include the oxygen saturation, peak expiratory flow rate (PEFR) and respiratory rate. During clinical reassessment, subjective parameters [i.e., Visual Analog Scale (VAS) and 5-point Likert scale of breathlessness] and the second reading of the objective parameters were measured. For the 5-point Likert scale, the patients were asked to describe their symptom relief as 1, much worse; 2, a little worse; 3, no change; 4, a little better; 5, much better.
Results
Out of the total of 32 patients enrolled, 17 patients (53%) were randomized to receive nebulized salbutamol and 15 (47%) to receive Symbicort®. For both treatment arms, by using paired t- and Wilcoxon signed rank tests, it was shown that there were statistically significant improvements in oxygen saturation, PEFR and respiratory rate within the individual treatment groups (pre- vs. post-treatment). Comparing the effects of Symbicort® vs. nebulized salbutamol, the average improvement of oxygen saturation was 1% in both treatment arms (p = 0.464), PEFR 78.67 l/min vs. 89.41 l/min, respectively (p = 0.507), and respiratory rate 2/min vs. 2/min (p = 0.890). For subjective evaluation, all patients reported improvement in the VAS (average 2.45 cm vs. 2.20 cm), respectively (p = 0.765). All patients in both treatment arms reported either "a little better" or "much better" on the 5-point Likert scale, with none reporting "no change" or getting worse.
Conclusion
This study suggests that there is no statistical difference between using Symbicort® vs. nebulized salbutamol as the reliever for the first 15 min post-intervention.
Similar content being viewed by others
Background
Defined as a chronic inflammatory airway disorder with bronchial hyper-responsiveness to a variety of stimuli, bronchial asthma is often punctuated with recurrent episodes of exacerbations with classical manifestations such as wheezing, breathlessness, chest tightness, and nocturnal or early morning cough. These episodes are usually associated with widespread but variable airflow obstruction within the lung that is often reversible either spontaneously or with treatment [1].
Conventionally, a nebulized short-acting β2 agonist (SABA) like salbutamol is often used as the reliever in acute exacerbations of asthma. However, the recent worldwide respiratory outbreaks, such as the H1N1 pandemic flu (in 2009) and the severe acute respiratory syndrome (SARS) outbreak (in 2002-2003), have discouraged routine nebulization because of its apparent association with air-borne infection spread [2].
Recent studies have shown that the similar bronchodilator delivered via a metered-dose inhaler, combined with a spacer, produces at least an equivalent improvement in lung function as the same dose as delivered via a nebulizer [3]. In fact, a previous study has found that with MDI and a spacer, the dose that reaches the lungs ranges from 15% to 20% of the total dose, whereas jet nebulizers deposit only 8% to 10% of the total dose [4].
Nonetheless, despite the effectiveness of delivering a bronchodilator via the metered dose route [3, 4], nebulized salbutamol is still very much preferred because of its convenience [5]. Most patients when visiting an emergency department expect to be given "something more" than the metered dose inhalers that they had been taking at home.
Formoterol is a long-acting β2-agonist (LABA) that has a rapid onset bronchodilatory effect within 1-3 min of inhalation [6]. Its rapid bronchodilatory effect is comparable with that of salbutamol, thus making it a suitable alternative for the treatment of acute asthma and to prevent exercise-induced bronchospasm [7]. In fact, formoterol has been shown to produce greater lung improvements than salbutamol up to 4 h after administration [8].
One of the reasons why formoterol exhibits a rapid onset is because formoterol acts as a full agonist to beta-2 receptors compared to salbutamol that acts as a partial beta-2 agonist [6]. Furthermore, there are additional non-bronchodilatory beneficial effects of formoterol including the effect of reducing plasma exudation by closing the gaps between endothelial cells, stabilizing the mast cells and reducing neutrophilic recruitment, and thus reducing the release of reactive oxygen species through the activated neutrophils [9].
Combined budesonide/formoterol in a single inhaler (Symbicort®, AstraZeneca) has been shown to be effective as a maintenance and reliever anti-asthmatic medication [10]. In fact, this combination therapy has recently been used in an effective novel approach called Symbicort® as Maintenance and Reliever Therapy (SMART) approach [9, 11]. SMART approach basically means using Symbicort® as a maintenance therapy twice daily with additional puffs of the combination inhaler as needed for symptom relief [9, 11].
Methods
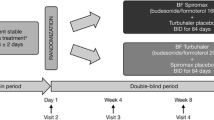
In this randomized, open-label study conducted from March until August 2011, we compared the effects of Symbicort® (at the strength of 160/4.5 mcg per inhalation) and nebulized salbutamol as the initial reliever in acute exacerbation of mild to moderate bronchial asthma in the Emergency Department, Hospital Universiti Sains Malaysia (HUSM). We defined mild and moderate asthmatic attack according to the guidelines outlined in the Global Initiative of Asthma (GINA) report on a global strategy for asthma management and prevention. Adult patients aged 18 years old and above who came to the Emergency Department of HUSM with mild to moderate asthmatic attacks were included in the study. We excluded patients referred from district hospitals or other health facilities (who may have been given reliever treatment prior to arrival), pregnancy, patients who are already started on any LABA medications as well as patients with persistent poor inhalational techniques despite being taught the proper technique in the Emergency Department. This study was approved by the hospital's ethics and research committee.
Patients who consented to participate in this study were randomized to receive either two puffs of Symbicort® or nebulized salbutamol. Patients randomized to receive Symbicort® were given proper instruction on the inhalational technique using the dummy turbuhaler devices supplied by AstraZeneca (Malaysia). Initial objective parameters obtained include the respiratory rate, oxygen saturation and peak expiratory flow rate (PEFR). The patients were then reassessed after 15 min. Objective and subjective variables related to the study were then recorded. Subjective parameters related to patients' perception of symptomatic relief were assessed using the Visual Analog Scale (VAS) and the 5-point Likert scale of breathlessness.
VAS is a widely used measurement scale for many symptoms including pain and breathlessness at a specific point in time. We recorded two readings (pre- and post-intervention) on a 10-cm scale with the spectrum of completely asymptomatic on one end and the most severe asthmatic symptoms ever experienced on the other end. In this study, we adopted the finding from a previous study where a change of 2.2 cm is defined as the minimal clinically significant improvement of asthma management in the emergency departments [12].
For the 5-point Likert scale, during reassessment of the patient 15 min after giving the allocated treatment, the patients were asked to describe their symptom relief on a 5-point scale of 1, much worse; 2, a little worse; 3, no change; 4, a little better; 5, much better
Subsequent management after the initial treatment and the decision for patient disposition were decided according to the discretion of the treating doctor based on the clinical improvement of the patient concerned. Patients who need admission are admitted accordingly, and patients with the nebulized salbutamol deemed fit for discharge are discharged with inhaled budesonide (Pulmicort® 100 mcg per inhalation, AstraZeneca) at a dose of two inhalations twice daily besides inhaled salbutamol on an as-needed basis for 30 days. Patients in the Symbicort® arm are discharged with two inhalations of Symbicort® as maintenance besides additional doses on an as-needed basis for up to eight doses for 30 days. All patients are given an appointment to attend a follow-up visit after 30 days to assess symptom progression.
All data were analyzed using Statistical Package for Social Sciences (SPSS®) Statistics software version 19.0.0. For categorical analysis, chi-square and Fisher's exact tests were used. For numerical analysis, dependent and independent Student's t-tests were used for parametric data, and Wilcoxon's signed rank test and Mann-Whitney test were used for nonparametric dependent and independent data, respectively. The block randomization process used in this study was generated using software available from the web [13].
Results
Out of the total of 32 patients enrolled, 17 (53%) were randomized to receive nebulized salbutamol and 15 (47%) to receive Symbicort® (Table 1). None of the patients required admission, and none of them required additional doses of relievers.
For patients within the Symbicort® arm, by using the paired t-test for PEFR (parametric) and Wilcoxon signed rank test for respiratory rate and SaO2 (non-parametric), it was shown that there were statistically significant improvements in SaO2 and PEFR, and reduction in the respiratory rate (improvement of tachypnea) in patients after using Symbicort® tubuhaler. Similar statistically significant changes were demonstrated in the nebulized salbutamol arm (Table 2).
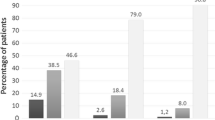
Arguably, although the statistically significant changes in this study may not be noticeable clinically, yet, on subjective assessment, regardless of the type of intervention, 18 out of 32 (56%) patients reported being "a little better" on the 5-point Likert scale, and another 14 out of the 32 (44%) reported a "much better" response. All patients reported improvement in the VAS with the mean VAS of 2.45 cm and 2.20 cm in the Symbicort® and nebulized salbutamol arms, respectively (Table 1).
Specifically, in the Symbicort® arm, on the 5-point Likert scale, nine patients (60%) said they felt "a little better," and another six patients (40%) said they felt "much better." Similarly, for the nebulized salbutamol arm, nine patients (52.9%) felt "a little better," and eight patients (47.1%) felt "much better" on the 5-point Likert scale. None of the patients in either arm reported feeling "no change," "a little worse" or "much worse" after interventions (Table 1). Incidentally, as all patients responded as being either "a little better" or "much better," we performed a post-hoc analysis using the chi-square test to compare the subjective improvement of Symbicort® versus nebulized salbutamol, and we found that there is no statistically significant difference between these two treatments (p = 0.688).
Discussion
In this study, both nebulized salbutamol and Symbicort® demonstrated objective and subjective clinical improvements in the first 15 min. Although not specifically designed to show non-inferiority of one drug over another, our study does suggest that Symbicort® is as effective as nebulized salbutamol when used as a reliever in the emergency department for the first 15 min, which is consistent with the findings from another study that Symbicort® is as effective as salbutamol in metered-dose inhalation [14].
Even though at this stage it is premature to state whether Symbicort® can or should be used as an alternative reliever in acute asthmatic attacks, nonetheless, we demonstrated that both Symbicort® and salbutamol offer similar improvement in objective and subjective assessments, and that there is no difference between the two.
This study is severely limited because of the inherent weaknesses of its methodology. It is an open-label, non-blinded trial involving a small sample size of 32 patients. Furthermore, in this study, the patient's comorbidities, duration of asthma and risk factors such as smoking status were not taken into consideration. However, this study may pave the way for larger studies in the future to study the beneficial effects of Symbicort® beyond the first 15 min and whether it can be used as an alternative reliever. A better alternative methodology would be to add a placebo inhaler for patients in the nebulized salbutamol arm and a placebo nebulization (e.g., saline) for the Symbicort® arm. In this way, the confounding bias due to the different drug delivery systems could be minimized.
Furthermore, one of the common problems in long-term asthma care is poor adherence to inhaled corticosteroid (ICS) maintenance therapy, resulting in under-treatment of the underlying airway inflammation. This is often because patients do not understand the difference between maintenance and reliever medications as well as the importance of regular ICS use [15, 16]. As a result, many patients tend to over-rely on short-acting β-2 agonist medications for quick relief of symptoms at the expense of omitting their ICS therapy, thus lowering anti-inflammatory protection and increasing the propensity for the development of severe and potentially life-threatening exacerbations [17]. If an alternative agent that combines both reliever and maintenance medications could be used, this would simplify treatment and provide a more convenient and effective way in which to deliver medications to the endobronchial tree.
Conclusion
In conclusion, this study shows that when Symbicort® is used as a reliever in acute mild to moderate asthmatic attacks, it does not demonstrate any statistical difference when compared to nebulized salbutamol in the first 15 min. Ultimately, with the recent development of many respiratory outbreaks, we hope that the findings in this study can translate into helping to minimize the occurrence of unnecessary nebulizations by using an alternative agent that is not only equally effective, but also well accepted by the patients.
References
GINA: Global strategy for asthma management and prevention (updated 2010). 2010. [http://www.ginasthma.org], Accessed on 4 November 2011
Varia M, Wilson S, Sarwal S, McGeer A, Gournis E, Galanis E, Henry B: Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ 2003,169(4):285–292.
Cates CJ, Crilly JA, Rowe BH: Holding chambers (spacers) versus nebulisers for betaagonist treatment of acute asthma. Cochrane Database Syst Rev 2006, (2):CD000052.
Newman SP: Therapeutic inhalation agents and devices. Effectiveness in asthma and bronchitis. Postgrad Med 1984,76(5):194–203–206–207.
Weber EJ, Silverman RA, Callaham ML, Pollack CV, Woodruff PG, Clark S, Camargo CA Jr: A prospective multicenter study of factors associated with hospital admission among adults with acute asthma. Am J Med 2002,113(5):371–378. 10.1016/S0002-9343(02)01242-1
Palmqvist M, Ibsen T, Mellen A, Lotvall J: Comparison of the relative efficacy of formoterol and salmeterol in asthmatic patients. Am J Respir Crit Care Med 1999,160(1):244–249.
Boonsawat W, Charoenratanakul S, Pothirat C, Sawanyawisuth K, Seearamroongruang T, Bengtsson T, Brander R, Selroos O: Formoterol (OXIS) Turbuhaler as a rescue therapy compared with salbutamol pMDI plus spacer in patients with acute severe asthma. Respir Med 2003,97(9):1067–1074. 10.1016/S0954-6111(03)00139-2
Bateman ED, Fairall L, Lombardi DM, English R: Budesonide/formoterol and formoterol provide similar rapid relief in patients with acute asthma showing refractoriness to salbutamol. Respir Res 2006, 7: 13. 10.1186/1465-9921-7-13
Barnes PJ: Scientific rationale for using a single inhaler for asthma control. Eur Respir J 2007,29(3):587–595. 10.1183/09031936.00080306
Aziz I, Lipworth BJ: A bolus of inhaled budesonide rapidly reverses airway subsensitivity and beta2-adrenoceptor down-regulation after regular inhaled formoterol. Chest 1999,115(3):623–628. 10.1378/chest.115.3.623
O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, Ekstrom T, Bateman ED: Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med 2005,171(2):129–136.
Karras DJ, Sammon ME, Terregino CA, Lopez BL, Griswold SK, Arnold GK: Clinically meaningful changes in quantitative measures of asthma severity. Acad Emerg Med 2000,7(4):327–334. 10.1111/j.1553-2712.2000.tb02231.x
Saghaei M: Random Allocation Software. Randomization Accessed: March 9, 2010 [http://mahmoodsaghaei.tripod.com/Softwares/randalloc.html#Version]
Balanag VM, Yunus F, Yang PC, Jorup C: Efficacy and safety of budesonide/formoterol compared with salbutamol in the treatment of acute asthma. Pulm Pharmacol Ther 2006,19(2):139–147. 10.1016/j.pupt.2005.04.009
Haughney J, Barnes G, Partridge M, Cleland J: The Living & Breathing Study: a study of patients' views of asthma and its treatment. Prim Care Respir J 2004,13(1):28–35. 10.1016/j.pcrj.2003.11.007
FitzGerald JM, Boulet LP, McIvor RA, Zimmerman S, Chapman KR: Asthma control in Canada remains suboptimal: the Reality of Asthma Control (TRAC) study. Can Respir J 2006,13(5):253–259.
Kaplan A, Ryan D: The role of budesonide/formoterol for maintenance and relief in the management of asthma. Pulm Pharmacol Ther 2010,23(2):88–96. 10.1016/j.pupt.2009.10.011
Acknowledgements
This research was made possible through a short-term research grant (grant no. 304/PPSP/61310047) from Universiti Sains Malaysia.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors received dummy turbuhaler devices from AstraZeneca (Malaysia) solely for the purpose of patient education. No other support was received from AstraZeneca (Malaysia).
Authors' contributions
KSC: involved in the initial conception, study design, data acquisition, statistical analysis, result interpretation and write-up of the manuscript. HK: contributed to the design of the study, completed the task of data acquisition as well as statistical analysis and data interpretation of the study. CWH contributed to the initial conception, design as well as intellectual contents of the study. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chew, K.S., Kamarudin, H. & Hashim, C.W. A randomized open-label trial on the use of budesonide/formoterol (Symbicort®) as an alternative reliever medication for mild to moderate asthmatic attacks. Int J Emerg Med 5, 16 (2012). https://doi.org/10.1186/1865-1380-5-16
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1865-1380-5-16