Abstract
Group I introns are intervening sequences that have invaded tRNA, rRNA and protein coding genes in bacteria and their phages. The ability of group I introns to self-splice from their host transcripts, by acting as ribozymes, potentially renders their insertion into genes phenotypically neutral. Some group I introns are mobile genetic elements due to encoded homing endonuclease genes that function in DNA-based mobility pathways to promote spread to intronless alleles. Group I introns have a limited distribution among bacteria and the current assumption is that they are benign selfish elements, although some introns and homing endonucleases are a source of genetic novelty as they have been co-opted by host genomes to provide regulatory functions. Questions regarding the origin and maintenance of group I introns among the bacteria and phages are also addressed.
Similar content being viewed by others
Introduction
Group I introns are structured self-splicing introns that in part persist in genomes by minimizing the impact of their insertion into host genes. This is accomplished by autocatalyzing their removal (splicing) from primary transcripts, restoring a contiguous and functional host transcript. The ability of group I introns to self-splice and therefore act as ribozymes was first demonstrated by Cech’s group for a group I intron inserted within the nuclear large subunit rRNA gene in the protozoan Tetrahymena thermophila [1]. At the same time Michel [2] recognized that organellar group I introns can fold into conserved secondary structures at the RNA level. These observations, when combined with the work by Cech’s group, led to a better understanding of how group I intron ribozymes promote their splicing from transcripts and the ligation of the adjoining exons [3]. Many group I introns can self-splice in vitro without assistance from protein co-factors, although splicing in vivo is dependent on, or enhanced by, intron- and/or host-encoded factors [4].
Group I introns can be divided into two general classes, those that encode open reading frames (ORFs) and those that do not. Group I introns with ORFs can function as mobile genetic elements that can move within and between genomes by inserting into cognate alleles that lack intron insertions [5]. Here, intron-encoded ORFs function as so-called homing endonucleases (HEases) that cleave intronless alleles to promote a DNA-based recombination-dependent mobility mechanism referred to as intron homing [5, 6]. The first experimental connection between DNA endonucleases and intron mobility stemmed from a detailed analysis of the mtDNA yeast omega (ω) locus [7–9]. Mating of two yeast, one with the ω locus and one without the locus, resulted in a much higher frequency of ω inheritance than would be anticipated from random assortment of alleles. Later characterization showed that intron movement was driven by the homing endonuclease encoded within the intron, generating a double-stranded break in the intronless allele at a position close to where the intron is inserted in the intron-containing allele (the intron insertion site). Similar findings of high frequency inheritance of introns were later found from mixed infections of intron-containing and intron-lacking bacteriophages [10]. It is generally assumed, yet infrequently shown experimentally, that these findings may also apply to organelles and to some degree towards bacterial introns.
The phylogenomic distribution of group I introns is diverse, as they are found in bacterial, phage, viral, organellar genomes and often nuclear rDNA genes of fungi, plants, and algae (Figure 1). Intriguingly, group I introns are scarce among early branching metazoan mitochondrial genomes [11], and so far have not yet been detected in the Archaea [12]. Bacterial group I introns are mostly confined to structural RNA genes (rRNA and tRNA) and are less frequently inserted within protein-coding genes. Group I introns have also been reported from a variety of bacteriophages [13–15] where they tend to be inserted within conserved protein-coding genes. Other intron and intron-like elements are encountered within prokaryotic genomes, such as group II introns, Archaeal tRNA introns, and bacterial rDNA intervening sequences [16–18], however this review will focus on group I introns.
The distribution and diversity of group I introns. A small subunit rDNA cladogram shows the biological host range for each group I intron subclass in bacteria (B) and viruses (V). Distribution of group I introns in Eukarya as well as the cellular location of each subclass is indicated (N, nucleus; M, mitochondria; C, chloroplast). This figure was generated based on the available information obtained from the Comparative RNA Website [http://www.rna.icmb.utexas.edu/] and Group I Intron Sequence and Structure Database [http://www.rna.whu.edu.cn/gissd/index.html].
Review
Core features of group I intron RNAs
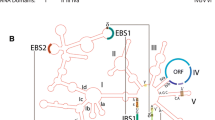
Group I introns are highly variable at the primary sequence level yet possess characteristic conserved secondary and tertiary structures. The secondary structure of group I introns consists of paired (P) elements designated P1 to P10 and single-stranded loop regions (Figure 2). Short, conserved sequences can be recognized in some intron sequences, and these are named P, Q, R, and S. These sequences participate in forming core helical regions, in which as shown in Figure 2 the P sequence pairs with Q (contributing towards the P4 helix) and R pairs with S (contributing towards the P7 helix) [2, 19]. The P1 and the P10 helices form the substrate-binding domain wherein the 5′ and 3′ splice sites are juxtaposed to each other [3, 20, 21]. In some group I introns, P2 is absent. The active core of the group I ribozyme is assembled by two helical domains P4/P6 (P4, P5 and P6), which is considered the scaffolding domain, and P3/P9 (P3, P7, P8 and P9) that form the catalytic domain [21–23]. The P3-P7-P9 helix contains the guanosine-5’-triphosphate (GTP) binding pocket and the exogenous GTP docks onto the G-binding site located in P7. Here the 3′–OH of an exogenous GTP is positioned so that it can attack the 5′-3′ phospodiester bond at the 5′ splice site located within the P1 fold. There is considerable evidence that at least one or more divalent metal ions (preferably Mg+2) are present at the active site and contribute towards the catalysis of the group I intron [24, 25].
Secondary structure model for group I introns. Generic secondary structure representations for group I introns highlighting the locations of intron-encoded proteins. (a) The blue lines indicate regions where ORFs that encode homing endonucleases are entirely located in loops. (b) In some group I introns, the endonuclease ORFs extend and overlap with intron core sequences. In both panels, stem regions are represented by solid black lines and single-stranded loop regions are represented by grey curved lines. Exon sequences are represented by black boxes. The ten pairing regions (P1 to P10) are also indicated. The solid green arrowheads indicate the intron-exon junctions (5′ and 3′ splicing sites). The positions of the internal guide sequence (IGS) and the so called P, Q, R and S sequence elements are indicated by thick orange lines. The guanosine-5’-triphosphate (GTP) binding pocket within the P7 helix is indicated by an asterisk.
Group I introns have been categorized into five classes, IA, IB, IC, ID and IE [26–28] based on conservation of core domains, alternative configurations of secondary structure elements, the presence of peripheral elements and features of the P7:P7′ helix (for example, P2, P7.1, P7.2) (see Figure 3). Each class is further subdivided based on the presence or absence of specific structural features (that is IA1, IA2 and IA3) [28]. Overall, 14 subgroups of introns have been recognized to date based on structural features [29], and over 20,000 group I introns have been identified or predicted in a variety of organisms. The secondary structures of some group I introns and a list of rDNA intron insertions sites have been compiled in the Comparative RNA Web Site [http://www.rna.ccbb.utexas.edu/] [30], and the group I intron sequence and structure database [28]. Among bacterial group I introns so far, representatives of the following intron subgroups have been noted: IA1, IA2, IA3, IB4, IC1, IC3, and ID [31–33]. When ORFs are present, they are usually entirely inserted in loops that protrude from the core secondary structure (see Figure 2) where the extra sequence associated with the ORF will not interfere with folding of the ribozyme core [34]. In cases where the intron ORF sequence extends into core intron sequences, expression of the intron ORF is tightly controlled so as not to interfere with intron folding and splicing [35, 36].
Differences between group I intron classes (IA to IE). Shown are secondary structure representatives for the group I intron classes [26–33]. The IA to ID classes are commonly found in bacteria. The IE class is also depicted for comparative purposes. For all group I intron RNA structures the catalytic core is highlighted in yellow. Beside each secondary structure model is a sequence logo alignment of the P7:P7′ pairing for the intron subclasses. The P7:P7′ pairing is important because it is a highly conserved region and is diagnostic for discriminating between various group I intron subclasses. With regards to the sequence logos the information content at each position (in bits, from 0 to 2) is represented by the height of the nucleotide. A score of 2 bits corresponds to high conservation, while a score of 0 corresponds to low conservation. The number of sequences used to generate each sequence logo is indicated below the intron subtype. Asterisks indicate the possible locations of peripheral insertions within the intron. The catalytic domain is highlighted in yellow.
The mechanism of group I intron splicing
Group I introns are removed from precursor RNA by an autocatalytic RNA splicing event that is mediated by the intron’s RNA tertiary structure. Base-pairing interactions between the 5′-end of the intron and flanking exon sequences define the location of the 5′ and 3′ splice sites. The Internal Guide Sequence (IGS), which is a short intronic sequence near the 5′-end that pairs with sequences of the upstream exon to form P1, determines the 5′ splice site (Figure 2). The 3′ splice site is determined by pairing of a short sequence of the downstream exon with a portion of the IGS, forming P10 and mediating interactions between P9 and the P3/P8 helices that form the catalytic core [3, 26, 37–39].
Splicing of the group I intron RNA is by a two-step transesterification reaction with an exogenous GTP (αG) with its 3′–OH acting as an initiating nucleophile (Figure 4). Binding of the αG in the G-binding site in P7 positions the 3′–OH of GTP to attack the 5′ splice site. During the first transesterification step the αG is attached to the 5′-end of the intron RNA by a 3′-5′ phosphodiester bond. This step is followed by conformational changes allowing the upstream exon′s terminal 3′ guanosine (ωG) to trade position with the αG and occupy the G-binding site to initiate the second transesterification reaction [26]. The 3′–OH of the upstream exon attacks the 3′ splice site (an interaction facilitated by the formation of P10) promoting the ligation of upstream and downstream exons and the release of the intron RNA [3, 40–42]. Splicing is absolutely dependent on a divalent metal ion to stabilize RNA secondary and tertiary structures and to activate the nucleophilic attack by the 3′–OH groups [24, 25]. Crystal structures of several group I introns have been resolved, including Azoarcus sp. BH72 pre-tRNAIle intron-exon complexes [24, 42, 43], Tetrahymena pre-rRNA apo enzyme [44, 45], and the bacteriophage Twort pre-mRNA ribozyme-product complex [22]. The crystal structures of these introns support the involvement of a two-metal ion mechanism in group I intron splicing.
Schematic representation of group I intron splicing. The splicing pathway consists of two sequential transesterification reactions. The first reaction is initiated by the 3′–OH group of an exogenous GTP (αG) that docks into the G-binding pocket located in the P7 region and the 3′–OH group attacks the 5′ splice site. In the second reaction, the 3′–OH of the released 5′ exon attacks the phosphodiester bond between the intronic terminal G (ωG) and the 3′ exon, resulting in the liberation of the intron and the ligation of the exons.
Intron- and host-encoded factors that facilitate splicing
Efficient in vivo splicing of group I introns often requires proteins with maturase function that can either be intron- or host-encoded [46–50]. The reliance on intron-encoded maturases or host factors implies that the intrinsic intron splicing rate may not be sufficient in a cellular context, and that introns have co-opted cellular factors to facilitate splicing to ensure little or no phenotypic effect on host gene function. For example, three nuclear mutations (cyt-4, cyt-18, cyt-19) were identified that showed cytochrome deficiencies due to defective splicing of the mL2449 group I intron in Neurospora crassa [51–53]. Cyt-4 was shown to be an RNase II-like protein that might be involved in the turnover of the excised group I intron [52], and Cyt-18 was revealed to be a tyrosyl-tRNA synthetase that promotes splicing by helping the intron RNA fold into a catalytically active structure [54, 55]. Cyt-19 is a member of the DEAD-box protein superfamily of RNA helicases that appears to be an ATP-dependent RNA chaperone that can recognize and destabilize non-native RNA folds that might arise during Cyt-18 mediated folding of group I intron RNAs [29, 56–59]. A general theme that emerges from these studies is that intron RNAs interact with cellular RNA-binding proteins to promote the formation of splicing-competent RNA structures.
With regard to bacterial group I introns, comparatively little is known about host- and intron-encoded splicing co-factors [46, 49, 50]. In the hyperthermophile Thermotoga neapolitana, the group I intron interrupting the 23S gene encodes a LAGLIDADG protein with maturase-like activity that stabilizes and activates its cognate intron at high temperatures [47]. Studies on Escherichia coli phage T4 introns revealed that host factors such as the StpA protein can act as an RNA chaperone and thus compensate for a group I intron splicing defect in vivo [46, 60, 61]. Ribosomal protein S12 was shown to facilitate the in vitro splicing of T4 introns [62], and translation initiation factor IF1 has RNA chaperone activity that can promote the splicing of the T4 phage thymidylate synthase intron [63]. In vitro work has shown that eukaryotic proteins such as Cyt-18 [29, 64], and DEAD-box proteins like Cyt-19, and Mss116p [59] promote splicing of some bacterial introns, suggesting that bacterial group I introns may benefit from interactions with proteins that assist in intron RNAs folding into splicing competent structures. There is also considerable evidence that the ribosome acts as an RNA chaperone for the T4 introns by sequestering upstream exon sequences that may otherwise compete with intron sequence to form non-productive RNA structures for splicing [65, 66]. Collectively, these observations also suggest that intron splicing and gene expression have to be coordinated and therefore introns may not be neutral with regards to their impact on their host cells [36, 66].
Intron-encoded HEases
Intron-encoded HEases are site-specific DNA endonucleases that recognize and cleave specific target sites (the homing site) in genomes that lack the intron (Figure 5a) [10, 67]. Homing sites are typically centered on the intron-insertion site, and include DNA sequences both up- and down-stream of the insertion site (that is in the up- and down-stream exons). The presence of a group I intron thus disrupts the homing site, rendering intron-containing alleles immune to cleavage by their encoded homing endonuclease, and providing a mechanism to discriminate self (intron-containing) from non-self (intronless) alleles. Most characterized HEases possess lengthy recognition sites (> 14 bp) that often encode codons specifying functionally critical amino acids or RNA sequences of the target gene [68–70]. Targeting of conserved sequences is one strategy to ensure that an appropriate homing site is present within closely related genomes. Moreover, many characterized HEases tolerate nucleotide substitutions within their homing sites, facilitating cleavage of variant cognate homing sites that arise by genetic drift.
Mobility pathways mediated by homing endonucleases. Schematics of different endonuclease-mediated mobility pathways between donor and recipient alleles. (a) group I intron homing mediated by intron-encoded endonucleases; (b) the collaborative or trans homing pathway; (c) the intronless homing pathway mediated by free-standing endonucleases. In all cases, the homing endonuclease gene is represented by a green rectangle, and the homing site of the endonuclease is shown by a grey filled rectangle. The green rectangle outlined with dashed line indicates the outcome of a recombination event whereby the endonuclease ORF becomes embedded within an endonuclease-lacking intron, creating a potential mobile group I intron.
Currently, there are six families of HEases, classified primarily on the basis of conserved amino acids that correspond to structural or active site residues; the LAGLIDADG, H-N-H, His-Cys box, GIY-YIG, PD-(D/E)xK, and EDxHD families [71–73]. The active site architecture of the His-Cys box and H-N-H families is very similar, and it has been suggested that they are divergent members of a ββα-metal motif. A similar argument can be made for a shared active site architecture of the PD-(D/E)xK and EDxHD families. The LAGLIDADG family is the largest and most diverse group with a wide host range including the organellar genomes of plants, fungi, protists, early branching metazoans, bacterial and archaeal genomes. The GIY-YIG, H-N-H, PD-(D/E)xK, and EDxHD enzymes are most often encoded within group I introns found in phage genomes, and less frequently in introns interrupting genes on bacterial chromosomes. His-Cys box enzymes have an extremely limited phylogenetic distribution, found almost exclusively in protists.
Intron mobility
Group I intron mobility is catalyzed by the intron-encoded HEases [6, 74, 75] (Figure 5). The HEases have specific target sites, with some allowance for sequence variation in their homing sites (Figure 5a). Recognition of variant homing sites ensures propagation in the face of substitutions that accumulate over time in the target site. Recently, trans-acting HEases have been described in T4 and related phages that can promote the homing of either group I introns lacking ORFs or group I introns that encode defunct (degenerated) HEases (Figure 5b) [67, 72, 76, 77]. Intron homing is initiated by the HEase that introduces a double-strand break (DSB), or nick, in an intronless allele [77]. The homing process is completed by host DSB-repair or synthesis-dependent strand annealing (SDSA) pathway [78–81] that use the intron-containing allele as a donor to repair the break in the recipient intronless allele (Figure 5). The end result is the nonreciprocal transfer of the mobile intron element into the intronless allele (that is recipient). As stated previously, nicking HEases can stimulate intron mobility but the actual mechanism of how a single-strand nick stimulates recombination is not understood. The homing event is frequently associated with co-conversion of markers flanking the intron insertion site, and the HEase can influence the extent of co-conversion by remaining bound to one of the cleavage products, preventing access of the recombination and repair machinery including exonucleases [79, 80, 82, 83]. It should be noted that homing endonuclease genes can be free-standing and move into new sites by a mechanism referred to as intronless homing, a mechanism that is similar to the one described above (see Figure 5c).
It is generally thought that group I introns propagate through a population of intronless alleles with ‘super-Mendelian’ inheritance, and that all available alleles for homing quickly become occupied. At this point, the HEase can quickly accumulate deleterious mutations that inactivate the enzyme, or the HEase assumes another function (possibly a maturase) to avoid loss. Alternatively, it is thought that group I introns can ‘escape’ to a new population of intronless alleles by transposition to new sites (ectopic integration) by reverse splicing. Reverse splicing is the reverse of the forward splicing reaction, and theoretically allows a group I intron RNA to insert into a RNA molecule with four to six complementary bases to the P1 stem of the intron RNA [84, 85]. This proposed pathway of RNA-based mobility also requires the additional steps of reverse transcription of the reverse-spliced intron and target RNA followed by integration of the cDNA into the genome by recombination, yet there is no direct experimental evidence to support this pathway. The best circumstantial evidence for reverse splicing has been documented for rDNA introns where related introns are inserted in two different locations within rDNA genes [55, 86].
Another mechanism for ectopic integration or transposition relates to the relaxed specificity of many intron-encoded HEases. For instance, cleavage at a site similar to a HEase’s native target site may promote intron mobility, and it has been shown that the cleavage specificity of the I-TevI HEase can be influenced by oxidative stress [87]. However, the low cleavage rates at ectopic sites will limit the frequency of intron movement by this mechanism. Because homologous recombination between unrelated sequences will be inefficient, it is thought that illegitimate recombination pathways would be necessary for intron transposition [88].
Domestication of group I introns and the formation of novel genetic elements
There are a few instances where group I introns or their components may have been domesticated by their host genomes, or by other types of mobile genetic elements. The bacterial DUF199/WhiA protein is a transcription factor and its N-terminal region contains the same protein fold as found in monomeric LAGLIDADG HEases encoded within group I introns [89, 90]. This similarity suggests that an invasive element was co-opted to serve as a regulatory protein [91]. The ability of group I intron RNAs to form complex tertiary structures has been harnessed in Clostridium difficile as a feature of a two-component riboswitch that involves c-di-GMP as an allosteric activator [92]. Here, in the 5′ untranslated region of an mRNA, a c-di-GMP binding aptamer is located upstream of a group I intron; the binding of c-di-GMP to its aptamer modifies the group I intron fold and shifts the 5′ splice site. In the presence of c-di-GMP, RNA processing yields an mRNA where the ribosome binding site is moved upstream of the start codon, whereas splicing without c-di-GMP results in a version of the transcript where the ribosome binding site is removed as part of the intron RNA [92]. In essence, the allosteric self-splicing intron has been domesticated as a metabolite sensor and genetic regulatory element.
A unique composite element has been described in some enterotoxin producing strains of C. difficile in the tcdA locus. The composite element, termed an IStron, is composed of a splicing-competent group I intron (IA2 subgroup) that has an insertion element (IS, of the IS605 element family) embedded within its 3′-end and encoding two transposases [93, 94]. One of the transposases is a TnpA-like protein that belongs to the HUH endonuclease superfamily [95]. TnpA can promote mobility events of the IS200/IS605 family of bacterial insertion elements by cleavage and rejoining of single-stranded DNA. These endonucleases cleave their target sites by cutting the lagging strand within a DNA replication fork [96, 97]. This mobility mechanism might be analogous to how the H-N-H family of nicking HEases promotes the mobility of group I introns. IStrons have the potential to transpose into genes but its capacity to self-splice should minimize its impact on the host gene [98]. Although IStrons appear to have the best of both worlds in the sense that they encode elements to promote spread (transposase) and aid in their persistence (self-splicing intron), they have limited phylogenetic distribution [99, 100].
Group I intron distribution in bacteria: genes and genomes
Within bacteria, group I introns are predominately inserted within structural RNA genes such as tRNA and rRNA genes [31–33, 101–107]. This bias has been explained in part by the conservation among structural RNA genes. Conversely, insertion of group I introns into protein-coding genes may be selected against, as the coupling of transcription and translation would interfere with folding of the group I intron to facilitate ribozyme formation and thus splicing [13, 108]. The presence of a stop codon in-frame with the upstream exon of many group I introns is viewed as evidence that stalling of the ribosome might be a strategy to facilitate intron RNA folding and splicing [98, 108–110]. Nevertheless, there have been reports of bacterial protein-coding genes that have been invaded by group I introns, such as the flagellin gene in a thermophilic Bacillus species [111, 112], recA and nrdE genes in various Bacillus species [99, 113], and some cyanobacterial nrdE genes [109, 110]. This trend of insertion into protein-coding genes is particularly evident in bacteriophages, as all introns observed to date are inserted in protein-coding genes, in spite of the presence of many phage-encoded tRNA genes [14, 100, 114–117]. This distribution may be related to the fact that optimal DNA targets for HEases occur within conserved protein-coding genes, which, in the context of the relatively small coding potential of many phage genomes, includes targets such as DNA polymerases, ribonucleotide reductases, and terminases.
Interestingly, group I introns have so far not been discovered in archaeal genomes, although group I intron derived HEase sequences are sometimes associated with archaeal introns [117–122]. The archaeal-specific introns are removed by a mechanism that involves tRNA splicing endonucleases [12, 123–126]. It has been suggested that the efficient protein-dependent splicing of archaeal introns may have outcompeted RNA-based self-splicing introns by minimizing any phenotypic effect on host genomes from slow in vivo splicing rates, and that self-splicing RNA introns became extinct in the archaeal lineage [12]. This scenario implies a cost associated to the host genome with maintaining group I ribozyme based splicing elements and/or their co-factors (maturases/chaperones), which may have limited their spread and persistence of self-splicing introns among the bacteria and their associated phages.
The persistence and spread of group I introns in prokaryotic genomes is dependent on a number of factors including (1) the phenotypic cost associated with the insertion of a group I intron, (2) the availability of intronless alleles for endonuclease-mediated homing, (3) the presence of efficient homology-based DSB repair systems, (4) the availability of DNA or RNA transfer mechanisms such as DNA uptake by natural transformation, conjugation and plasmid transfer, and phages. Interestingly, recent work on the Bacillus cereus group suggested that some of the genomic recA, nrdE, nrdF introns are similar to phage introns, indicating that phage infection could serve as a vector system for the lateral movement of introns among different genomes [100]. However, there is little evidence to show that bacterial introns are moved horizontally among bacterial species. One study [127] showed that placing a group I intron from Tetrahymena into the E. coli 23S gene resulted in the reduction of the growth rate which was correlated with poor splicing of the Tetrahymena intron. Moreover, the intron RNA was shown to associate with the 50 S ribosomal subunit and possibly interfere with translation. Clearly, there are barriers to intron spread in bacteria [13] that are curiously absent from organellar genomes where group I introns are very abundant.
The evolution of a composite mobile element
One of the most intriguing questions about mobile group I introns concerns their evolutionary origin. The current consensus is that HEases and group I introns had distinct evolutionary origins, and that HEases have on multiple independent occasions invaded an endonuclease-free intron. The alternative scenario, that group I introns always possessed an endonuclease gene is problematic for a number of reasons, including the fact that many group I introns do not contain ORFs, and the notion that group I introns were direct descents of catalytic RNAs from the RNA world. Moreover, the finding that HEases can exist outside of the protective confines of introns, as so-called free-standing homing endonucleases, lent credibility to the hypothesis that these free-standing enzymes could be a potential source of the ‘invading’ endonuclease. Two mechanisms that would lead to the formation of such a composite mobile intron have been proposed. Loizos et al. [128] noted that in the sunY gene of the T4 phage the intron sequences flanking the HEase ORF (I-TevII) were similar to the exon junction sequences that comprise the I-TevII target sequence. Importantly, they were able to demonstrate that a synthetic construct that included the fused sequence composed of the up- and down-stream sequences that flank the I-TevII ORF was indeed cleaved by I-TevII. This result provided strong circumstantial evidence for the ‘endonuclease-gene invasion’ hypothesis whereby a free-standing HEase cut an intron sequence that fortuitously contained a similar HEase target site. During the recombination-based repair process, the endonuclease gene sequence was inserted into the cleaved intron sequence, thus generating a composite potentially mobile intron.
Recent studies [72, 76] provide a second mechanism, termed collaborative homing, for the origin of mobile introns. Work on two different phages revealed systems where a free- standing HEase and an ORF-less group I intron converged on the same conserved target site (Figure 5b). That is, the target site of the endonuclease corresponded to the intron-insertion site. Thus, the endonuclease was ‘pre-adapted’ to target the intron-insertion site, and an illegitimate recombination event that moved the free-standing endonuclease gene into the intron would quickly create an efficient composite mobile intron capable of mobility [76].
Regardless of the origin of mobile group I introns, one would assume that endonuclease invasion would have a deleterious effect on intron splicing. In this respect, it is interesting to note that many endonuclease ORFs are inserted in loops that presumably do not interfere with folding and splicing. It is also possible that the intron-encoded endonucleases and/or host factors were able to compensate by stabilizing the intron tertiary RNA structure or discouraging misfolding of the intron RNAs [129–132]. This would effectively stabilize the intron/endonuclease relationship within the genome as splicing competency would be under a strong selective pressure if the intron was inserted in a functionally important gene. Long-term persistence of the composite element is dependent on the opportunity to invade intronless alleles, as detailed by Goddard and Burt and others [132, 133].
This returns us to the enigma of why group I introns and their associated HEases have been successful in spreading among the organellar genomes of plants, protozoans, and fungi but have very limited representation among bacterial and phage genomes. Koonin [134] proposed that group I introns evolved as parasitic selfish-RNAs (ribozymes) in abiotic compartments that housed early forms of the ‘RNA world’. If indeed these elements are ancient, it is surprising that now they have such a limited distribution, being absent in the Archaea and only rarely encountered among bacteria. One intriguing possibility is that the CRISPR/Cas RNA-based genome defense system, that restricts foreign DNAs such as plasmids or phage DNAs, has a role in limiting the spread of mobile group I introns present on these elements, specifically the type III CRISPR systems can target ssRNA in addition to DNA [135–137]. An interesting observation is that CRISPR/Cas systems are extremely prevalent in Archaea, but less so in bacteria, correlating with the absence of group I introns from Archaea.
Conclusions
The mechanisms that promote and prevent group I introns from proliferating among bacterial genomes are poorly understood, as is the long-term impact of introns on organismal viability. When present, it is assumed that introns are phenotypically neutral, yet the co-opting of intron functions by a riboswitch or the domestication of intron-encoded homing endonuclease as a regulatory protein (WhiA) indicates that introns can be a source of genetic novelty. Future research efforts directed at understanding the effect of group I introns on host gene expression, mechanisms of mobility to ectopic sites and their spread among bacterial genomes and phages will lead to valuable insights regarding the dynamics and evolution of group I introns.
Abbreviations
- bp:
-
base pair
- c-di-GMP:
-
cyclic diguanylate
- DSB:
-
double-strand break
- GTP:
-
guanosine-5′-triphosphate
- HEase:
-
homing endonuclease
- HEG:
-
homing endonuclease gene
- HUH:
-
endonuclease motif
- IGS:
-
Internal Guide Sequence
- IS:
-
insertion element
- ORF:
-
open-reading frame
- rRNA:
-
ribosomal RNA tRNA, transfer RNA
- SDSA:
-
synthesis-dependent strand annealing.
References
Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR: Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena . Cell 1982, 31: 147-157. 10.1016/0092-8674(82)90414-7
Michel F, Jacquier A, Dujon B: Comparison of fungal mitochondrial introns reveals extensive homologies in RNA secondary structure. Biochimie 1982, 64: 867-881. 10.1016/S0300-9084(82)80349-0
Cech TR: Self-splicing of group-I introns. Annu Rev Biochem 1990, 55: 599-629.
Lang BF, Laforest MJ, Burger G: Mitochondrial introns: a critical view. Trends Genet 2007, 23: 119-125. 10.1016/j.tig.2007.01.006
Dujon B: Group I introns as mobile genetic elements: facts and mechanistic speculation - a review. Gene 1989, 82: 91-114. 10.1016/0378-1119(89)90034-6
Belfort M, Roberts RJ: Homing endonucleases: keeping the house in order. Nucleic Acids Res 1997, 25: 3379-3388. 10.1093/nar/25.17.3379
Dujon B, Bolotin-Fukuhara M, Coen D, Deutsch J, Netter P, Slonimski PP, Weill L: Mitochondrial genetics. XI. Mutations at the mitochondrial locus omega affecting the recombination of mitochondrial genes in Saccharomyces cerevisiae . Mol Gen Genet 1976, 143: 131-165. 10.1007/BF00266918
Jacquier A, Dujon B: The intron of the mitochondrial 21S rRNA gene: distribution in different yeast species and sequence comparison between Kluyveromyces thermotolerans and Saccharomyces cerevisiae . Mol Gen Genet 1983, 192: 487-499. 10.1007/BF00392195
Colleaux L, d’Auriol L, Betermier M, Cottarel G, Jacquier A, Galibert F, Dujon B: Universal code equivalent of a yeast mitochondrial intron reading frame is expressed into E. coli as a specific double strand endonuclease. Cell 1986, 44: 521-533. 10.1016/0092-8674(86)90262-X
Belfort M, Derbyshire V, Parker MM, Cousineau B, Lambowitz AM: Mobile introns: pathways and proteins. In Mobile DNA II. Edited by: Craig NL, Craigie R, Gellert M, Lambowitz AM. Washington DC: ASM Press; 2002:761-783.
Haugen P, Simon DM, Bhattacharya D: The natural history of group I introns. Trends Genet 2005, 21: 111-119. 10.1016/j.tig.2004.12.007
Tocchini-Valentini GD, Fruscoloni P, Tocchini-Valentini GP: Evolution of introns in the archaeal world. Proc Natl Acad Sci U S A 2011, 108: 4782-4787. 10.1073/pnas.1100862108
Edgell DR, Belfort M, Shub DA: Barriers to intron promiscuity in bacteria. J Bacteriol 2000, 182: 5281-5289. 10.1128/JB.182.19.5281-5289.2000
Edgell DR, Gibb EA, Belfort M: Mobile DNA elements in T4 and related phages. Virol J 2010, 27: 290.
Lavigne R, Vandersteegen K: Group I introns in Staphylococcus bacteriophages. Future Virol 2013, 8: 997. 10.2217/fvl.13.84
Zimmerly S: Mobile introns and retroelements in bacteria. In The Dynamic Bacterial Genome. Edited by: Mullany P. Cambridge UK: Cambridge University Press; 2005:121-148.
Raghavan R, Minnick MF: Group I introns and inteins: disparate origins but convergent parasitic strategies. J Bacteriol 2009, 191: 6193-6202. 10.1128/JB.00675-09
Lambowitz AM, Zimmerly S: Group II introns: mobile ribozymes that invade DNA. In Cold Spring Harbor Perspectives in Biology - The RNA World. Volume 1. 4th edition. Edited by: Gesteland RF, Cech TR, Atkins JF. Woodbury NY: Cold Spring Harbor Laboratory Press; 2011:a003616.
Burke JM, Belfort M, Cech TR, Davies RW, Schweyen RJ, Shub DA, Szostak JW, Tabak HF: Structural conventions for group-I introns. Nucleic Acids Res 1987, 15: 7217-7222. 10.1093/nar/15.18.7217
Cech TR, Damberger SH, Gutell ER: Representation of the secondary and tertiary structure of group I introns. Nat Struc Biol 1994, 1: 273-280. 10.1038/nsb0594-273
Woodson SA: Structure and assembly of group I introns. Curr Opin Struct Biol 2005, 15: 324-330. 10.1016/j.sbi.2005.05.007
Golden BL, Kim H, Chase E: Crystal structure of a phage Twort group I ribozyme-product complex. Nat Struct Mol Biol 2005, 12: 82-89. 10.1038/nsmb868
Vicens Q, Cech TR: Atomic level architecture of group I introns revealed. Trends Biochem Sci 2006, 31: 41-51. 10.1016/j.tibs.2005.11.008
Stahley MR, Strobel SA: Structural evidence for a two-metal-ion mechanism of group I intron splicing. Science 2005, 309: 1587-1590. 10.1126/science.1114994
Stahley MR, Strobel SA: RNA splicing: group I intron crystal structures reveal the basis of splice site selection and metal ion catalysis. Curr Opin Struct Biol 2006, 16: 319-326. 10.1016/j.sbi.2006.04.005
Michel F, Westhof E: Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J Mol Biol 1990, 216: 585-610. 10.1016/0022-2836(90)90386-Z
Suh S-Q, Jones KG, Blackwell M: A group I intron in the nuclear small subunit rRNA gene of Cryptendoxyla hypophloia , an Ascomycetes fungus: evidence for a new major class of group I introns. J Mol Evol 1999, 48: 493-500. 10.1007/PL00006493
Zhou Y, Lu C, Wu Q-J, Wang Y, Sun Z-T, Deng J-C, Zhang Y: GISSD: group I intron sequence and structure database. Nucleic Acids Res 2008,36(Database issue):D31-D37.
Vicens Q, Paukstelis PJ, Westhof E, Lambowitz AM, Cech TR: Toward predicting self-splicing and protein-facilitated splicing of group I introns. RNA 2008, 14: 2013-2029. 10.1261/rna.1027208
Cannone JJ, Subramanian S, Schnare MN, Collett JR, D’Souza LM, Du Y, Feng B, Lin N, Madabusi LV, Müller KM, Pande N, Shang Z, Yu N, Gutell RR: The Comparative RNA Web (CRW) Site: an online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinformatics 2002, 3: 2. 10.1186/1471-2105-3-2
Haugen P, Bhattacharya D, Palmer JD, Turner S, Lewis LA, Pryer KM: Cyanobacterial ribosomal RNA genes with multiple, endonuclease-encoding group I introns. BMC Evol Biol 2007, 7: 159. 10.1186/1471-2148-7-159
del Campo EM, Casano LM, Gasulla F, Barreno E: Presence of multiple group I introns closely related to bacteria and fungi in plastid 23S rRNAs of lichen-forming Trebouxia . Int Microbiol 2009, 12: 59-67.
Salman V, Amann R, Shub DA, Schulz-Vogt HN: Multiple self-splicing introns in the 16S rRNA genes of giant sulfur bacteria. Proc Natl Acad Sci U S A 2012, 109: 4203-4208. 10.1073/pnas.1120192109
Schäfer B: Genetic conservation versus variability in mitochondria: the architecture of the mitochondrial genome in the petite-negative yeast Schizosaccharomyces pombe . Curr Genet 2003, 43: 311-326. 10.1007/s00294-003-0404-5
Gibb EA, Edgell DR: Better late than early: delayed translation of intron-encoded endonuclease I-TevI is required for efficient splicing of its host group I intron. Mol Microbiol 2010, 78: 35-46. 10.1111/j.1365-2958.2010.07216.x
Edgell DR, Chalamcharla VR, Belfort M: Learning to live together: mutualism between self-splicing introns and their hosts. BMC Biol 2011, 9: 22. 10.1186/1741-7007-9-22
Ikawa Y, Shiraishi H, Inoue T: Minimal catalytic domain of a group I self-splicing intron RNA. Nat Struct Biol 2000, 7: 1032-1035. 10.1038/80947
Ikawa Y, Naito D, Shiraishi H, Inoue T: Structure-function relationships of two closely related group IC3 intron ribozymes from Azoarcus and Synechococcus pre-tRNA. Nucleic Acids Res 2000, 28: 3269-3277. 10.1093/nar/28.17.3269
Rangan P, Masquida B, Westhof E, Woodson SA: Architecture and folding mechanism of the Azoarcus group I pre-tRNA. J Mol Biol 2004, 339: 41-51. 10.1016/j.jmb.2004.03.059
Saldanha R, Mohr G, Belfort M, Lambowitz AM: Group I and group II introns. FASEB J 1993, 7: 15-24.
Guo F, Cech TR: In vivo selection of better self-splicing introns in Escherichia coli : the role of the P1 extension helix of the Tetrahymena intron. RNA 2002, 8: 647-658. 10.1017/S1355838202029011
Adams PL, Stahley MR, Gill ML, Kosek AB, Wang J, Strobel SA: Crystal structure of a group I intron splicing intermediate. RNA 2004, 10: 1867-1887. 10.1261/rna.7140504
Adams PL, Stahley MR, Kosek AB, Wang J, Strobel SA: Crystal structure of a self splicing group I intron with both exons. Nature 2004, 430: 45-50. 10.1038/nature02642
Guo F, Gooding AR, Cech TR: Structure of the Tetrahymena ribozyme: base triple sandwich and metal ion at the active site. Mol Cell 2004, 16: 351-362.
Guo F, Gooding AR, Cech TR: Comparison of crystal structure interactions and thermodynamics for stabilizing mutations in the Tetrahymena ribozyme. RNA 2006, 12: 387-395. 10.1261/rna.2198206
Waldsich C, Grossberger R, Schroeder R: RNA chaperone StpA loosens interactions of the tertiary structure in the td group I intron in vivo . Genes Dev 2002, 16: 2300-2312. 10.1101/gad.231302
Mo D, Wu L, Xu Y, Ren J, Wang L, Huang L, Wu QJ, Bao P, Xie MH, Yin P, Liu BF, Liang Y, Zhang Y: A maturase that specifically stabilizes and activates its cognate group I intron at high temperatures. Biochimie 2011, 93: 533-541. 10.1016/j.biochi.2010.11.008
Caprara MG, Waring RB: Group I introns and their maturases: uninvited, but welcome guests. In Homing endonucleases and inteins. Edited by: Belfort M, Derbyshire V, Stoddard BL, Wood DL. New York: Springer; 2005:103-119.
Prenninger S, Schroeder R, Semrad K: Assaying RNA chaperone activity in vivo in bacteria using a ribozyme folding trap. Nat Protoc 2006, 1: 1273-1277. 10.1038/nprot.2006.189
Moll I, Leitsch D, Steinhauser T, Bläsi U: RNA chaperone activity of the Sm-like Hfq protein. EMBO Rep 2003, 4: 284-289. 10.1038/sj.embor.embor772
Bertrand H, Bridge P, Collins RA, Garriga G, Lambowitz AM: RNA splicing in Neurospora mitochondria. Characterization of new nuclear mutants with defects in splicing the mitochondrial large rRNA. Cell 1982, 29: 517-526. 10.1016/0092-8674(82)90168-4
Turcq B, Dobinson KF, Serizawa N, Lambowitz AM: A protein required for RNA processing and splicing in Neurospora mitochondria is related to gene products involved in cell cycle protein phosphatase functions. Proc Natl Acad Sci U S A 1992, 89: 1676-1680. 10.1073/pnas.89.5.1676
Mohr G, Rennard R, Cherniack AD, Stryker J, Lambowitz AM: Function of the Neurospora crassa mitochondrial tyrosyl-tRNA synthetase in RNA splicing. Role of the idiosyncratic N-terminal extension and different modes of interaction with different group I introns. J Mol Biol 2001, 307: 75-92. 10.1006/jmbi.2000.4460
Akins RA, Lambowitz AM: A protein required for splicing group I introns in Neurospora mitochondria is mitochondrial tyrosyl-tRNA synthetase or derivative thereof. Cell 1987, 50: 331-345. 10.1016/0092-8674(87)90488-0
Mohr G, Lambowitz AM: Integration of a group I intron into a ribosomal RNA sequence promoted by a tyrosyl-tRNA synthetase. Nature 1991,354(6349):164-167. 10.1038/354164a0
Mohr S, Stryker JM, Lambowitz AM: A DEAD-box protein functions as an ATP-dependent RNA chaperone in group I intron splicing. Cell 2002, 109: 769-779. 10.1016/S0092-8674(02)00771-7
Cao W, Coman MM, Ding S, Henn A, Middleton ER, Bradley MJ, Rhoades E, Hackney DD, Pyle AM, De La Cruz EM: Mechanism of Mss116 ATPase reveals functional diversity of DEAD-Box proteins. J Mol Biol 2011, 409: 399-414. 10.1016/j.jmb.2011.04.004
Jarmoskaite I, Russell R: DEAD-box proteins as RNA helicases and chaperones. Wiley Interdiscip Rev RNA 2011, 2: 135-152. 10.1002/wrna.50
Sinan S, Yuan X, Russell R: The Azoarcus group I intron ribozyme misfolds and is accelerated for refolding by ATP-dependent RNA chaperone proteins. J Biol Chem 2011, 286: 37304-37312. 10.1074/jbc.M111.287706
Zhang A, Derbyshire V, Salvo JL, Belfort M: Escherichia coli protein StpA stimulates self-splicing by promoting RNA assembly in vitro . RNA 1995, 1: 783-793.
Mayer O, Waldsich C, Grossberger R, Schroeder R: Folding of the td pre-RNA with the help of the RNA chaperone StpA. Biochem Soc Trans 2002, 30: 1175-1180.
Coetzee T, Herschlag D, Belfort M: Escherichia coli proteins, including ribosomal protein S12, facilitate in vitro splicing of phage T4 introns by acting as RNA chaperones. Genes Dev 1994, 8: 1575-1588. 10.1101/gad.8.13.1575
Croitoru V, Semrad K, Prenninger S, Rajkowitsch L, Vejen M, Laursen BS, Sperling-Petersen HU, Isaksson LA: RNA chaperone activity of translation initiation factor IF1. Biochimie 2006, 88: 1875-1882. 10.1016/j.biochi.2006.06.017
Paukstelis PJ, Chen JH, Chase E, Lambowitz AM, Golden BL: Structure of a tyrosyl-tRNA synthetase splicing factor bound to a group I intron RNA. Nature 2008, 451: 94-97. 10.1038/nature06413
Semrad K, Schroeder R: A ribosomal function is necessary for efficient splicing of the T4 phage thymidylate synthase intron in vivo . Genes Dev 1998, 12: 1327-1337. 10.1101/gad.12.9.1327
Sandegren L, Sjöberg BM: Self-splicing of the bacteriophage T4 group I introns requires efficient translation of the pre-mRNA in vivo and correlates with the growth state of the infected bacterium. J Bacteriol 2007, 189: 980-980. 10.1128/JB.01287-06
Wilson GW, Edgell DR: Phage T4 mobE promotes trans homing of the defunct homing endonuclease I-TevIII. Nucleic Acids Res 2009, 37: 7110-7123. 10.1093/nar/gkp769
Edgell DR, Stanger MJ, Belfort M: Coincidence of cleavage sites of intron endonuclease I-TevI and critical sequences of the host thymidylate synthase gene. J Mol Biol 2004, 343: 1231-1241. 10.1016/j.jmb.2004.09.005
Edgell DR, Stanger MJ, Belfort M: Importance of a single base pair for discrimination between intron-containing and intronless alleles by endonuclease I-BmoI. Curr Biol 2003, 13: 973-978. 10.1016/S0960-9822(03)00340-3
Scalley-Kim M, McConnell-Smith A, Stoddard BL: Coevolution of a homing endonuclease and its host target sequence. J Mol Biol 2007, 2: 1305-1319.
Skowronek KJ, Bujnicki JM: Restriction and homing endonucleases. In Industrial Enzymes, Structure, Function and Applications. Edited by: Polaina J, MacCabe AP. Dordrecht The Netherlands: Springer-Verlag; 2007:357-378.
Zeng Q, Bonocora RP, Shub DA: A free-standing homing endonuclease targets an intron insertion site in the psb A gene of cyanophages. Curr Biol 2009, 19: 218-222.
Stoddard BL: Homing endonucleases: from microbial genetic invaders to reagents for targeted DNA modification. Structure 2011, 19: 7-15. 10.1016/j.str.2010.12.003
Dujon B: Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell 1980, 20: 185-197. 10.1016/0092-8674(80)90246-9
Bell-Pedersen D, Quirk S, Clyman J, Belfort M: Intron mobility in phage T4 is dependent upon a distinctive class of endonucleases and independent of DNA sequences encoding the intron core: mechanistic and evolutionary implications. Nucleic Acids Res 1990, 18: 3763-3770. 10.1093/nar/18.13.3763
Bonocora RP, Shub DA: A likely pathway for formation of mobile group I introns. Curr Biol 2009, 19: 223-228. 10.1016/j.cub.2009.01.033
Landthaler M, Lau NC, Shub DA: Group I intron homing in Bacillus phages SPO1 and SP82: a gene conversion event initiated by a nicking homing endonuclease. J Bacteriol 2004, 186: 4307-4314. 10.1128/JB.186.13.4307-4314.2004
Mueller JE, Smith D, Bryk M, Belfort M: Intron-encoded endonuclease I-TevI binds as a monomer to effect sequential cleavage via conformational changes in the td homing site. EMBO J 1995, 14: 5724-5735.
Mueller JE, Smith D, Belfort M: Exon coconversion biases accompanying intron homing: battle of the nucleases. Genes Dev 1996, 10: 2158-2166. 10.1101/gad.10.17.2158
Mueller JE, Clyman J, Huang YJ, Parker MM, Belfort M: Intron mobility in phage T4 occurs in the context of recombination-dependent DNA replication by way of multiple pathways. Genes Dev 1996, 10: 351-364. 10.1101/gad.10.3.351
Huang YJ, Parker MM, Belfort M: Role of exonucleolytic degradation in group I intron homing in phage T4. Genetics 1999, 153: 1501-1512.
Parker MM, Court DA, Preiter K, Belfort M: Homology requirements for double-strand break-mediated recombination in a phage lambda-td intron model system. Genetics 1996, 143: 1057-1068.
Brok-Volchanskaya VS, Kadyrov FA, Sivogrivov DE, Kolosov PM, Sokolov AS, Shlyapnikov MG, Kryukov VM, Granovsky IE: Phage T4 SegB protein is a homing endonuclease required for the preferred inheritance of T4 tRNA gene region occurring in co-infection with a related phage. Nucleic Acids Res 2008, 36: 2094-2105. 10.1093/nar/gkn053
Roman J, Woodson SA: Reverse splicing of the Tetrahymena IVS: evidence for multiple reaction sites in the 23S rRNA. RNA 1995, 1: 478-490.
Birgisdottir AB, Johansen S: Site-specific reverse splicing of a HEG-containing group I intron in ribosomal RNA. Nucleic Acids Res 2005, 33: 2042-2051. 10.1093/nar/gki341
Bhattacharya D, Reeb V, Simon DM, Lutzoni F: Phylogenetic analysis suggests reverse splicing spread of group I introns in fungal ribosomal DNA. BMC Evol Biol 2005, 5: 68. 10.1186/1471-2148-5-68
Robbins JB, Smith D, Belfort M: Redox-responsive zinc finger fidelity switch in homing endonuclease and intron promiscuity in oxidative stress. Curr Biol 2011, 21: 243-248. 10.1016/j.cub.2011.01.008
Parker MM, Belisle M, Belfort M: Intron homing with limited exon homology. Illegitimate double-strand-break repair in intron acquisition by phage t4. Genetics 1999, 153: 1513-1523.
Kaiser BK, Clifton MC, Shen BW, Stoddard BL: The structure of a bacterial DUF199/WhiA protein: domestication of an invasive endonuclease. Structure 2009, 17: 1368-1376. 10.1016/j.str.2009.08.008
Taylor GK, Stoddard BL: Structural, functional and evolutionary relationships between homing endonucleases and proteins from their host organisms. Nucleic Acids Res 2012, 40: 5189-5200. 10.1093/nar/gks226
Bush MJ, Bibb MJ, Chandra G, Findlay KC, Buttner MJ: Genes required for aerial growth, cell division, and chromosome segregation are targets of WhiA before sporulation in Streptomyces venezuelae . Ambio 2013,4(5):e00684-13.
Lee ER, Baker JL, Weinberg Z, Sudarsan N, Breaker RR: An allosteric self-splicing ribozyme triggered by a bacterial second messenger. Science 2010, 329: 845-848. 10.1126/science.1190713
Braun V, Mehlig M, Moos M, Rupnik M, Kalt B, Mahony DE, von Eichel-Streiber C: A chimeric ribozyme in Clostridium difficile combines features of group I introns and insertion elements. Mol Microbiol 2000, 36: 1447-1459.
Hasselmayer O, Nitsche C, Braun V, von Eichel-Streiber C: The IStron CdISt1 of Clostridium difficile : molecular symbiosis of a group I intron and an insertion element. Anaerobe 2004, 10: 85-92. 10.1016/j.anaerobe.2003.12.003
Chandler M, de la Cruz F, Dyda F, Hickman AB, Moncalian G, Ton-Hoang B: Breaking and joining single-stranded DNA: the HUH endonuclease superfamily. Nat Rev Microbiol 2013, 11: 525-538. 10.1038/nrmicro3067
Ton-Hoang B, Pasternak C, Siguier P, Guynet C, Hickman AB, Dyda F, Sommer S, Chandler M: Single-stranded DNA transposition is coupled to host replication. Cell 2010, 142: 398-408. 10.1016/j.cell.2010.06.034
He S, Guynet C, Siguier P, Hickman AB, Dyda F, Chandler M, Ton-Hoang B: IS200/IS605 family single-strand transposition: mechanism of IS608 strand transfer. Nucleic Acids Res 2013, 41: 3302-3313. 10.1093/nar/gkt014
Hasselmayer O, Braun V, Nitsche C, Moos M, Rupnik M, von Eichel-Streiber C: Clostridium difficile IStron CdISt1: discovery of a variant encoding two complete transposase-like proteins. J Bacteriol 2004, 186: 2508-2510. 10.1128/JB.186.8.2508-2510.2004
Tourasse NJ, Helgason E, Økstad OA, Hegna IK, Kolstø AB: The Bacillus cereus group: novel aspects of population structure and genome dynamics. J Appl Microbiol 2006, 101: 579-593. 10.1111/j.1365-2672.2006.03087.x
Tourasse NJ, Kolstø AB: Survey of group I and group II introns in 29 sequenced genomes of the Bacillus cereus group: insights into their spread and evolution. Nucleic Acids Res 2008, 36: 4529-4548. 10.1093/nar/gkn372
Kuhsel MG, Strickland R, Palmer JD: An ancient group I intron shared by eubacteria and chloroplasts. Science 1990, 250: 1570-1573. 10.1126/science.2125748
Xu MQ, Kathe SD, Goodrich-Blair H, Nierzwicki-Bauer SA, Shub DA: Bacterial origin of a chloroplast intron: conserved self-splicing group I introns in cyanobacteria. Science 1990, 250: 1566-1570. 10.1126/science.2125747
Zaug AJ, McEvoy MM, Cech TR: Self-splicing of the group I intron from Anabaena pre-tRNA: requirement for base-pairing of the exons in the anticodon stem. Biochemistry 1993, 32: 7946-7953. 10.1021/bi00082a016
Paquin B, Kathe SD, Nierzwicki-Bauer SA, Shub DA: Origin and evolution of group I introns in cyanobacterial tRNA genes. J Bacteriol 1997, 179: 6798-6806.
Bonocora RP, Shub DA: A novel group I intron-encoded endonuclease specific for the anticodon region of tRNA(fMet) genes. Mol Microbiol 2001, 39: 1299-1306. 10.1111/j.1365-2958.2001.02318.x
Rudi K, Fossheim T, Jakobsen KS: Nested evolution of a tRNA(Leu)(UAA) group I intron by both horizontal intron transfer and recombination of the entire tRNA locus. J Bacteriol 2002, 184: 666-671. 10.1128/JB.184.3.666-671.2002
Nesbø CL, Doolittle WF: Active self-splicing group I introns in 23S rRNA genes of hyperthermophilic bacteria, derived from introns in eukaryotic organelles. Proc Natl Acad Sci U S A 2003, 100: 10806-10811. 10.1073/pnas.1434268100
Ӧhman-Hedén M, Ahgren-Stålhandske A, Hahne S, Sjöberg BM: Translation across the 5′-splice site interferes with autocatalytic splicing. Mol Microbiol 1993, 7: 975-982. 10.1111/j.1365-2958.1993.tb01189.x
Meng Q, Zhang Y, Liu XQ: Rare group I intron with insertion sequence element in a bacterial ribonucleotide reductase gene. J Bacteriol 2007, 189: 2150-2154. 10.1128/JB.01424-06
Fujisawa T, Narikawa R, Okamoto S, Ehira S, Yoshimura H, Suzuki I, Masuda T, Mochimaru M, Takaichi S, Awai K, Sekine M, Horikawa H, Yashiro I, Omata S, Takarada H, Katano Y, Kosugi H, Tanikawa S, Ohmori K, Sato N, Ikeuchi M, Fujita N, Ohmori M: Genomic structure of an economically important cyanobacterium, Arthrospira ( Spirulina ) platensis NIES-39. DNA Res 2010, 17: 85-103. 10.1093/dnares/dsq004
Hayakawa J, Ishizuka M: A group I self-splicing intron in the flagellin gene of the thermophilic bacterium Geobacillus stearothermophilus . Biosci Biotechnol Biochem 2009, 73: 2758-2761. 10.1271/bbb.90400
Hayakawa J, Ishizuka M: Temperature-dependent self-splicing group I introns in the flagellin genes of the thermophilic Bacillus species. Biosci Biotechnol Biochem 2012, 76: 410-413. 10.1271/bbb.110741
Ko M, Choi H, Park C: Group I self-splicing intron in the recA gene of Bacillus anthracis . J Bacteriol 2002, 184: 3917-3922. 10.1128/JB.184.14.3917-3922.2002
Goodrich-Blair H, Scarlato V, Gott JM, Xu MQ, Shub DA: A self-splicing group I intron in the DNA polymerase gene of Bacillus subtilis bacteriophage SPO1. Cell 1990, 63: 417-424. 10.1016/0092-8674(90)90174-D
Landthaler M, Begley U, Lau NC, Shub DA: Two self-splicing group I introns in the ribonucleotide reductase large subunit gene of Staphylococcus aureus phage Twort. Nucleic Acids Res 2002, 30: 1935-1943. 10.1093/nar/30.9.1935
Landthaler M, Shub DA: The nicking homing endonuclease I-BasI is encoded by a group I intron in the DNA polymerase gene of the Bacillus thuringiensis phage Bastille. Nucleic Acids Res 2003, 31: 3071-3077. 10.1093/nar/gkg433
Rogers J: Introns in archaebacteria. Nature 1983, 304: 685. 10.1038/304685a0
Armbruster DW, Daniels CJ: Splicing of intron-containing tRNATrp by the archaeon Haloferax volcanii occurs independent of mature tRNA structure. J Biol Chem 1997, 272: 19758-19762. 10.1074/jbc.272.32.19758
Morinaga Y, Nomura N, Sako Y: Population dynamics of archaeal mobile introns in natural environments: a shrewd invasion strategy of the latent parasitic DNA. Microbes Environ 2002, 17: 153-163. 10.1264/jsme2.17.153
Nomura N, Morinaga Y, Kogishi T, Kim EJ, Sako Y, Uchida A: Heterogeneous yet similar introns reside in identical positions of the rRNA genes in natural isolates of the archaeon Aeropyrum pernix . Gene 2002, 295: 43-50. 10.1016/S0378-1119(02)00802-8
Nomura N, Morinaga Y, Shirai N, Sako Y: I-ApeI: a novel intron-encoded LAGLIDADG homing endonuclease from the archaeon, Aeropyrum pernix K1. Nucleic Acids Res 2005, 33: e116. 10.1093/nar/gni118
Tocchini-Valentini GD, Fruscoloni P, Tocchini-Valentini GP: Coevolution of tRNA intron motifs and tRNA endonuclease architecture in Archaea. Proc Natl Acad Sci U S A 2005, 102: 15418-15422. 10.1073/pnas.0506750102
Lykke-Andersen J, Aagaard C, Semionenkov M, Garrett RA: Archaeal introns: splicing, intercellular mobility and evolution. Trends Biochem Sci 1997, 22: 326-331. 10.1016/S0968-0004(97)01113-4
Xue S, Calvin K, Li H: RNA recognition and cleavage by a splicing endonuclease. Science 2006, 312: 906-910. 10.1126/science.1126629
Calvin K, Li H: RNA-splicing endonuclease structure and function. Cell Mol Life Sci 2008, 65: 1176-1185. 10.1007/s00018-008-7393-y
Popow J, Schleiffer A, Martinez J: Diversity and roles of (t)RNA ligases. Cell Mol Life Sci 2012, 69: 2657-2670. 10.1007/s00018-012-0944-2
Nikolcheva T, Woodson SA: Association of a group I intron with its splice junction in 50S ribosomes: implications for intron toxicity. RNA 1997, 3: 1016-1027.
Loizos N, Tillier ER, Belfort M: Evolution of mobile group I introns: recognition of intron sequences by an intron-encoded endonuclease. Proc Natl Acad Sci U S A 1994, 91: 11983-11987. 10.1073/pnas.91.25.11983
Bassi GS, de Oliveira DM, White MF, Weeks KM: Recruitment of intron-encoded and co-opted proteins in splicing of the bI3 group I intron RNA. Proc Natl Acad Sci U S A 2002, 99: 128-133. 10.1073/pnas.012579299
Belfort M: Two for the price of one: a bifunctional intron-encoded DNA endonuclease-RNA maturase. Genes Dev 2003, 17: 2860-2863. 10.1101/gad.1162503
Geese WJ, Kwon YK, Wen X, Waring RB: In vitro analysis of the relationship between endonuclease and maturase activities in the bi-functional group I intron-encoded protein, I-AniI. Eur J Biochem 2003, 270: 1543-1554. 10.1046/j.1432-1033.2003.03518.x
Goddard MR, Burt A: Recurrent invasion and extinction of a selfish gene. Proc Natl Acad Sci U S A 1999, 96: 13880-13885. 10.1073/pnas.96.24.13880
Gogarten JP, Hilario E: Inteins, introns, and homing endonucleases: recent revelations about the life cycle of parasitic genetic elements. BMC Evol Biol 2006, 6: 94. 10.1186/1471-2148-6-94
Koonin EV, Senkevich TG, Dolja VV: The ancient virus world and evolution of cells. Biol Direct 2006, 1: 29. 10.1186/1745-6150-1-29
Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P, Moineau S, Mojica FJ, Wolf YI, Yakunin AF, van der Oost J, Koonin EV: Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol 2011, 9: 467-477. 10.1038/nrmicro2577
Chylinski K, Le Rhun A, Charpentier E: The tracrRNA and Cas9 families of type II CRISPR-Cas immunity systems. RNA Biol 2013, 10: 726-737. 10.4161/rna.24321
Barrangou R: CRISPR-Cas systems and RNA-guided interference. Wiley Interdiscip Rev RNA 2013, 4: 267-278. 10.1002/wrna.1159
Acknowledgements
This work was supported by a CIHR Operating Grant (MOP-97780) and a CIHR New Investigator Salary Award to DRE. GH’s research on mobile introns is supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada. MH would like to acknowledge support by the Egyptian Ministry of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Author’s contributions
GH: conception and design, figure preparation, manuscript writing and final approval of manuscript. MH: conception and design, compilation of data for Figures 1, 2 and 3, final approval of manuscript. DRE: conception and design, figure preparation, manuscript writing and final approval of manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hausner, G., Hafez, M. & Edgell, D.R. Bacterial group I introns: mobile RNA catalysts. Mobile DNA 5, 8 (2014). https://doi.org/10.1186/1759-8753-5-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1759-8753-5-8