Abstract
Background
Using blood lactate monitoring for risk assessment in the critically ill patient remains controversial. Some of the discrepancy is due to uncertainty regarding the appropriate reference interval, and whether to perform a single lactate measurement as a screening method at admission to the hospital, or serial lactate measurements. Furthermore there is no consensus whether the sample should be drawn from arterial, peripheral venous, or capillary blood. The aim of this review was:
1) To examine whether blood lactate levels are predictive for in-hospital mortality in patients in the acute setting, i.e. patients assessed pre-hospitally, in the trauma centre, emergency department, or intensive care unit.
2) To examine the agreement between arterial, peripheral venous, and capillary blood lactate levels in patients in the acute setting.
Methods
We performed a systematic search using PubMed, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and CINAHL up to April 2011. 66 articles were considered potentially relevant and evaluated in full text, of these ultimately 33 articles were selected.
Results and Conclusion
The literature reviewed supported blood lactate monitoring as being useful for risk assessment in patients admitted acutely to hospital, and especially the trend, achieved by serial lactate sampling, is valuable in predicting in-hospital mortality. All patients with a lactate at admission above 2.5 mM should be closely monitored for signs of deterioration, but patients with even lower lactate levels should be considered for serial lactate monitoring. The correlation between lactate levels in arterial and venous blood was found to be acceptable, and venous sampling should therefore be encouraged, as the risk and inconvenience for this procedure is minimal for the patient. The relevance of lactate guided therapy has to be supported by more studies.
Similar content being viewed by others
Introduction
Handheld point-of-care testing (POCT) has made it possible to assess blood lactate easy and fast, but the use of blood lactate monitoring for risk assessment in the critically ill patient remains controversial. In the intensive care unit (ICU), blood lactate monitoring is widely used as an indirect marker of tissue hypoxia [1, 2]. In the assessment of the traumatized patient [3] and the critically ill patient admitted to Emergency Department (ED) [2, 4], some hospitals also include blood lactate monitoring. The different policies between hospitals and departments reflect the controversy regarding the usefulness of the blood sample. Some of the discrepancy is due to uncertainty regarding the appropriate reference interval, and whether to perform a single lactate measurement as a screening method at admission to the hospital, or serial lactate measurements. Furthermore there is some discrepancy whether the sample should be obtained from arterial, central venous, peripheral venous, or capillary blood. Previous reviews do not address the question of single versus serial sampling, cut off levels, or point of sampling [1, 5–8].
The aim of this review was to appraise the scientific literature to uncover whether elevated blood lactate levels are predictive for in-hospital mortality in patients in the acute setting, i.e. patients assessed pre-hospitally, in the trauma center, ED, or ICU. Furthermore to examine the agreement between arterial, peripheral venous, and capillary lactate levels in patients in the acute setting.
Biochemistry
The blood lactate concentration reflects a balance between production and uptake of lactate in tissues, and is normally between 0.5-1.8 mM. Lactate is formed by reduction of pyruvate, and is metabolized by oxidation to pyruvate in the reaction catalyzed by the cytosolic NAD-dependent lactate dehydrogenase (Figure 1). The metabolic fate of pyruvate is mainly mitochondrial oxidation to carbon dioxide and water with accompanying energy production in the respiratory chain. The latter sequence of reactions are oxygen requiring, and with insufficient oxygen supply, or if pyruvate production for other reasons exceeds the capacity of oxidative metabolism, pyruvate will be diverted to lactate. This assures regeneration of NAD+ from NADH, which will enable glycolysis, and the accompanying ATP production to proceed. Due to the central role of the NAD-redox state for lactate production and metabolism, any metabolic condition giving rise to a steady-state increase in the cytosolic NADH/NAD+ ratio, will cause an increased net lactate production. This applies not only to conditions of hypoxia/anoxia in all tissues, but is also observed e.g. during extensive muscular work, and during alcohol metabolism by the liver. Lactate is released from tissues accompanied by a proton, and because lactic acid is fully dissociated at pH above approximately 6, excessive lactate production may thus give rise to lactic acidosis. The uptake of lactate from plasma takes place predominantly in liver and heart, where lactate will be used as an energy producing substrate or, in case of the liver, as a precursor for glucose formation.
Methods
Eligibility criteria
Due to the lack of studies of high quality, it was a priori decided to allow the inclusion of all study types. Randomized, controlled trials were prioritized, but also non-randomized trials, cohort studies, and case-control studies were considered. Case studies, studies with fewer than 40 patients, and abstracts where full text articles were not available were excluded. This review was restricted to English language publications. Study characteristics were defined according to PICO:
Participants: patients in the acute setting, i.e. patients assessed pre-hospitally, in the trauma centre, ED, or ICU.
Intervention/exposure: elevated lactate levels in venous, arterial or capillary blood.
Comparisons: patients with lactate within the normal range. Outcome: in-hospital mortality.
Inclusion and exclusion criteria are listed in Table 1.
Data sources
Studies were identified by a systematic search using PubMed, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and CINAHL up to April 2011. Reference lists of relevant peer-reviewed literature were hand searched to identify any appropriate articles that may have been missed by the electronic search.
Search strategy
We searched PUBMED systematically using the Medical Subject Headings (MeSH),
("Lactic Acid/blood"[Mesh] OR "Lactic Acid/diagnostic use"[Mesh] OR "Acidosis, Lactic/blood"[Mesh] OR "Acidosis, Lactic/classification"[Mesh] OR "Acidosis, Lactic/complications"[Mesh] OR "Acidosis, Lactic/diagnosis"[Mesh] OR "Acidosis, Lactic/drug therapy"[Mesh] OR "Acidosis, Lactic/etiology"[Mesh] OR "Acidosis, Lactic/metabolism"[Mesh] OR "Acidosis, Lactic/mortality"[Mesh] OR "Acidosis, Lactic/physiopathology"[Mesh] OR "Acidosis, Lactic/prevention and control"[Mesh] OR "Acidosis, Lactic/surgery"[Mesh]) AND ("triage"[Mesh] OR "Injury Severity Score"[Mesh] OR "Mortality"[Mesh] OR "Blood Component Transfusion"[Mesh] OR "Emergency medical services"[Mesh] OR "Emergency Service, Hospital"[Mesh] OR "Sepsis"[Mesh] OR "Point-of-care systems"[Mesh] OR "Intensive care units"[Mesh] OR "Intensive care"[Mesh] OR "Length of stay"[Mesh] OR "Early diagnosis"[Mesh] OR "Critical illness"[Mesh] OR "Shock"[Mesh] OR "Treatment Outcome"[Mesh] OR "Veins"[Mesh] OR "Arteries"[Mesh] OR "Capillaries"[Mesh])
Study selection and data extraction
Two experts independently screened the selected studies for inclusion. Studies were included only if both experts considered the study as relevant for answering the study objectives.
Information was extracted from each included study on (1) characteristics of participants; (2) characteristics of blood lactate assessment; (3) lactate cut off value; (4) type of outcome measure; (5) statistics supporting the main findings of the study. To assess the quality of the included studies, methods validated for internal validity, precision, and applicability (external validity) were applied [9]. The methodological quality and clinical relevance of each study was graded as high, moderate, or low. The review was presented according to 'Preferred Reporting Items for Systematic Reviews and Meta-Analysis: 'The PRISMA statement' [10].
Results
The initial search identified 1.677 abstracts, which were evaluated for relevance. 66 articles were considered potentially relevant and evaluated in full text. Ultimately 33 articles were selected.
The flow diagram in Figure 2 illustrates the study selection.
The articles that were ultimately selected were divided in 2 groups; each group referring to one of the two research questions. The groups were:
1) Studies investigating single or serial blood lactate assessment (Table 2).
2) Studies comparing arterial, venous and capillary lactate (Table 3).
Single blood lactate assessment
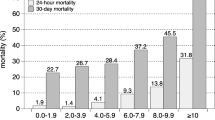
The predictive value of a single elevated blood lactate on mortality is demonstrated in a number of studies in patients admitted to ICU [11–16]. Four of these studies were of moderate quality and two were of low quality. Admission lactate ≥ 2 mM was a significant independent predictor of mortality in adult patients admitted to ICU in a large retrospective study by Khosravani et al. [11]. Odds Ratio (OR) for mortality increased form 1.94 to 10.89 dependent on the level of hyperlactatemia, compared to patient with admission lactate < 2 mM. Studies by Nichol et al. and Smith et al. also demonstrated a highly significant effect of an elevated admission lactate above 2.0 mM respectively 1.5 mM on mortality in unselected ICU patients [12, 13]. In patients admitted acutely to ED or trauma centre several studies have demonstrated a significant effect of elevated admission lactate on in-hospital mortality [2–4, 17–26]. Ten of these studies were of moderate quality, and three were of low quality. The admission lactate in this group of patients was either assessed pre-hospitally, by admission to the ED, or the ICU. The cut-off value was chosen between 2.0 and 4.0 mM. Two minor studies were not able to demonstrate a significant difference between survivors and non-survivors based on a single elevated lactate measurement [22, 25]. In addition to in-hospital mortality as the primary outcome measure, some studies also demonstrated a significant effect on secondary outcome measures; for instance length of hospital stay [26, 27] and the need for blood transfusion within the first 24 hours [21]. Several of the studies express a dose-response relationship between lactate levels and mortality [2, 4, 11, 12, 17].
Serial blood lactate assessment
Sustained hyperlactatemia among ICU patients, demonstrated by serial measurements, has shown to be predictive for in-hospital mortality [12–16, 28–30]. Five of these studies were of moderate quality and three were of low quality. In these studies, high lactate levels in serial measurements and prolonged time to normalize lactate, predicted a higher mortality rate. Most studies used 2.0 mM as cut off point. In a large study by Nichol et al., however, sustained lactate levels as low as 0.75-1.0 mM were associated with doubling of the risk of adverse outcome (OR = 2.0, p < 0.0001) [12]. In 2004 Kliegel et al. examined patients who were resuscitated from cardiac arrest and survived at least 48 hours [31]. They concluded that sustained hyperlactatemia (> 2.0 mM after 48 hours) was predictive for mortality as well as poor neurological outcome. Only one single study has evaluated the value of serial lactate monitoring pre-hospitally [18]. In this study, Jansen et al. assessed lactate at the site of injury and at arrival to the hospital, and found that mortality was significantly higher in patients with lactate > 3.5 mM at the site of injury (41% vs. 12%, p < 0.001), or at admission to the hospital (47% vs. 15%; p < 0.001) compared to patients with lactate levels < 3.5 mM. Pre-hospital lactate had better prognostic value than vital signs such as blood pressure alone. The effect of lactate guided treatment was studied in a randomized controlled study of ICU patients [32]. The administration of erythrocytes, dobutamine, and fluid substitution was administrated following an algorithm targeting a SvO2 at 70%. In the intervention group further therapy with vasodilators was started if lactate clearance was less than 20% in 2 hours. When adjusted for predefined risk factors, in-hospital mortality was significantly lower in the intervention group (hazard ratio 0.61; 95% CI 0.43-0.87, p = 0.006). No further reduction in mortality was observed when lactate levels were reduced to less than 2 mM. In a randomized controlled study by Jones et al., septic patients and other patients with hypoperfusion were treated by two different resuscitation protocols, one guided by SvO2 and the other guided by lactate levels [33]. There was no significant difference in mortality between the two groups.
Comparison of arterial, venous, and capillary lactate
Point-of-care testing has enabled monitoring of lactate levels close to the patients in the general ward, in the ambulance, or in the ED. Six studies investigating arterial, venous, and capillary lactate were included. All studies were of moderate quality. High accuracy was demonstrated in a number of studies, when lactate was analyzed using POCT compared to laboratory results in the same type of blood (venous, arterial, or capillary) [34–36]. The correlation between arterial and capillary values of lactate was evaluated in 2001 by Boldt et al., who showed a moderate correlation between lactate measured in capillary blood compared to arterial values (r2 = 0,80) in 40 intensive care patients [34]. They recommended that capillary lactate should not substitute arterial lactate in critically ill patients due to the risk of overestimating lactate levels. The correlation between arterial and peripheral venous lactate has been investigated by Lavery et al., who found no significant difference between arterial and venous levels of lactate, the highest correlation being between the radial artery and a peripheral vein (r = 0.988) [26]. In a study with patients admitted to ED, Gallagher et al. found a correlation at r2 = 0.89 between arterial and venous lactate [37]. Venous lactate tended to be overestimated (bias 0.22 mM, 95% CI (0.04-0.41)). Younger et al. found a moderate correlation between arterial and venous lactate (r2 = 0.71) in patients admitted to ED, also with tendency of venous lactate being overestimated (bias 0.18 mM, 95% CI (0.012-0.372)) [38]. The sensitivity and specificity of peripheral venous lactate for hyperlactatemia were found to be 94% and 57% respectively in the study by Gallagher et al., while sensitivity was 100% and specificity was 86% in the study by Younger et al. [37, 38]. No difference was found in peripheral venous lactate level when comparing sampling with or without the use of tourniquet [26, 37].
Discussion
Single versus serial lactate monitoring
Most studies reviewed here support single lactate measurements, assessed at admission to the hospital, as being useful in terms of predicting adverse outcome. The predictive value of blood lactate is further supported by the fact that several studies demonstrate a dose-response relationship; the higher the lactate levels, the higher the mortality rates. The predictive value of a single lactate assessed at admission is, however, controversial, as several studies were not able to show a significant predictive value of admission lactate [16, 22, 25]. Furthermore there is great heterogeneity between studies when defining, at what stage an admission lactate should be taken; some studies choosing the first patient contact, others when the patient is admitted to ICU. In the population admitted to ICU, close monitoring of all patients are mandatory, and it seems reasonable to perform serial lactate measurement on all patients. However in trauma patients or patients admitted to ED, a decision based on a single lactate measurement could be useful in terms of deciding the level of observation and treatment, and to select patients for serial lactate monitoring. Sustained hyperlactatemia has been shown to be predictive for adverse outcome in a number of studies, and therefore serial lactate measurements might be a useful approach to monitor the critically ill patient. Another advantage by using serial measurements is that patients having a temporary and non-pathologically elevated lactate, for instance as a result of a high adrenaline level [39], or alcohol intake [40], are excluded from the population. There is no agreement about the optimal time interval between serial lactate sampling. Jansen et al. aimed to reduce lactate by 20% per 2 hours, and managed to reduce mortality significantly in the intervention group [32]. Therefore, based on the reviewed articles, an interval between two and six hours seems reasonable to be able to follow the trend.
Lactate cut off point
This study was not designed to specify the appropriate lactate cut off values. Setting the cut off point for lactate in order to predict patient outcome, is a question of getting the optimal relationship between sensitivity and specificity. This is most easily visualized by the receiver operating characteristics (ROC) [41]. A test with high sensitivity carries the risk of many false positive and hence over-triage. On the other hand, if the test is used for screening purposes, or as a part of multifactorial risk assessment, it might be desirable with a certain level of over-triage. The higher the cut off point, the better predictive value of a positive test, and the greater risk for getting false negative results. Several of the studies demonstrating a significant predictive value of single lactate, assessed at admission to hospital, had a cut off point at 2.5 mM [2, 4, 21]. Studies which used a higher cut off point, also found significant predictive value of admission lactate [18, 24]. When 2.0 mM was used as cut off point, the predictive value of admission lactate became more controversial. This indicates that the cut off point for lactate, assessed at admission to hospital, probably should lie in the interval 2.0-2.5 mM. The cut off point for sustained hyperlactatemia are for several studies 2.0 mM [12, 14–16, 31]. A few studies even found significantly increased mortality for a sustained lactate as low as 0.75-1.0 mM [12, 13]. More studies are needed to support this very low cut off point; it will inevitably lead to the risk of over-triage. It is possible however, that the limit for serial lactate should be considered lower than for admission lactate.
Lactate measured in capillary, arterial, or venous blood
The golden standard in assessing lactate levels is in arterial blood. In a previous review, the authors concluded that most studies found a satisfactory agreement between the capillary, peripheral venous, and arterial lactate [1]. The studies available, however, are all quite small, and they present conflicting conclusions in relation to whether the capillary lactate could replace the arterial, due to tendency of a higher lactate result in capillary blood. Peripheral venous lactate demonstrates a good correlation to the arterial lactate, as well as a very high sensitivity and acceptable specificity for hyperlactatemia. Bland-Altman plots show bias between 0.18-0.22 mM, with the tendency of venous lactate being higher than arterial lactate [37, 38]. Setting the same cut-off for arterial and venous lactate levels will lead to more false positive results when using venous lactate, but makes lactate in peripheral blood a reasonable screening test, because all patients with hyperlactatemia are found. An arterial puncture requires training of the personnel, can be time consuming, expensive, and subject to pain and inconvenience for the patient [26, 34]. A venous or capillary lactate would increase the accessibility of the test, because the sample could be drawn together with other venous blood samples at admission to hospital, or in the ambulance. Furthermore a venous or capillary sample carries minimal risk and inconvenience for the patient, and minimal training of the personnel. The use of capillary lactate carries the risk of over-triage, unless the cut off point is set higher than for arterial lactate.
Strengths and limitations
The strength of this review is due to the systematic search and inclusion of studies. The quality of the studies reviewed is, however, not optimal. The majority of the studies presented here are of moderate or low quality, and suffers from a sample size of uncertain adequacy; some of the studies are quite small and probably underpowered. Furthermore convenience samples are used in the studies comparing lactate levels in capillary, arterial, and venous blood with the associated risk of getting selection bias. The lactate taken at admission is not clearly defined; the blood sample is taken in the ED, the ICU, or pre-hospitally depending on the study design. In addition, the population is heterogeneous and in different phases of the acute process. We find, that an attempt to evaluate the strength of the recommendation for monitoring lactate, for instance using the internationally accepted GRADE system, is not possible due to the heterogeneity between studies [42]. This is in accordance with the conclusion of Jansen et al, in a recent review discussing the use of lactate monitoring [1]
Future research
The lactate cut off values should be confirmed by larger, prospective studies. These should be followed by interventional studies to determine if therapy directed towards a reduction in lactate levels improve patient outcome compared to a control group. Lactate level is a surrogate parameter for the insufficiency of the circulation. Therefore all interventions aiming at improving the circulation (fluids, inotropes etc.) will have an effect on lactate levels. It is probably not the elevated lactate per se, but the underlying condition that increases the risk for adverse outcome. This needs to be further investigated. Concerning the discussion about the use of capillary and peripheral lactate, a study with a larger number of patients is wanted. The study should aim to take arterial, peripheral venous, and capillary lactate within a short time relative to each other, and preferably serial measurements over time. It would be beneficial to include a subpopulation of patients with hypotension, since peripheral constriction, triggered by increased sympathetic activity, could lead to artificially high lactate values when measured on capillary blood.
Conclusions
Blood lactate monitoring is useful for risk assessment in patients admitted acutely to hospital, and especially the trend in serial lactate monitoring is valuable in predicting in-hospital mortality. All patients with a lactate at admission above 2.5 mM should be closely monitored for signs of deterioration, and patients with even lower lactate levels should be considered for serial lactate monitoring. Peripheral venous blood lactate is highly correlated with arterial blood lactate, and venous sampling should therefore be encouraged, as the risk and inconvenience for this procedure is minimal for the patient. The relevance of lactate guided therapy has to be supported by more studies.
Abbreviations
- CI:
-
confidence interval
- ED:
-
emergency department
- GCS:
-
Glasgow coma scale
- ICU:
-
intensive care unit
- IQR:
-
interquartile range
- ISS:
-
injury severity score
- LOS:
-
length of stay
- NPV:
-
negative predictive value
- OR:
-
odds ratio
- POCT:
-
point-of-care testing
- PPV:
-
positive predictive value
- RR:
-
relative risk
- ROC:
-
receiver operating characteristic
- Sens.:
-
sensitivity
- Spec.:
-
specificity
- SvO2:
-
central venous oxygen saturation
References
Jansen TC, van BJ, Bakker J: Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med. 2009, 37 (10): 2827-39. 10.1097/CCM.0b013e3181a98899.
Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al: Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005, 45 (5): 524-8. 10.1016/j.annemergmed.2004.12.006.
Callaway DW, Shapiro NI, Donnino MW, Baker C, Rosen CL: Serum lactate and base deficit as predictors of mortality in normotensive elderly blunt trauma patients. J Trauma. 2009, 66 (4): 1040-4. 10.1097/TA.0b013e3181895e9e.
Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI: Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007, 33 (11): 1892-9. 10.1007/s00134-007-0680-5.
Hung KK: Best Evidence Topic report. BET 2. Serum lactate as a marker for mortality in patients presenting to the emergency department with trauma. Emerg Med J. 2009, 26 (2): 118-9.
Kaplan LJ, Frangos S: Clinical review: Acid-base abnormalities in the intensive care unit -- part II. Crit Care. 2005, 9 (2): 198-203.
Mercier D, Jones J: Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Serum lactate as a marker for mortality in patients presenting to the emergency department with infection. Emerg Med J. 2007, 24 (6): 431-2.
Rixen D, Siegel JH: Bench-to-bedside review: oxygen debt and its metabolic correlates as quantifiers of the severity of hemorrhagic and post-traumatic shock. Crit Care. 2005, 9 (5): 441-53. 10.1186/cc3526.
Bilaga 1. Mallar etc för bedömning av studiernas kvalitet. [http://www.sbu.se/upload/Publikationer/Content0/1/Bilagor_triage.pdf]
Moher D, Liberati A, Tetzlaff J, Altman DG: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009, 3 (3): e123-e130.
Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB: Occurrence and adverse effect on outcome of hyperlactatemia in the critically ill. Crit Care. 2009, 13 (3): R90-10.1186/cc7918.
Nichol AD, Egi M, Pettila V, Bellomo R, French C, Hart G, et al: Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. 2010, 14 (1): R25-10.1186/cc8888.
Smith I, Kumar P, Molloy S, Rhodes A, Newman PJ, Grounds RM, et al: Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intensive Care Med. 2001, 27 (1): 74-83. 10.1007/s001340051352.
Suistomaa M, Ruokonen E, Kari A, Takala J: Time-pattern of lactate and lactate to pyruvate ratio in the first 24 hours of intensive care emergency admissions. Shock. 2000, 14 (1): 8-12. 10.1097/00024382-200014010-00002.
Hatherill M, McIntyre AG, Wattie M, Murdoch IA: Early hyperlactataemia in critically ill children. Intensive Care Med. 2000, 26 (3): 314-8. 10.1007/s001340051155.
Cerovic O, Golubovic V, Spec-Marn A, Kremzar B, Vidmar G: Relationship between injury severity and lactate levels in severely injured patients. Intensive Care Med. 2003, 29 (8): 1300-5. 10.1007/s00134-003-1753-8.
del Portal DA, Shofer F, Mikkelsen ME, Dorsey PJ, Gaieski DF, Goyal M, et al: Emergency department lactate is associated with mortality in older adults admitted with and without infections. Acad Emerg Med. 2010, 17 (3): 260-8. 10.1111/j.1553-2712.2010.00681.x.
Jansen TC, van BJ, Mulder PG, Rommes JH, Schieveld SJ, Bakker J: The prognostic value of blood lactate levels relative to that of vital signs in the pre-hospital setting: a pilot study. Crit Care. 2008, 12 (6): R160-10.1186/cc7159.
Kaplan LJ, Kellum JA: Initial pH, base deficit, lactate, anion gap, strong ion difference, and strong ion gap predict outcome from major vascular injury. Crit Care Med. 2004, 32 (5): 1120-4. 10.1097/01.CCM.0000125517.28517.74.
Pal JD, Victorino GP, Twomey P, Liu TH, Bullard MK, Harken AH: Admission serum lactate levels do not predict mortality in the acutely injured patient. J Trauma. 2006, 60 (3): 583-7. 10.1097/01.ta.0000205858.82575.55.
Vandromme MJ, Griffin RL, Weinberg JA, Rue LW, Kerby JD: Lactate is a better predictor than systolic blood pressure for determining blood requirement and mortality: could prehospital measures improve trauma triage?. J Am Coll Surg. 2010, 210 (5): 861-9. 10.1016/j.jamcollsurg.2010.01.012.
Arnold RC, Shapiro NI, Jones AE, Schorr C, Pope J, Casner E, et al: Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock. 2009, 32 (1): 35-9. 10.1097/SHK.0b013e3181971d47.
Guyette F, Suffoletto B, Castillo JL, Quintero J, Callaway C, Puyana JC: Prehospital serum lactate as a predictor of outcomes in trauma patients: a retrospective observational study. J Trauma. 2011, 70 (4): 782-6. 10.1097/TA.0b013e318210f5c9.
Trzeciak S, Dellinger RP, Chansky ME, Arnold RC, Schorr C, Milcarek B, et al: Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med. 2007, 33 (6): 970-7. 10.1007/s00134-007-0563-9.
Kaplan LJ, Kellum JA: Comparison of acid-base models for prediction of hospital mortality after trauma. Shock. 2008, 29 (6): 662-6.
Lavery RF, Livingston DH, Tortella BJ, Sambol JT, Slomovitz BM, Siegel JH: The utility of venous lactate to triage injured patients in the trauma center. J Am Coll Surg. 2000, 190 (6): 656-64. 10.1016/S1072-7515(00)00271-4.
van Beest PA, Mulder PJ, Oetomo SB, van den Broek B, Kuiper MA, Spronk PE: Measurement of lactate in a prehospital setting is related to outcome. Eur J Emerg Med. 2009, 16 (6): 318-22. 10.1097/MEJ.0b013e32832dbe54.
Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al: Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004, 32 (8): 1637-42. 10.1097/01.CCM.0000132904.35713.A7.
Claridge JA, Crabtree TD, Pelletier SJ, Butler K, Sawyer RG, Young JS: Persistent occult hypoperfusion is associated with a significant increase in infection rate and mortality in major trauma patients. J Trauma. 2000, 48 (1): 8-14. 10.1097/00005373-200001000-00003.
Jansen TC, van BJ, Mulder PG, Lima AP, van der Hoven B, Rommes JH, et al: Prognostic value of blood lactate levels: does the clinical diagnosis at admission matter?. J Trauma. 2009, 66 (2): 377-85. 10.1097/TA.0b013e3181648e2f.
Kliegel A, Losert H, Sterz F, Holzer M, Zeiner A, Havel C, et al: Serial lactate determinations for prediction of outcome after cardiac arrest. Medicine (Baltimore). 2004, 83 (5): 274-9. 10.1097/01.md.0000141098.46118.4c.
Jansen TC, van BJ, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, et al: Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010, 182 (6): 752-61. 10.1164/rccm.200912-1918OC.
Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA: Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010, 303 (8): 739-46. 10.1001/jama.2010.158.
Boldt J, Kumle B, Suttner S, Haisch G: Point-of-care (POC) testing of lactate in the intensive care patient. Accuracy, reliability, and costs of different measurement systems. Acta Anaesthesiol Scand. 2001, 45 (2): 194-9. 10.1034/j.1399-6576.2001.450210.x.
Perez EH, Dawood H, Chetty U, Esterhuizen TM, Bizaare M: Validation of the Accutrend lactate meter for hyperlactatemia screening during antiretroviral therapy in a resource-poor setting. Int J Infect Dis. 2008, 12 (5): 553-6. 10.1016/j.ijid.2008.03.007.
Shapiro NI, Fisher C, Donnino M, Cataldo L, Tang A, Trzeciak S, et al: The feasibility and accuracy of point-of-care lactate measurement in emergency department patients with suspected infection. J Emerg Med. 2010, 39 (1): 89-94. 10.1016/j.jemermed.2009.07.021.
Gallagher EJ, Rodriguez K, Touger M: Agreement between peripheral venous and arterial lactate levels. Ann Emerg Med. 1997, 29 (4): 479-83. 10.1016/S0196-0644(97)70220-8.
Younger JG, Falk JL, Rothrock SG: Relationship between arterial and peripheral venous lactate levels. Acad Emerg Med. 1996, 3 (7): 730-4. 10.1111/j.1553-2712.1996.tb03502.x.
Luchette FA, Jenkins WA, Friend LA, Su C, Fischer JE, James JH: Hypoxia is not the sole cause of lactate production during shock. J Trauma. 2002, 52 (3): 415-9. 10.1097/00005373-200203000-00001.
Dunne JR, Tracy JK, Scalea TM, Napolitano LM: Lactate and base deficit in trauma: does alcohol or drug use impair their predictive accuracy?. J Trauma. 2005, 58 (5): 959-66. 10.1097/01.TA.0000158508.84009.49.
Hanley JA, McNeil BJ: The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982, 143 (1): 29-36.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al: Grading quality of evidence and strength of recommendations. BMJ. 2004, 328 (7454): 1490-
Blow O, Magliore L, Claridge JA, Butler K, Young JS: The golden hour and the silver day: detection and correction of occult hypoperfusion within 24 hours improves outcome from major trauma. J Trauma. 1999, 47 (5): 964-9. 10.1097/00005373-199911000-00028.
Lee SW, Hong YS, Park DW, Choi SH, Moon SW, Park JS, et al: Lactic acidosis not hyperlactatemia as a predictor of in hospital mortality in septic emergency patients. Emerg Med J. 2008, 25 (10): 659-65. 10.1136/emj.2007.055558.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors contributed to study concept and design and acquisition, analysis and interpretation of the data. Finally all authors read and approved the submitted manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kruse, O., Grunnet, N. & Barfod, C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med 19, 74 (2011). https://doi.org/10.1186/1757-7241-19-74
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1757-7241-19-74