Abstract
Background
Both BRCA1 and angiotensin II type 1 receptor (AGTR1) play a critical role in ovarian cancer progression. However, the crosstalk between BRCA1 and AGTR1 signaling pathways remains largely unknown.
Methods
BRCA1 promoter methylation was analyzed by bisulfite sequence using primers focused on the core promoter region. Expression levels of BRCA1 and AGTR1 were assessed by immunohistochemistry and real-time PCR. Regression analysis was used to examine the possible relationship between BRCA1 and AGTR1 protein levels. Knockdown or overexpression of BRCA1 was achieved by using a lentiviral vector in 293 T cells and SKOV3 ovarian carcinoma cells, and primary non-mutated and BRCA1-mutated ovarian cancer cells.
Results
BRCA1 dysfunction (BRCA1 mutation or hypermethylated BRCA1 promoter) ovarian cancer showed decreased AGTR1 levels compared to normal tissue. In contrast, AGTR1 expression was increased in non-BRCA1-mutated ovarian cancer. Notably, BRCA1 activation was an effective way to induce AGTR1 expression in primary ovarian cancer cells and a positive correlation exists between BRCA1 and AGTR1 expression in human ovarian cancer specimens.
Conclusions
These results indicate that BRCA1 may be a potential trigger involved in the transcriptional regulation of AGTR1 in the development of ovarian cancer.
Similar content being viewed by others
Background
Ovarian cancer is the most lethal gynecological malignancy in women worldwide [1]. To date, although the exact cause of ovarian cancer remains largely unknown, BRCA mutations are the main known hereditary factor [2], and the risk of ovarian cancer conferred by BRCA mutations can be regulated by both genetic and environmental components [3]. The angiotensin II type 1 receptor (AGTR1) is a novel component of the renin-angiotensin system, and has a direct effect on blood pressure and heart hypertrophy [4]. Recently, AGTR1 has drawn considerable interest, not only in the field of cardiovascular risk but also in several types of gynecological malignancies, such as endometrial cancer [5, 6], cervical carcinoma [7], and especially ovarian cancer [8–10]. Accumulating evidence also indicates that an increased risk of ovarian cancer and poor patient outcome are associated with AGTR1 expression [9, 11]. Our previous study has found that AGTR1 interacts with genetic and environmental factors, which exert a potent effect on the proliferation and survival of the estrogen-induced Ishikawa cell line [12]. Several recent studies also support a possible role for AGTR1 in regulating cell proliferation during cancer development [13]. Additionally, an increasing amount of evidence suggests that BRCA1 haploinsufficiency mutations are more likely to result in cancer, due to an extraordinary ability for clonal growth and proliferation [14]. However, the complex interrelationship between AGTR1 and BRCA1 remains to be elucidated. Therefore, the present study was undertaken to investigate AGTR1 expression from genetic (BRCA1 mutated or not) and epigenetic (BRCA1 promoter methylated or not) aspects in ovarian cancer, and to provide novel insights into the regulatory mechanism of AGTR1.
Methods
Patients and tissue collection
This study was approved by the Institutional Review Board at China Medical University. Serous ovarian cancer patients were enrolled between 2010 and 2012, and all patients gave informed consent. Fresh tumor samples, adjacent normal ovarian tissues, ascites and blood samples were obtained at the time of primary surgery before any chemotherapy or radiotherapy. Hematoxylin and eosin staining of the samples for histopathological diagnosis and grading were determined by three staff pathologists using the World Health Organization criteria. All patients were screened for BRCA1 mutations by multiplex polymerase chain reaction (PCR) with complete sequence analysis using methods reported by Bi and Simard [15, 16], Their characteristics are given in Additional file 1: Table S1.
Cell culture and lentiviral transfection
Primary ovarian cancer cells were obtained from ascites for 15 BRCA1-mutated and 15 non-mutated patients undergoing surgery for ovarian cancer and cultured in RPMI 1640 with 10% fetal bovine serum (Invitrogen, CA USA) using methods reported by Szlosarek [17]. Primary ovarian cancer cells used in all experiments were passage 2. The proliferation rate is shown in Additional file 2: Figure S1 (methods shown in Additional file 3). Human 293 T cells and SKOV3 ovarian carcinoma cells were maintained in DMEM with 10% fetal bovine serum (Invitrogen). Each experiment was repeated four times for primary ovarian cancer cells of each patient, 293 T cells and SKOV3 cells. Lentiviral vectors expressing short hairpin RNAs (shRNAs) against BRCA1 (NM_007299) were obtained from GeneChem Co., Ltd (Shanghai, China), and synthesized as follows: Forward: 5′-CCGGAACCTGTCTCCACAAAGTGTGCTCGAGCACACTTTGT GGAGACAGGTTTTTTTG-3′, and Reverse: 5′-AATTCAAAAAAACCTGTCTCCACAAAGTGTGCTCGAGCACACTTTGTGGAGACAGGTT-3′. The non-silencing siRNA sequence (TTCTCCGAACGTGTCACGT) was used as a negative control. For overexpression of BRCA1, the open reading frame of BRCA1 (NM_007299) was cloned into the lentiviral vector GV287 (Ubi-MCS-3FLAG-SV40-EGFP) (GeneChem, Shanghai, China). Transfections were performed using polybrene and enhanced infection solution (GeneChem) according to the manufacturer’s recommended protocol. The efficiency of BRCA1 knockdown and overexpression is shown in Additional file 4: Figure S2 (methods shown in Additional file 3).
Real-time quantitative PCR
Total RNA was extracted using Trizol reagents (Invitrogen) according to the manufacturer’s protocol. DNA contamination was removed by adding DNase I (Invitrogen) according to the manufacturer’s protocols. Total RNA was then reverse-transcribed from 2 μg of RNA using the PrimeScript RT Master Mix kit (TaKaRa, Dalian, China) and amplified by SYBR Premix Ex TaqTM II (TaKaRa) in a Roche LightCycler 2.0 instrument (Roche Diagnostics, Mannheim, Germany). The specific primer sequences were as follows: AGTR1: 5′-CCTCAGATAATGTAAGCTCATCCAC-3′ (F) and 5′-GCTGCAGAGGAATGTTCTCTT-3′ (R); BRCA1: 5′-GGCTATCCTCTCAGAGTGACATTT-3′ (F) and 5′-GCTTTATCAGGTTATGTTGCATGG-3′ (R); GAPDH: 5′-AGGTGAAGGTCGGAGTCA-3′ (F) and 5′-GGTCATTGATGGCAACAA-3′(R).
GAPDH mRNA was amplified as an internal control for normalization of each sample. All samples were analyzed in triplicate using the 2–∆∆CT method.
Immunohistochemistry
The standard SP kit (Zhongshan, Beijing, China) was used for immunohistochemical staining. Briefly, serial 4-μm sections were obtained from each paraffin-embedded tissue block. Following deparaffinization and rehydration, sections were subjected to microwave antigen retrieval. The primary antibody were rabbit polyclonal anti-AGTR1 (sc-1173) (1:100; Santa, Cruz Biotechnologies, USA) and rabbit polyclonal anti-BRCA1 (sc-642) (1:100; Santa), and the sections were incubated overnight at 4°C with this antibody. 3,3‘-diaminobenzidine was used as the chromogen. Nuclei were counterstained with hematoxylin, and slides were dried and mounted. Negative controls were incubated with phosphate-buffered saline instead of the antibody. Immunostaining was evaluated by two independent pathologists, blinded to the identity of subject groups. Area quantification was made with a light microscope at a magnification of 400 × and analyzed by Image-Pro Plus 6.0 (Media 2 Cybernetics, USA). Intensity of the staining was divided into 10 units.
Bisulfite sequencing for BRCA1 promoter
All the tissues were used for bisulfite sequencing from the non-BRCA1-mutated cases. Genomic DNA extracted from ovarian cancer and normal ovarian tissue with a TIANamp Genomic DNA kit (Tiangen biotech, Beijing, China) was subjected to bisulfite conversion using the EZ DNA Methylation-Direct kit (Zymo research, Orange, USA) following the manufacturer’s instructions; the conversion efficiency was estimated to be at least 99.6%. It was then amplified by nested PCR. After gel purification, cloning and transformation into E. coli Competent Cells JM109 (TaKaRa), ten positive clones of each sample were sequenced to ascertain the methylation patterns of each CpG locus. The following primers were used for BRCA1 gene (Accession number: NG_005905; GRCh37/hg19) promoter: round I, F: 5′-TTGTAGTTTTTTTAAAGAGT-3′ and R: 5′-TACTACCTTTACCCAAAACAAAA-3′; and round II, F: 5′-GTAGTTTTTTTAAAGAGTTGTA-3′ and R: 5′-ACCTTTACCCAAAACAAAAA-3′. The conditions were as follows: 95°C for 2 min, 40 cycles of 30s at 95°C, 30s at 56°C and 45 s at 72°C, then 72°C for 7 min.
Statistical analysis
Regression analysis was used to examine the possible relationship between AGTR1 and BRCA1 expression. The data are presented as means ± SD. Statistical differences in the data were evaluated by Student’s t test or one-way ANOVA as appropriate, and were considered significant at P < 0.05.
Results
BRCA1 can regulate AGTR1 expression in primary ovarian cancer cells
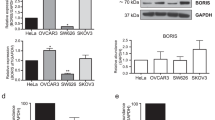
To confirm the role of BRCA1 in the regulation of AGTR1, the effects of overexpression or knockdown of BRCA1 were observed in 293 T cells, human ovarian carcinoma cell line SKOV3, primary ovarian cancer cells with identified BRCA1 mutations or non-mutation. The results indicated that there were no significant changes in the expression of AGTR1 after overexpression or knockdown of BRCA1 in 293 T and SKOV3 cells (Figure 1A-B). Interestingly, we observed that overexpression of BRCA1 was an effective way to induce an increase in AGTR1 levels in primary non-mutated and BRCA1-mutated ovarian cancer cells, although AGTR1 levels were not sensitive to the BRCA1 knockdown (Figure 1C-D).
Differences in expression patterns of AGTR1 in BRCA1-mutated and non-mutated ovarian cancer
Real-time PCR and immunohistochemical analysis showed that the levels of AGTR1 mRNA and protein were increased in non-BRCA1-mutated ovarian cancer compared to adjacent normal tissue (Figure 2A and B, P < 0.05). It is, however, interesting to note that BRCA1-mutated ovarian cancer as compared to their adjacent normal tissue reduced the expression of AGTR1 (Figure 2C and D, P < 0.05).
AGTR1 expression patterns in BRCA1 -mutated and non-mutated ovarian cancer . A and C, relative AGTR1 mRNA levels were measured in non-mutated or BRCA1-mutated ovarian cancer compared to their adjacent normal tissue, respectively (each group, n = 15). Bi and Di, sections were subjected to immunostaining for AGTR1 in non-mutated or BRCA1-mutated ovarian cancer compared to their adjacent normal tissue, respectively (each group, n = 15). Arrow shows positive staining for AGTR1 in the membrane. Bii and Dii, summary of the percentages of AGTR1-positive cells from the measurements shown in Bi and Di, respectively. Intensity of the staining was divided into 10 units. Magnification is 400 ×.
Hypermethylated BRCA1 promoter-mediated decreased expression of BRCA1 is correlated with AGTR1 levels
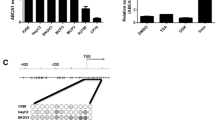
In mammals, promoter methylation at CpG dinucleotides is an important feature regulating gene expression [18]. Consistent with this idea, we showed that ovarian cancer tissue with a hypermethylated BRCA1 promoter displayed decreased expression of BRCA1 in comparison with adjacent normal tissue (Figure 3Aii and Bi, P < 0.05). However, no significant BRCA1 expression differences were observed in ovarian cancer with unmethylated BRCA1 promoter as compared to adjacent normal tissue (Figure 3Aiii and Ci, P > 0.05). Based on these considerations, the low levels of BRCA1 appeared to be mediated by promoter hypermethylation, making this an appropriate model to investigate the physiological relationship between BRCA1 and AGTR1. Notably, the expression levels of AGTR1 were decreased markedly (Figure 3Biii), along with hypermethylated promoter-mediated BRCA1 deficiency in ovarian cancer (Figure 3Bii). In contrast, AGTR1 expression was increased in ovarian cancer tissue (Figure 3Ciii), along with no significant difference of BRCA1 promoter methylation and expression (Figure 3Ci and Cii).
AGTR1 expression patterns in ovarian cancer with hypermethylated promoter-mediated BRCA1 dysfunction . Ai, location of CpG sites in the core promoter region of BRCA1. Genomic coordinates are shown, along with the primer-amplified fragments, GC percentage, location of individual CpG dinucleotides (dashes) and BRCA1 RefSeq gene (exon 1 shown as a blue box and intron shown as an arrowed line). The arrow indicates the direction of transcription. Aii and Aiii, comparative analysis of methylation patterns in the core promoter region of BRCA1 and their adjacent normal tissue (each group, n = 15). The circles correspond to the CpG sites denoted by black dashes in Figure 3Ai. Closed circles, methylation; open circles, unmethylated. Ten individual clones were sequenced for each sample. Bi and Ci, summary of the methylation levels of BRCA1 core promoter from the measurements shown in Aii and Aiii, respectively. Bii and Cii, relative BRCA1 mRNA levels were measured in ovarian cancer with identified hypermethylated or unmethylated BRCA1 promoter compared to their adjacent normal tissue, respectively (each group, n = 15). Biii and Ciii, relative AGTR1 mRNA levels were measured in ovarian cancer in the presence or absence of BRCA1 dysfunction, respectively (each group, n = 15). Bar graphs show mean ± SD. *P < 0.05 vs. Normal.
AGTR1 is positively correlated with BRCA1 expression in ovarian cancer samples
Of particular interest and potential clinical relevance, the relationship between BRCA1 and AGTR1 expression was studied in 63 human ovarian cancer specimens. Our results showed that there is a significant positive association between BRCA1 and AGTR1 protein expression (Figure 4A and B).
Correlation between the expression levels of BRCA1 and AGTR1 in ovarian cancer samples. A, Correlation between the BRCA1 and AGTR1 protein levels in 63 human ovarian cancer tissue samples. Intensity of staining was divided into 10 units. B, Examples of immunohistochemical staining showing the positive correlation between the expression levels of BRCA1 and AGTR1 in ovarian cancers. Magnification is 400 ×.
Discussion
In this study, we report for the first time an association between BRCA1 and AGTR1 status in ovarian cancer: (i) AGTR1 expression was increased in non-BRCA1-mutated ovarian cancer, but BRCA1 dysfunction (such as via BRCA1 mutation or promoter hypermethylation) was associated with decreased AGTR1 levels; (ii) there was a positive correlation between BRCA1 and AGTR1 expression in ovarian cancer specimens; and (iii) BRCA1 activation was effective at inducing AGTR1 expression in primary ovarian cancer cells. These results suggest that BRCA1 may be a potential trigger for AGTR1. Interestingly, the activation effects of BRCA1 were primarily observed in cells originating from primary ovarian cancer, especially BRCA1-mutated ovarian cancer cells, but 293 T and SKOV3 cells were insensitive to the overexpression or knockdown of BRCA1. Accordingly, a specific intracellular environment may exist, and AGTR1 expression is likely to be the long-term result of a complex interaction of multiple factors in BRCA1-related ovarian cancer. Notably, a body of evidence suggests that there is extensive crosstalk among BRCA1 signaling pathways and hormone receptors. For example, the insulin-like growth factor 1 receptor (IGF1R) gene is a downstream target for BRCA1, as wild-type BRCA1 expression suppresses promoter activity and endogenous IGF1R levels [3]; BRCA1 can lead to degradation of the progesterone receptor by counteracting the action of progesterone; multiple mechanisms are involved in BRCA1-mediated estrogen receptor repression [19, 20]. However, to date, there have been few reports about the interactions between BRCA1 and AGTR1. Moreover, mounting evidence indicates that BRCA1 tumor suppressor gene dysfunction has an important role in promoting cell proliferation and survival [14, 21–23]. The mechanism may involve: 1) inducing insulin-like growth factor 1 expression [3, 24] in an estrogen receptor α-dependent manner [24, 25]; and 2) stimulating progesterone receptor activity by facilitating progesterone binding to the progesterone response elements [26]. AGTR1 also plays a key role in regulating cell growth and proliferation during the initiation and progression of cancer [27, 28]. Therefore, the discovery of BRCA1-mediated AGTR1 expression will stimulate new interest in the study of BRCA1-related cellular proliferation, although the details remain unclear. To date, it is not fully understood how BRCA1 activates AGTR1 gene expression at the molecular level. However, some insight may be gained by further study, and preliminary data suggest that a direct interaction between miR-155 and AGTR1 (Additional file 5: Figure S3, methods shown in Additional file 3). Specifically, miR-155 is a regulatory target for BRCA1 [29, 30]; BRCA1 knockdown results in a two- to three-fold increase in miR-155 levels [29, 31], which may be involved in AGTR1 transcriptional repression, but there are still some details that need to be considered.
Conclusion
Our results indicate that BRCA1 may be a potential regulator of AGTR1 in ovarian cancer cells. Based on these findings, there are some interesting issues that need to be considered in future studies, such as how BRCA1 affects AGTR1 transcription and whether other factors could cooperate with BRCA1 in controlling AGTR1 expression. Also, the complex interactions between BRCA1 and AGTR1 signaling pathways need to be clarified. All of this may improve our understanding of the basic molecular mechanism of BRCA1-related ovarian cancer.
Abbreviations
- AGTR1:
-
Angiotensin II type 1 receptor
- PCR:
-
Polymerase chain reaction
- shRNAs:
-
Short hairpin RNAs
References
Siegel R, Naishadham D, Jemal A: Cancer statistics, 2012. CA Cancer J Clin 2012, 62: 10–29. 10.3322/caac.20138
Pruthi S, Gostout BS, Lindor NM: Identification and management of women with BRCA mutations or hereditary predisposition for breast and ovarian cancer. Mayo Clin Proc 2010, 85: 1111–1120. 10.4065/mcp.2010.0414
Werner H, Bruchim I: IGF-1 and BRCA1 signalling pathways in familial cancer. Lancet Oncol 2012, 13: e537-e544. 10.1016/S1470-2045(12)70362-5
Herichova I, Szantoova K: Renin-angiotensin system: upgrade of recent knowledge and perspectives. Endocr Regul 2013, 47: 39–52. 10.4149/endo_2013_01_39
Piastowska-Ciesielska AW, Płuciennik E, Wójcik-Krowiranda K, Bieńkiewicz A, Bednarek A, Ochędalski T: Analysis of the expression of angiotensin II type 1 receptor and VEGF in endometrial adenocarcinoma with different clinicopathological characteristics. Tumour Biol 2012, 33: 767–774. 10.1007/s13277-011-0292-0
Piastowska-Ciesielska AW, Płuciennik E, Wójcik-Krowiranda K, Bieńkiewicz A, Nowakowska M, Pospiech K, Bednarek AK, Domińska K, Ochędalski T: Correlation between VEGFR-2 receptor kinase domain-containing receptor (KDR) mRNA and angiotensin II receptor type 1 (AT1-R) mRNA in endometrial cancer. Cytokine 2013, 61: 639–644.
Kikkawa F, Mizuno M, Shibata K, Kajiyama H, Morita T, Ino K, Nomura S, Mizutani S: Activation of invasiveness of cervical carcinoma cells by angiotensin II. Am J Obstet Gynecol 2004, 190: 1258–1263. 10.1016/j.ajog.2003.12.013
De Nuccio I, Salvati G, Genovesi G, Paolini P, Marcellini L, Schiavello V, Re M: Physiopathology of the renin-angiotensin system in the ovary. Minerva Endocrinol 1999, 24: 77–81.
Ino K, Shibata K, Kajiyama H, Yamamoto E, Nagasaka T, Nawa A, Nomura S, Kikkawa F: Angiotensin II type 1 receptor expression in ovarian cancer and its correlation with tumour angiogenesis and patient survival. Br J Cancer 2006, 94: 552–560. 10.1038/sj.bjc.6602961
Suganuma T, Ino K, Shibata K, Kajiyama H, Nagasaka T, Mizutani S, Kikkawa F: Functional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal dissemination. Clin Cancer Res 2005, 11: 2686–2694. 10.1158/1078-0432.CCR-04-1946
Song L, Zhang SL, Bai KH, Yang J, Xiong HY, Li X, Liu T, Liu HR: Serum agonistic autoantibodies against type-1 angiotensin II receptor titer in patients with epithelial ovarian cancer: a potential role in tumor cell migration and angiogenesis. J Ovarian Res 2013, 6: 22. 10.1186/1757-2215-6-22
Yang Q, Su Q, Wang G, Bi F, Sa R: Effect of AT1R knockdown on ishikawa cell proliferation induced by estrogen. Arch Gynecol Obstet 2012, 286: 481–487. 10.1007/s00404-012-2305-7
Chen X, Meng Q, Zhao Y, Liu M, Li D, Yang Y, Sun L, Sui G, Cai L, Dong X: Angiotensin II type 1 receptor antagonists inhibit cell proliferation and angiogenesis in breast cancer. Cancer Lett 2013, 328: 318–324. 10.1016/j.canlet.2012.10.006
Burga LN, Tung NM, Troyan SL, Bostina M, Konstantinopoulos PA, Fountzilas H, Spentzos D, Miron A, Yassin YA, Lee BT, Wulf GM: Altered proliferation and differentiation properties of primary mammary epithelial cells from BRCA1 mutation carriers. Cancer Res 2009, 69: 1273–1278. 10.1158/0008-5472.CAN-08-2954
Bi FF, Li D, Yang Q: Promoter hypomethylation, especially around the E26 transformation-specific motif, and increased expression of poly (ADP-ribose) polymerase 1 in BRCA-mutated serous ovarian cancer. BMC Cancer 2013, 13: 90. 10.1186/1471-2407-13-90
Simard J, Tonin P, Durocher F, Morgan K, Rommens J, Gingras S, Samson C, Leblanc JF, Bélanger C, Dion F, Liu Q, Skolnick M, Goldgar D, Shattuck-Eidens D, Labrie F, Narod SA: Common origins of BRCA1 mutations in Canadian breast and ovarian cancer families. Nat Genet 1994, 8: 392–398. 10.1038/ng1294-392
Szlosarek PW, Grimshaw MJ, Kulbe H, Wilson JL, Wilbanks GD, Burke F, Balkwill FR: Expression and regulation of tumor necrosis factor alpha in normal and malignant ovarian epithelium. Mol Cancer Ther 2006, 5: 382–390. 10.1158/1535-7163.MCT-05-0303
Suvà ML, Riggi N, Bernstein BE: Epigenetic reprogramming in cancer. Science 2013, 339: 1567–1570. 10.1126/science.1230184
Ma Y, Fan S, Hu C, Meng Q, Fuqua SA, Pestell RG, Tomita YA, Rosen EM: BRCA1 regulates acetylation and ubiquitination of estrogen receptor-alpha. Mol Endocrinol 2010, 24: 76–90. 10.1210/me.2009-0218
Ma Y, Hu C, Riegel AT, Fan S, Rosen EM: Growth factor signaling pathways modulate BRCA1 repression of estrogen receptor-alpha activity. Mol Endocrinol 2007, 21: 1905–1923. 10.1210/me.2006-0397
Garcia AI, Buisson M, Bertrand P, Rimokh R, Rouleau E, Lopez BS, Lidereau R, Mikaelian I, Mazoyer S: Down-regulation of BRCA1 expression by miR-146a and miR-146b-5p in triple negative sporadic breast cancers. EMBO Mol Med 2011, 3: 279–290. 10.1002/emmm.201100136
Promkan M, Liu G, Patmasiriwat P, Chakrabarty S: BRCA1 modulates malignant cell behavior, the expression of survivin and chemosensitivity in human breast cancer cells. Int J Cancer 2009, 125: 2820–2828. 10.1002/ijc.24684
Srinivas G, Annab LA, Gopinath G, Banerji A, Srinivas P: Antisense blocking of BRCA1 enhances sensitivity to plumbagin but not tamoxifen in BG-1 ovarian cancer cells. Mol Carcinog 2004, 39: 15–25. 10.1002/mc.10164
Kang HJ, Yi YW, Kim HJ, Hong YB, Seong YS, Bae I: BRCA1 negatively regulates IGF-1 expression through an estrogen-responsive element-like site. Cell Death Dis 2012, 3: e336. 10.1038/cddis.2012.78
Wen J, Li R, Lu Y, Shupnik MA: Decreased BRCA1 confers tamoxifen resistance in breast cancer cells by altering estrogen receptor-coregulator interactions. Oncogene 2009, 28: 575–586. 10.1038/onc.2008.405
Katiyar P, Ma Y, Riegel A, Fan S, Rosen EM: Mechanism of BRCA1 -mediated inhibition of progesterone receptor transcriptional activity. Mol Endocrinol 2009, 23: 1135–1146. 10.1210/me.2008-0347
Arrieta O, Pineda-Olvera B, Guevara-Salazar P, Hernández-Pedro N, Morales-Espinosa D, Cerón-Lizarraga TL, la Rosa CH G-D, Rembao D, Segura-Pacheco B, Sotelo J: Expression of AT1 and AT2 angiotensin receptors in astrocytomas is associated with poor prognosis. Br J Cancer 2008, 99: 160–166. 10.1038/sj.bjc.6604431
Redondo-Müller MA, Stevanovic-Walker M, Barker S, Puddefoot JR, Vinson GP: Anti-cancer actions of a recombinant antibody (R6313/G2) against the angiotensin II AT1 receptor. Endocr Relat Cancer 2008, 15: 277–288. 10.1677/ERC-07-0068
Chang S, Wang RH, Akagi K, Kim KA, Martin BK, Cavallone L, Haines DC, Basik M, Mai P, Poggi E, Isaacs C, Looi LM, Mun KS, Greene MH, Byers SW, Teo SH, Deng CX, Sharan SK, Kathleen Cuningham foundation consortium for research into familial breast cancer (kConFab): Tumor suppressor BRCA1 epigenetically controls oncogenic microRNA-155 . Nat Med 2011, 17: 1275–1282. 10.1038/nm.2459
Stefansson OA, Esteller M: BRCA1 as a tumor suppressor linked to the regulation of epigenetic states: keeping oncomiRs under control. Breast Cancer Res 2012, 14: 304.
Chang S, Sharan SK: Epigenetic control of an oncogenic microRNA, miR-155, by BRCA1 . Oncotarget 2012, 3: 5–6.
Acknowledgements
This work was supported by the 973 Program of China (No. 2011CB933504), Natural Science Foundation of China (No. 81071072) and the Higher Specialized Research Fund for Doctoral Program of Ministry of Education of China (No. 20122104110027).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DL and QY conceived of the study, participated in its design and drafted the manuscript. DL, FFB and CC carried out data acquisition and interpretation. FFB, CC and CYL participated in the design of the study and performed the statistical analysis. All authors read and approved the final manuscript.
Electronic supplementary material
13048_2013_211_MOESM1_ESM.pdf
Additional file 1: Table S1: Clinical characteristics for the 15 BRCA1-mutated serous ovarian cancer patients. (PDF 29 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Bi, FF., Li, D., Cao, C. et al. Regulation of angiotensin II type 1 receptor expression in ovarian cancer: a potential role for BRCA1. J Ovarian Res 6, 89 (2013). https://doi.org/10.1186/1757-2215-6-89
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1757-2215-6-89