Abstract
MicroRNAs (miRNAs) represent a class of small non-coding RNAs that control gene expression by targeting messenger RNA (mRNA). Recently, it has been demonstrated that miRNA expression is altered in many human diseases including cancers, suggesting that miRNA may play a potential role in the pathogenesis of different diseases. It has also been reported that there is a unique expression pattern of miRNAs in the disease state differing from the normal state. In this review, we focus on the miRNA signatures in different human diseases including cancers. Such signatures may be used as diagnostic and prognostic markers.
Similar content being viewed by others
Discovery of microRNA
Discovered in 1993 from nematode Caenorhabditis elegans, miRNA are small, non-coding RNAs composed of 18–24 nucleotides. They act by negatively regulating gene expression at the post-transcriptional level through modulation of mRNA expression. Lee et al. [1] found that lin-4, an important gene that is important for post-embryonic development of C. elegans, does not code for a protein. Instead, it is transcribed into a 22-nucleotide RNA molecule. Seven years later, a second miRNA, let-7, also emerged from C. elegans genetic screening. The discovery of many new miRNAs has continued there after, with evidence emerging to suggest these small RNAs play a crucial role in gene regulation. This role has been confirmed by the identification of hundreds of conserved miRNA sequences in nematodes, flies, and mammals [2].
To date, more than 3% of the genes in humans have been found to encode for miRNAs. Anywhere from 40% to 90% of the human protein encoding genes are under miRNA-mediated gene regulation [3]. MiRNAs exert their effect by negatively regulating gene expression through one of two mechanisms, i.e., mRNA degradation or translational suppression. The mechanism by which the miRNA proceeds is dependent on the complementarity between the miRNA and its mRNA target [4]. A high level of complementarity corresponds to the degradation of the target mRNA via the RNA-mediated interference (RNAi) pathway. When there is inadequate complementarity, miRNAs bind to the untranslated region of the target mRNAs and translational suppression occurs through a RNA-induced silencing complex (RISC). Plants more commonly utilize the first mechanism while animals employ the second [2].
MiRNAs play an important role in different biological process such as cell proliferation during development, cell growth, and organization as well as apoptosis, biogenesis, transcription, signal transduction, cell cycle, neurogenesis, and fat metabolism. The deregulation of miRNA function can lead to human diseases as the target genes of miRNA are involved in several metabolic disorders such as various forms of cancers, lymphomas, diabetes and neurological disorders such as Parkinson's and Alzheimer's diseases. Recently, there has been considerable interest in understanding the mechanism of the RNA regulatory phenomena and how miRNA functions in carcinogenesis. Specifically, determining how the regulation of miRNA may be targeted to develop cancer therapies has been a focus. In this review, we discuss recent discoveries related to miRNA in different cancer diagnoses and, in particular, treatment related to ovarian cancer (OVCA).
Biogenesis of miRNA
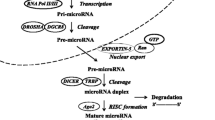
Like proteins, miRNAs are transcribed from DNA into a primary (Pri) RNA transcript. In the first step, formation of Pri-miRNA inside the nucleus occurs from the transcription of the miRNA gene by RNA polII. The Pri-miRNA is capped, polyadenylated, and then processed by RNAase III endonuclease Drosha and the double stranded (ds) RNA-binding protein Pasha. This processing results in the formation of a 60 to 70 nucleotide stem loop precursor (Pre) called Pre-miRNA. The Pre-miRNA is then transported to the cytoplasm by the Ras-related nuclear protein-guanosine-5'-triphosphate (RAN-GTP)-dependent export receptor Exportin-5. Next, the Pre-miRNA is cleaved at the base of the loop by a second RNase III endonuclease, a cytoplasmic Dicer [5]. This forms a transient, double stranded miRNA (dsmiRNA) of 22 nucleotides in length. The dsmiRNA complex then enters the miRNA-associated multiprotein RISC (miRISC), which contains the Argonuate 2 protein, and the mature miRNA is retained in the miRISC complex [2]. Argonuate proteins are active RNase enzymes and extremely conserved in the RISC. The mature miRNA is now capable of regulating its target mRNAs (See Fig. 1). Deregulation of this processing results in perturbation in miRNA genesis and may have oncogenic consequences.
Role of miRNAs in disease
Gene regulation by miRNA can affect a wide variety of cell functions, such as regulation of cell differentiation, proliferation, and apoptosis. MiRNAs are important for normal cell functioning; dysfunction of the miRNA regulation system results in disruption of the normal cellular activity leading to induction of a disease.
MiRNAs in neurodegenerative diseases
Neurodegenerative diseases are the result of the deterioration of neurons, which leads to disabilities and the possibility of death. Studies have shown that gene defects play an important role in the pathogenesis of neurodegenerative diseases such as Parkinson's, Alzheimer's, and Huntington's [6].
Parkinson's disease is the second most prevalent age-associated neurodegenerative disorder. Like Alzheimer's disease, gene mutations can result in inherited forms of Parkinson's disease [7]. Kim et al. [8] suggest that miRNAs are essential for maintaining the dopaminergic neurons in the brain. Given that Parkinson's disease is characterized by an extreme loss of dopaminergic neurons, it could be possible that miRNAs play a role in the disease pathogenesis. These investigators [8] hypothesized that inducing alterations in the miRNA networks of the brain results in neurodegenerative disorders to some extent. Based on the results of this study and from information obtained in previous work performed with mice [9], flies [10] and cultured neurons [8] in which the enzyme Dicer was genetically inactivated, Kim et al. [8] concluded that loss of Dicer results in complete inactivation of miRNA and is tremendously detrimental [11]. Moreover, Dicer was shown to be important for neuronal survival, as the loss of miRNAs may result in development and/or progression of Parkinson's disease [8, 9].
Spinal muscular atrophy (SMA) is a common genetic disease characterized by the progressive degeneration of motor neurons. It has been demonstrated that deletion or loss-of-function mutations in the survival of motor neuron (SMN) protein results in SMA [12]. Gemin 3 and Gemin 4 are shared components of SMN and miRNA-containing ribonucleoprotein particles (miRNPs). Deletion or loss-of-function mutations of SMN in SMA may affect the activity of miRNPs due to possible redistribution or changes in the levels of Gemin 3 and 4.
Using RNA immunoprecipitation 3'-end labeling and miRNA cloning, Dostie and colleagues [13] identified 53 novel miRNAs from mouse and human cell lines, out of which several were found to be phylogenetically conserved. Additionally, several miRNAs that were discovered to be components of miRNPs in these cells seem to represent distinct subfamilies, all of which contain multiple copies of these miRNAs on different chromosomes. As such, it can be proposed that these miRNAs function to regulate gene expression.
Julie Bilen and colleagues [14] isolated miRNA bantam (ban) in the genetic screen for the modulators of pathogenecity of the human neurodegenerative disease model in Drosophila. They demonstrated that upregulation of ban mitigated degeneration induced by the pathogenic polyglutamine (polyQ) protein Ataxin 3, which was mutated in human polyQ disease spinocerebellar ataxia type 3 (SCA 3). They also showed that loss of all miRNAs by Dicer mutation dramatically enhances pathogenic polyQ protein toxicity in flies and human HeLa cells. These studies suggest that miRNAs may be important for neuronal survival in relation to neurodegenerative disease.
MiRNAs in heart diseases
The heart responds to chronic and acute injury by hypertrophic growth. Cardiomyocyte hypertrophy is the dominant cellular response to virtually all forms of hemodynamic overload, endocrine disorders, myocardial injury, or inherited mutations in the various structural and contractile proteins [15]. Multiple genes are aberrantly expressed in hypertrophic cardiac cells. Using microarray analysis, miRNA expression pattern in hearts revealed that miR-1, miR-29, miR-30, miR-133, and miR-150 have often been found to be down-regulated while miR-21, miR-125, miR-195, miR-199, and miR-214 are up-regulated with hypertrophy.
MiR-21 is found to be consistently induced by cardiac stress and appears to function as a regulator of cardiac growth and fetal gene activation in primary cardiomyocytes in vitro. Its role in myocyte hypertrophy was demonstrated by Cheng et al. in 2007 [16]. Their study showed that knockout of miR-21 by an inhibitor suppresses cardiomyocyte hypertrophy induced by angiotensin II in cell culture experiments [16]. This indicates that overexpression of miR-21 has a functional role in cardiac hypertrophy. Tatsuguchi et al. [17] also confirmed the role of miR-21 in cardiac hypertrophy using locked nucleic acid modified oligos. Recently, Sayed et al. [18] reported that miR-21 modulates the cellular outgrowths during hypertrophy through the regulation of sprouty 2, an intracellular inhibitor of mitogen-activated protein-kinase signaling.
Another miRNA that is consistently up regulated in rodent and human hypertrophic hearts is miR-195. Forced expression of miRNA-195 in transgenic mice is sufficient to induce hypertrophic growth and also results in dilated cardiomyopathy and heart failure [19].
MiR-208 is encoded within intron 27 of the α major histocompatibility complex (MHC) gene and plays a key role in the expression of β MHC in response to cardiac stress [20]. Although the expression level of miR-208 remains stable during cardiac stress, this miRNA appears to fulfill a dominant function in regulating cardiac hypertrophy and remodeling. In response to pressure overload by thoracic aortic constriction, the knock-out mice show virtually no hypertrophy of cardiomyocytes or fibrosis and are unable to up regulate the β MHC expression [19]. MiR-208 is supposed to act by repressing the expression of a common component of stress-response and thyroid hormone signaling pathways in the heart, that of the thyroid-hormone-receptor (TR) co-regulator TR-associated protein 1 (THRAP1).
MiR-1 is a muscle-specific miRNA that forms a bicistronic cluster with miR-133. Both miRNAs belong to the same transcriptional unit and have been found to play a crucial role in determining when cardiomyocyte hypertrophy occurs [21]. While both miRNAs are expressed at low levels in the mouse and human models of cardiac hypertrophy, their overexpression can inhibit cardiac hypertrophy.
MiRNAs in diabetes
Diabetes is a chronic disease caused by defects in insulin production, secretion, and signaling [22]. Poy et al. [12] reported that overexpression of miR-375 resulted in suppressed glucose-stimulated insulin secretion and its inhibition resulted in enhanced insulin secretion. Myotrophin, a protein that has a role in exocytosis, was identified as one of the targets of miR-375. Inhibition of Myotrophin production has similar effects of miR-375 on insulin secretion, making miR-375 an important modulator of β-cell function. Keller et al. [23] suggested the additional targets of miR-375 could be the pancreatic transcription factors pdx-1 and NeuroD1. This indicates that miR-375 may play important role in interacting with transcription factors that control pancreatic maintenance and development [24].
MiRNA and carcinogenesis
Recent studies show that several miRNAs are differentially expressed in several human cancers, which indicate that miRNAs may have a role in the carcinogenic processes (See Table 1). In 2002, Calin et al. [25] correlated aberrant miRNA expression with cancer. The first study that was reported regarding the abnormalities in miRNA expression was in miR-15a and miR-16-1 in B-cell chronic lymphocytic leukemia (CLL); [25]. The study showed that miRNA 15 and miRNA 16 genes are located in a deleted region present in more than half of B-cell CLL cases and that both of the genes are deleted/mutated in more than half of the in CLL cases. In addition, miR-17-92, which is present as a cluster of miRNAs on chromosome 13, was reported to be associated with B cell lymphoma [26]. Other miRNAs that have been discovered to be abnormally expressed in cancerous tissues include: miR-145, which is down regulated in breast cancer [27] ovarian cancer [4]; miR-10b, which is found to be mostly down regulated in breast cancer [27]; and miR-145, which has reduced expression in colorectal cancer [28].
MiRNAs participate in various biological processes including cell differentiation, proliferation, apoptosis, stress resistance, and fat metabolism [29] and often have dual functions. For instance, in some types of cancers, they possess oncogenic activity, while in others they can suppress tumor development. Michael et al. [28] showed that miR-143 and mi-R-145 have been associated with the reduction of some malignancies, suggesting their potential tumor suppressing activities. Additional studies demonstrated that miRNAs were found to suppress BCL2, an anti-apoptotic gene [30]; supporting the idea that miRNA can also have oncogenic activity. Furthermore, miR-372 and miR-373 cooperate with oncogenic Ras in the cellular transformation of testicular germ cell tumors [31]. Additionally, in the Let-7 family, Let 7a-2 and Let 7a-3 are down regulated in lung cancer and Ras is frequently mutated [32]. Let-7b is involved in Ras regulation and inhibits cell cycle progression in melanoma [33, 34].
MiR-17-92 has been shown to function in embryogenesis [35] and is overexpressed in lung cancer, especially in small cell lung cancer, which is characterized by neuroendocrine differentiation. In addition to being overexpressed, there are an increased number of gene copies. This suggests they have a role in the development of lung cancer [36]. MiR-34a, which is absent in pancreatic cells, was directly trans-activated by the p53 gene and proved to be associated with modulation of gene expression initiated by p53 [37]. C-MYC, a proto-oncogene, encodes for a transcription factor that is responsible for cell proliferation and growth. A mutation in this gene results in most common abnormalities associated with human cancers. Finally, using microarray-based large scale profiling, Volinia et al. [38] analyzed the miRNA expression pattern in 540 normal and tumor samples from six types of tissues (lung, breast, stomach, prostate, colon, and pancreas). They proved that the abnormal expression pattern of many miRNAs is connected to carcinogenesis.
For additional information regarding differential expression of miRNAs in various cancers including glioblastomas, colorectal neoplasia, hepatocellular carcinoma, papillary thyroid carcinoma etc. ' [see Additional file 1]'.
MiRNAs in human ovarian carcinoma (OVCA)
OVCA is the most common of the gynecologic malignancies, but little is known about the molecular genetics of its initiation and progression. Epithelial OVCA (EOC), which accounts for approximately 90% of OVCAs, continues to be the leading cause of death resulting from gynecological malignancies [39]. Histologically, OVCA is divided into different subgroups such as serous, mucinous, endometrioid, clear cell, Brenner, and undifferentiated carcinomas [40]. The high mortality rate of this disease is due to late stage diagnosis for more than 70% of affected patients (Chemoresistance). Trans-vaginal ultrasound and serum CA-125 remain poor tests for diagnosing the disease in its early stage and, as a result, most cases are still more likely to be detected at the advanced stage [41].
New, more effective diagnostic tests must be developed to identify OVCA in its early stage. If OVCA is diagnosed in the early stage, current treatments will be more successful, which in turn will significantly reduce the high mortality rate. The discovery that miRNAs are expressed at different levels in normal versus malignant tissues offers the possibility of identifying new methods of diagnosing early stage incidence and discovering new therapies for the treatment of OVCA.
With the advent of new miRNA technology, it is possible to plan appropriate treatments and predict chemotherapy outcomes. Studies have shown that miRNAs, which are noncoding regulatory RNA products, are aberrantly expressed in many cancer types including breast [27], pancreatic [42], and lung cancer [4]. In addition to these forms, a study conducted by Iorio et al. in 2007, [4] confirmed that miRNAs are also abnormally expressed in OVCA (See Table 2). It was concluded that in the cancerous tissues, i.e., miR-141, miR-200a, miR-200b, and miR-200c, had the highest level of up-regulation while miR-125b, miR-140, miR-145, and miR-199a were the most down-regulated. Moreover, this study demonstrated that the levels of expression between the normal and malignant ovaries were so drastic that expression levels alone could distinctly distinguish between tissue types. This finding is extremely significant because it points to the possibility that miRNAs may have oncogenic or tumor suppressing activities.
In addition to the difference in expression levels between normal and cancerous tissues, it has been shown that miRNAs can also be histotype-specific. In endometrioid tumors, miR-21, miR-182, and miR-205 are considerably overexpressed while miR-144, miR-222, and miR-302a have reduced expression compared to normal. However, in serous and clear cell tumors, these miRNAs are normally expressed [4]. These findings suggest that while all types of OVCA are currently treated with the same standard therapies, a more effective course of treatment could be targeted towards specific histotypes of various cancers.
The above tests are invasive procedures because they require a tissue sample to determine the expression levels of miRNAs for diagnostic purposes. However, a new non-invasive approach has recently been discovered that only requires a serum sample from the patient. In the serum of OVCA patients, miRNAs 21, 29a, 92, 93, and 126 were overexpressed while miRNAs 127, 155, and 99b were under expressed as compared to controls. This indicates that a patient's serum miRNA level could be used to potentially diagnose OVCA. Additionally, miRNAs 21, 92, and 93 were also overexpressed in patients with normal CA125 levels. This suggests that earlier diagnosis of OVCA may be possible through the identification of these miRNAs even though patients may present with normal CA-125 levels [43].
Another new non-invasive method with the potential for diagnosing OVCA involves isolating tumor-derived exosomes. Exosomes are saucer shaped nanovesicles (30–100 nm) that are normally released by multiple mammalian cell types and have a significant role in cell-to-cell communication. In addition to exosomes released by normal proliferating cells, tumor cells also release exosomes. Tumor cells release exosomes at a much higher level than normal cells and the exosomes also display numerous tumor antigens that mirror the primary tumor cells. Therefore, it is proposed that these tumor-derived exosomes could be used as a diagnostic biomarker. In addition to the antigens, circulating tumor-derived exosomes have been shown to contain miRNAs that parallel those of the original tumor [44]. This demonstrates that miRNA profiling can be executed and accurately represent the originating tumor through a blood sample rather than requiring the more invasive tissue sample. It has been shown that eight particular miRNAs are of diagnostic significance in OVCA. MiRNA 21, 141, 200a, 200b, 200c, 203, 205, and 214 are all overexpressed in tumor cells and tumor-derived exosomes. The levels are different enough to clearly distinguish between benign and malignant tumors as well [44].
The current treatment utilized for patients with OVCA consists of primary cytoreductive surgery followed by platinum and taxane combination chemotherapy treatments. This method of therapy is initially successful; however, most of the patients become resistant to the platinum-based chemotherapy [45]. Recent studies indicate that there are significant differences in the level of miRNA expression between chemotherapeutic sensitive and resistant OVCA cell lines and tissues. Boren et al. [45] found that 27 miRNAs were connected to OVCA cell line sensitivity to platinum-based chemotherapeutic agents and the differential expression of these miRNAs leads to chemoresistance. In the cells resistant to the drugs, 18 miRNAs were up-regulated and 9 were down-regulated. It was determined that increased expression of miR-181a, miR-181b, and miR-213 in OVCA cell lines results in resistance to doxorubicin and gemcitabine and increased expression of miR-99b and miR-14 leads to docetaxel and paclitaxel resistance. Eitan et al. [46] also discovered several miRNAs that were differentially expressed in stage 3 ovarian tumors. It was established that increased expression of miR-23a, miR-27a, miR-30c, let-7g, and miR-199a-3p corresponds to resistance to platinum-based chemotherapy while reduced expression of miR-378 and miR-625 relates to resistance.
The difference in miRNA expression levels between chemotherapy-sensitive and resistant cells is clinically significant. New treatments could exploit these differences if the mechanism behind regulating miRNA expression is determined. Then, for instance, miR-378, which is down-regulated in malignant ovarian tissue, could be induced to over express to a normal level, possibly making the cells more sensitive to platinum-based chemotherapy. Moreover, identification of specific miRNAs between types of cancers (lung, ovarian, pancreatic) and even within types (endometrioid, clear cell, serious in ovarian) could allow for more personalized treatments, which certainly present the chance for a better prognosis.
While there is not much information currently available on the mechanisms responsible for inducing aberrant miRNA expression, there is a new possible explanation for their overexpression in OVCA. Iorio et al. [4] recently proved that treating OVCAR3 cells with 5-aza-2' deoxycytidine, a demethylating treatment, results in the overexpression of miR-21, miR-203, and miR-205. These miRNAs are also up-regulated in ovarian malignant tumors. This indicates that the mechanism causing their up-regulation in vivo could be DNA hypomethylation.
Conclusion
The discovery of aberrant miRNA expression between control patients and those with OVCA holds great promise for improved patient care and more positive outcomes. Thus, identification of altered miRNA expression, as well as their targets, provides new opportunities for therapeutic strategies. MiRNA-based gene therapy targeting deregulated miRNAs will be the future tool for cancer treatment. Using data from miRNA profiling and knowledge of familial history of a certain diseases will help in developing miRNA-based personalized therapy. Detailed understanding of the characteristic miRNA abnormalities could contribute to novel approaches in early diagnosis and treatment of different diseases including cancer and can serve as a biomarker for cancer. miRNA expression profiling eventually assist physicians in providing patients with accurate diagnosis, personalized therapy, and treatment outcome.
Abbreviations
- (miRNA):
-
MicroRNA
- (mRNA):
-
messenger RNA
- (RNAi):
-
RNA-mediated interference
- (RISC):
-
RNA-induced silencing complex
- (Pri):
-
primary
- (Pre):
-
precursor
- (RAN):
-
Ras-related nuclear protein
- (GTP):
-
guanosine-5'-triphosphate
- (ds):
-
double stranded
- (dsmiRNA):
-
double stranded miRNA
- (miRISC):
-
miRNA-associated multiprotein RISC
- (SMA):
-
Spinal muscularatrophy
- (SMN):
-
survival of motor neuron
- (miRNP):
-
miRNA-containingribonucleoprotein particle
- (ban):
-
bantam
- (polyQ):
-
polyglutamine
- (SCA 3):
-
spinocerebellar ataxia type 3
- (MHC):
-
major histocompatibility complex
- (TR):
-
thyroid-hormone-receptor
- (THRAP1):
-
thyroid-hormone-receptor co-regulator TR-associated protein 1
- (CLL):
-
chronic lymphocytic leukemia
- (EOC):
-
Epithelial ovarian cancer
- (OVCA):
-
ovarian cancer.
References
Lee RC, Feinbaum RL, Ambros V: The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75: 843–854. 10.1016/0092-8674(93)90529-Y
Esquela-Kerscher A, Slack FJ: Oncomirs -microRNAs with a role in cancer. Nat Rev Cancer 2006,6(4):259–269. 10.1038/nrc1840
Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O: Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet 2005, 37: 766–770. 10.1038/ng1590
Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, Calin GA, Menard S: MicroRNA signatures in human ovarian cancer. Cancer Res 2007, 67: 8699–8707. 10.1158/0008-5472.CAN-07-1936
Pillai R: MicroRNA functions: Multiple mechanisms for a tiny RNA? RNA Journal 2005, 11: 1753–1761. 10.1261/rna.2248605
Bertram L, Tanzi R: The genetic epidemiology of neurodegenerative disease. J Clin Invest 2005,115(6):1449–1457. 10.1172/JCI24761
Mandemakers W, Morais VA, De Strooper B: A cell biological perspective on mitochondrial dysfunction in Parkinson disease and other neurodegenerative diseases. J Cell Sci 2007,120(Pt 10):1707–1716. 10.1242/jcs.03443
Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A: A MicroRNA feedback circuit in midbrain dopamine neurons. Science 2007,317(5842):1220–1224. 10.1126/science.1140481
Schaefer A, O'Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, Greengard P: Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med 2007,204(7):1553–1558. 10.1084/jem.20070823
Bilen J, Liu N, Burnett BG, Pittman RN, Bonini NM: MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Mol Cell 2006,24(1):157–163. 10.1016/j.molcel.2006.07.030
Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ: Dicer is essential for mouse development. Nat Genet 2003,35(3):215–217. 10.1038/ng1253
Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M: A pancreatic islet-specific microRNA regulates insulin secretion. Nature 2004, 432: 226–230. 10.1038/nature03076
Dostie J, Mourelatos Z, Yang M, Sharma A, Dreyfuss G: Numerous microRNPs in neuronal cells containing novel MicroRNAs. RNA 2003,9(2):180–186. 10.1261/rna.2141503
Bilen J, Liu N, Bonini NM: A New Role for MicroRNA Pathways: Modulation of Degeneration Induced by Pathogenic Human Disease Proteins. Cell Cycle 2006,5(24):2835–2838.
Ahmad F, Seidman JG, Seidman CE: The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet 2005, 6: 185–216. 10.1146/annurev.genom.6.080604.162132
Cheng Y: MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol 2007, 170: 1831–1840. 10.2353/ajpath.2007.061170
Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen JF, Newman M, Rojas M, Hammond SM, Wang DZ: Expression of microRNAs is dynamically regulated during Cardiomyocyte hypertrophy. J Mol Cell Cardiol 2007,42(6):1137–1141. 10.1016/j.yjmcc.2007.04.004
Sayed D, Rane S, Lypowy J, He M, Chen IY, Vashistha H, Yan L, Malhotra A, Vatner D, Abdellatif M: MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Mol Biol Cell 2008, 19: 3272–3282. 10.1091/mbc.E08-02-0159
Van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN: A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA 2006,103(48):18255–18260. 10.1073/pnas.0608791103
Van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN: Control of stress-dependent cardiac growth and gene expression by microRNA. Science 2007, 316: 575–579. 10.1126/science.1139089
Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G: MicroRNA-133 controls cardiac hypertrophy. Nat Med 2007,13(5):541. 10.1038/nm1582
Cohen A, Horton ES: Progress in the treatment of type 2 diabetes: new pharmacologic approaches to improve glycemic control. Curr Med Res Opin 2007, 23: 905–917. 10.1185/030079907X182068
Keller DM, McWeeney S, Arsenlis A, Drouin J, Wright CV, Wang H, Wollheim CB, White P, Kaestner KH, Goodman RH: Characterization of pancreatic transcription factor Pdx-1 binding sites using promoter microarray and serial analysis of chromatin occupancy. J Biol Chem 2007, 282: 32084–32092. 10.1074/jbc.M700899200
Chakrabarti SK, Mirmira RG: Transcription factors direct the development and function of pancreatic beta cells. Trends Endocrinol Metab 2003, 14: 78–84. 10.1016/S1043-2760(02)00039-5
Calin GA: Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 2002, 99: 15524–15529. 10.1073/pnas.242606799
He L: A microRNA polycistron as a potential human oncogene. Nature 2005, 435: 828–833. 10.1038/nature03552
Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri A, Musiani P, Volinia S, Nenci I, Calin GA, Querzzoli P: MicroRNA gene expression deregulation in human breast cancer. Cancer Res 2005, 65: 7065–7070. 10.1158/0008-5472.CAN-05-1783
Michael MZ, O'Connor SM, Van Holst Pellekaan NG, Young GP, James RJ: Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res 2003, 1: 882–891.
Ambros V: MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell 2003, 113: 673–676. 10.1016/S0092-8674(03)00428-8
Cimmino A, Calin GA, Fabbri M: miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA 2005, 102: 13944–13949. 10.1073/pnas.0506654102
Voorhoeve PM, le Sage C, Schrier M: Genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell 2006, 124: 1169–1181. 10.1016/j.cell.2006.02.037
Takamizawa J, Konishi H, Yanagisawa K: Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 2004, 64: 3753–3756. 10.1158/0008-5472.CAN-04-0637
Johnson SM, Grosshans H, Shingara J: RAS is regulated by the let-7 microRNA family. Cell 2005, 120: 635–647. 10.1016/j.cell.2005.01.014
Schultz J, Lorenz P, Gross G, Ibrahim S, Kunz M: MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Res 2008, 18: 549–557. 10.1038/cr.2008.45
Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP: The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science 2005,310(5755):1817–21. 10.1126/science.1121158
Hayashita Y: A polycistronic microRNA cluster, miR-17–92, is over expressed in human lung cancers and enhances cell proliferation. Cancer Res 2005, 65: 9628–9632. 10.1158/0008-5472.CAN-05-2352
Chang TC: Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Molecular Cell 2007, 26: 745–752. 10.1016/j.molcel.2007.05.010
Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM: A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 2006,103(7):2257–2261. 10.1073/pnas.0510565103
Zhang L: MicroRNAs exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci USA 2006, 103: 9136–9141. 10.1073/pnas.0508889103
Seidman JD, Kurman RJ: Pathology of ovarian carcinoma. Hematol Oncol Clin North Am 2003, 17: 909–925. 10.1016/S0889-8588(03)00061-3
Olivier RI, Lubsen-Brandsma MA, Verhoef S, van Beurden M: CA125 and transvaginal ultrasound monitoring in high-risk women cannot prevent the diagnosis of advanced ovarian cancer. Gynecol Oncol 2006,100(1):20–26. 10.1016/j.ygyno.2005.08.038
Gironella M, Seux M, Xie MJ, Cano C, Tomasini R, Gommeaux J, Garcia S, Nowak J, Yeung ML, Jeang KT, Chaix A, Fazli L: Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci USA 2007, 104: 16170–16175. 10.1073/pnas.0703942104
Resnick K: The detection of differentially expressed MicroRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol 2009, 112: 55–59. 10.1016/j.ygyno.2008.08.036
Taylor D, Gercel-Taylor C: MicroRNA signatures of tumor-derived exosome as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 2008, 110: 13–21. 10.1016/j.ygyno.2008.04.033
Boren T, Xiong Yin, Hakam Ardeshir: MicroRNAs and their target messenger RNAs associated with ovarian cancer response to chemotherapy. Gynecol Oncol 2009, 113: 249–255. 10.1016/j.ygyno.2009.01.014
Eitan R: Tumor microRNA expression patterns associated with resistance to platinum based chemotherapy and survival in ovarian cancer patients. Gynecol Oncol 2009. doi: 10.1016/j.ygyno.2009.04.024
Cummins JM, Velculescu VE: Implications of micro-RNA profiling for cancer diagnosis. Oncogene 2006, 25: 6220–6227. 10.1038/sj.onc.1209914
Akao Y, Nakagawa Y, Naoe T: MicroRNAs 143 and 145 are possible common onco-microRNAs in human cancers. Oncol Rep 2006, 16: 845–850.
Bandres E, Cubedo E, Agirre X, Malumbres R, Zarate R, Ramirez N, Abajo A: Identification by Real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and nontumoral tissues. Mol Cancer 2006, 5: 29. 10.1186/1476-4598-5-29
Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, Calin GA, Liu CG: Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 2006, 9: 189–198. 10.1016/j.ccr.2006.01.025
Calin GA, Ferracin M, Cimmino A: A microRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med 2005, 353: 1793–801. 10.1056/NEJMoa050995
He H, Jazdzewski K, Li W, Liyanarachchi S, Nagi R, Volinia S, Calin GA: The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA 2005, 102: 19075–19080. 10.1073/pnas.0509603102
Chan JA, Krichevsky AM, Kosik KS: MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 2005, 65: 6029–6033. 10.1158/0008-5472.CAN-05-0137
Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, Jacob ST: Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem 2006, 99: 671–678. 10.1002/jcb.20982
Gramantieri L, Ferracin M, Fornari F, Veronese A, Sabbioni S, Liu CG, Calin GA: Cyclin G1 is a target of miR-122a, microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res 2007, 67: 6092–6099. 10.1158/0008-5472.CAN-06-4607
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T: MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular carcinoma. Gastroenterology 2007, 133: 647–658. 10.1053/j.gastro.2007.05.022
Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, Roberts LR: Association of microRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res 2008, 14: 419–427. 10.1158/1078-0432.CCR-07-0523
Gottardo F, Liu CG, Ferracin M, Calin GA, Fassan M, Bassi P, Sevignani C, Byrne D, Negrini M, Pagano F, Gomella LG, Croce CM: Micro-RNA profiling in kidney and bladder cancers. Urol Oncol 2007, 25: 387–392.
Tran N, McLean T, Zhang X, Zhao CJ, Thomson JM, O'Brien C, Rose B: MicroRNA expression profiles in head and neck cancer cell lines. Biochem Biophys Res Commun 2007, 358: 12–17. 10.1016/j.bbrc.2007.03.201
Cheng AM, Byrom MW, Shelton J, Ford LP: Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res 2005, 33: 1290–1297. 10.1093/nar/gki200
Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ, Schmittgen TD: Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer 2007, 120: 1046–1054. 10.1002/ijc.22394
Fulci V, Chiaretti S, Goldoni M, Azzalin G, Carucci N, Tavolaro S, Castellano L, Magrelli A, Citarella F, Messina M, Maggio R, Peragine N: Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood 2007, 109: 4944–4951. 10.1182/blood-2006-12-062398
Boren T, Xiong Y, Hakam A, Wenham R, Apte S, Wei Z, Kamath S, Chen DT, Dressman H, Lancaster JM: MicroRNAs and their target messenger RNAs associated with endometrial carcinogenesis. Gynecol Oncol 2008, 110: 206–215. 10.1016/j.ygyno.2008.03.023
Schulte JH, Horn S, Otto T, Samans B, Heukamp LC, Eilers UC, Krause M, Astrahantseff K, Klein-Hitpass L, Buettner R, Schramm A, Christiansen H: MYCN regulates oncogenic MicroRNAs in neuroblastoma. Int J Cancer 2008, 122: 699–704. 10.1002/ijc.23153
Landais S, Landry S, Legault P, Rassart E: Oncogenic potential of the miR-106–363 cluster and its implication in human T-cell leukemia. ancer Res 2007, 67: 5699–5707. 10.1158/0008-5472.CAN-06-4478
Matsubara H, Takeuchi T, Nishikawa E, Yanagisawa K, Hayashita Y, Ebi H, Yamada H, Suzuki M, Nagino M, Nimura Y, Osada H, Takahashi T: Apoptosis induction by antisense oligonucleotides against miR-17–5p and miR-20a in lung cancers over expressing miR-17–92. Oncogene 2007, 26: 6099–6105. 10.1038/sj.onc.1210425
Zanette DL, Rivadavia F, Molfetta GA, Barbuzano FG, Proto-Siqueira R, Silva WA Jr: miRNA expression profiles in chronic lymphocytic and acute lymphocytic leukemia. Braz J Med Biol Res 2007, 40: 1435–1440.
Dahiya N, Sherman-Baust CA, Wang TL, Davidson B, Shih IeM, Zhang Y, Wood W 3rd, Becker KG, Morin PJ: MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS One 2008,3(6):e2436. 10.1371/journal.pone.0002436
Acknowledgements
The authors thank Mr. Andrew Marsh for critical editing of the manuscript. This work was supported by a grant from NIH/NCI 124630 (SSK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PPS drafted the manuscript. LEH contributed in writing part of the manuscript. SSK conceptualized, edited, and revised the manuscript. All authors have read and approved the final manuscript.
Electronic supplementary material
13048_2009_20_MOESM1_ESM.doc
Additional file 1: Differentially expressed miRNAs in various diseases. The data provided represents the differential expression of miRNAs in various diseases. Also includes cited references for this file. (DOC 104 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Shah, P.P., Hutchinson, L.E. & Kakar, S.S. Emerging role of microRNAs in diagnosis and treatment of various diseases including ovarian cancer. J Ovarian Res 2, 11 (2009). https://doi.org/10.1186/1757-2215-2-11
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1757-2215-2-11