Abstract
Background
Although a previous meta-analysis reported no association between metabolic syndrome (MetS) and prostate cancer risk, a number of studies suggest that MetS may be associated with the aggressiveness and progression of prostate cancer. However, these results have been inconsistent. This systematic review and meta-analysis investigated the nature of this association.
Methods
We systematically searched MEDLINE, EMBASE and bibliographies of retrieved studies up to January 2013 using the keywords “metabolic syndrome” and “prostate cancer”. We assessed relative risks (RRs) of the prostate cancer, several parameters of prostate cancer aggressiveness and progression associated with MetS using 95% confidence intervals (95% CIs).
Results
The literature search produced 547 hits from which 19 papers were extracted for the meta-analysis. In cancer-free population with and without MetS, the combined adjusted RR (95% CI) of prostate cancer risk and prostate cancer-specific mortality in longitudinal cohort studies is 0.96 (0.85 ~ 1.09) and 1.12 (1.02 ~ 1.23) respectively. In the prostate cancer patients with and without MetS, the combined unadjusted OR (95% CI) of high grade Gleason prostate cancer is 1.44 (1.20 ~ 1.72), the OR of advanced prostate cancer is 1.37 (1.12 ~ 1.68) and the OR of biochemical recurrence is 2.06 (1.43 ~ 2.96).
Conclusions
The overall analyses revealed no association between MetS and prostate cancer risk, although men with MetS appear more likely to have high-grade prostate cancer and more advanced disease, were at greater risk of progression after radical prostatectomy and were more likely to suffer prostate cancer-specific death. Further primary studies with adjustment for appropriate confounders and larger, prospective, multicenter investigations are required.
Similar content being viewed by others
Background
In men, prostate cancer (PCa) is the most frequently diagnosed malignancy in industrialized countries [1] and it is the second most commonly diagnosed cancer and the sixth leading cause of cancer death worldwide [2]. There is a clear need for a better understanding of the risk factors related to PCa development and progression. Age, race and family history are the only established prostate cancer risk factors and these factors are all non-modifiable. Recently, modifiable lifestyle factors such as physical activity and diet have been investigated. Because a higher incidence of PCa was associated with a higher prevalence of “western” lifestyle, it has been suggested that these lifestyle factors play a significant role in the pathogenesis of PCa [3].
Metabolic syndrome (MetS) is a cluster of cardiovascular risk factors that includes hypertension, diabetes mellitus, obesity, hypertriglyceridemia, and low high-density lipoprotein cholesterol, with insulin resistance as the underlying hallmark feature [4]. The prevalence of MetS has been increasing worldwide and has become a major public health problem in many western countries. For example, 35%-41% of adults in the USA are reported to exhibit MetS [5]. Recently, increasing evidences suggests that MetS may be involved in the development and progression of certain types of cancer as an independent etiologic factor including breast cancer [6], endometrial cancer [7], colorectal cancer [8], pancreatic cancer [9] and prostate cancer [10]. MetS was firstly observed as a composite factor associated with prostate cancer risk in 2004 [11], and more studies have since reported the association between MetS and prostate cancer. However, the studies investigating the association between MetS and prostate cancer risk have reported inconsistent findings [12–21].
It is crucial to review and evaluate the magnitude to which MetS affects the development and progression of PCa, as proper management of this modifiable lifestyle factor may help improve PCa outcomes.
A recently performed meta-analysis study summarized the association between MetS and the incidence of some common cancer types, including prostate cancer. The results, based on 14 databases, revealed that MetS was not associated with prostate cancer risk [22]. However, a new investigation on MetS and prostate cancer risk was published recently [19], and much increasing evidence in the latest investigations suggests that MetS may be associated with the aggressiveness and progression of PCa; prostate cancer patients with MetS may suffer more aggressive disease and adverse clinical outcomes [19, 23–27]. However, inverse results [28] or no significant associations [14, 20, 29, 30] have been reported in other studies. Therefore, to thoroughly investigate the nature of this association, we focused on longitudinal cohort studies and conducted a new meta-analysis to confirm the association between MetS and prostate cancer risk by searching the latest literature. Subsequently, we performed another meta-analysis to quantitatively summarize several parameters of PCa aggressiveness and progression, including Gleason score, clinical stage, biochemical recurrence and prostate cancer-specific mortality associated with MetS.
Methods
Search strategy
We systematically searched MEDLINE, EMBASE through January 2013 for human studies on the association between MetS and PCa with the following medical subject heading terms and/or text words: “metabolic syndrome”, “insulin resistance syndrome”, or “syndrome X”, combined with “prostate cancer”, “prostatic cancer”, “prostate neoplasm”, or “prostatic neoplasm”. We also manually searched relevant journals, bibliographies, and reviews for additional articles. The search had no language restriction.
Inclusion criteria
The eligibility of each study was assessed independently by two investigators (YX and HX). We included only cohort studies of MetS and prostate cancer risk or prostate cancer-specific mortality and clinical studies of MetS and Gleason score or clinical stage at diagnosis or biochemical recurrence after treatment. We included studies that reported standardized forms of relative risk, risk ratio, hazard ratio or odds ratio with estimates of confidence intervals (CIs) or with sufficient data to estimate CIs. We used relative risks (RRs) to represent various effect estimates in a cohort study in this meta-analysis.
Exclusion criteria
We excluded reviews, editorials, meta-analysis and animal studies. Among the 23 studies that underwent full-text reviews, we excluded a study on MetS and prostate cancer risk of re-biopsy [31], a study that did not use a standard definition of MetS [32, 33] and one case-control study on MetS and prostate cancer risk [21]. For studies previously published on the same database [34, 35], we included only the most recent findings [19, 20]. All of the studies on which we focused reported RRs with 95% CIs or sufficient data to estimate them.
Data extraction
The data extracted included publication data (the first author’s last name, year of publication, and country of the population studied), study design, population resources, number of cases, risk estimates with their corresponding CIs, and variables controlled for by matching or in the most adjusted model. Abstractions of the data elements were conducted separately by two authors; discordant results were resolved by consensus.
Statistical analysis
Firstly, we updated the data and attempted to analyze the association of MetS with the prostate cancer risk in longitudinal cohort studies only. Subsequently, we assessed the association between MetS and prostate cancer-specific mortaligy in cohort studies and between MetS and high grade Gleason PCa and/or advanced PCa or biochemical recurrence in clinical studies. We pooled all of the RRs for MetS and assessed the heterogeneity between the studies by Q and I2 statistics, which are distributed as x2 statistics [36]. A value of P < 0.10 was used to indicate lack of homogeneity (heterogeneity) among effects. We used a fixed-effects model if I2 value significance was <0.1; otherwise, we used a random-effect model. Sensitivity analysis was conducted by omitting one study at a time, generating the pooled estimates and comparing with the original estimates. Funnel plots and both Begg’s and Egger’s tests were used to evaluate publication bias. All analyses were performed using STATA version 9.0 statistical software (Stata, College Station, Texas, USA). All statistical comparisons were 2-sided, and a p-value < 0.05 was considered statistically significant).
Results
Study characteristics
Nineteen studies met the search inclusion and exclusion criteria. The characteristics of included studies are presented in Tables 1 and 2.
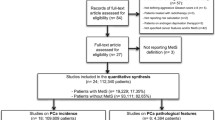
Detailed search steps are described in Figure 1. Briefly, from the initial literature search we identified 547 abstracts. Twenty-three articles were considered of interest and full text of each article was retrieved for detailed evaluation. Eleven studies investigated the association between MetS and prostate cancer [11–21]. Nine of them were longitudinal cohort studies that reported the RRs of PCa in cancer-free population with and without MetS [7–15]. Seven studies evaluated MetS and pathological and clinical stages of PCa, of these studies, 7/7 investigate Gleason score [20, 23–26, 28, 29] and 4/7 investigated clinical stage [20, 23, 24, 29]. Two case-control studies explored biochemical recurrence after primary treatment [23, 27], and three longitudinal cohort studies focused on prostate cancer-specific mortality [14, 19, 30].
Main findings
Prostate cancer risk
Result from a meta-analysis based on nine longitudinal cohort studies revealed that there was no association between MetS and prostate cancer risk (RR = 0.96, 95% CI 0.85-1.09 n = 9 studies) (Figure 2).
Prostate cancer aggressiveness
High grade Gleason score
The definition of high grade Gleason score is ≥ 7 or > 7. A trend for a 36% increased risk of a high Gleason score in patients with MetS (OR = 1.36, 95% CI 0.90-2.06 n = 7 studies) was identified based on a meta-analysis of seven total relative databases (Figure 3).
Advanced clinical stage
Advanced clinical stage was defined as a clinical stage ≥ T3. Four databases were included in the analysis of the association of MetS with advanced clinical stage. The analysis revealed that MetS was significantly associated with a 37% increased risk of advanced clinical stage (OR = 1.37, 95% CI: 1.12 ~ 1.68; n = 4 studies) (Figure 4).
Prostate cancer progression
Biochemical recurrence
Only two databases [23, 27] focused on the association of MetS which biochemical recurrence. The Individual study results and the overall summary results are presented in Figure 5. The result indicates that MetS was significantly associated with 2-folds of increased risk of biochemical recurrence (OR = 2.06, 95% CI: 1.43-2.96, n = 2 studies).
Prostate cancer-specific mortality
Three cohort studies [14, 19, 30] investigated how MetS affected prostate cancer-specific mortality. The meta-analysis revealed that MetS was significantly associated with a higher risk of the prostate cancer-specific death (RR = 1.12, 95% CI: 1.02 ~ 1.23; n = 3 studies) (Figure 6).
Sensitivity analysis
We conducted sensitivity analysis by omitting one study at a time, generating the pooled estimates and comparing the pooled estimates with the original estimates. Omitting any one of nine studies concerning MetS and prostate cancer risk or omitting any one of four studies concerning MetS and advanced clinical stage produced no dramatic influence on the original pooled RRs. Omitting Jeon 2012 database [28] in the 7 studies concerning MetS and Gleason score produced a significant OR = 1.44 (95% CI: 1.20 ~ 1.72), whereas none of the remaining severn studies exhibited a significant influence on the original estimates. For biochemical recurrence and prostate cancer-specific mortality, there were too few studies to do a sensitivity analysis.
Publication bias
Visual inspection of the Begg funnel plot for both PCR and Gleason score did not reveal the asymmetry typically associated with publication bias (Figure 7). Evidence of publication bias was also not seen with the Egger or Begg tests (Egger P = 0.27 and 0.64 for prostate cancer risk and Gleason score respectively).
Discussion
In 2007, Hsing et al. summarized five studies on MetS and prostate cancer risk and concluded that the epidemiologic evidence was insufficient to suggest a link between MetS and PCa [37]. In 2012, Esposito et al. performed a systematical review and meta-analysis on the association of MetS and cancer risk including prostate cancer. The authors also concluded that MetS was not associated with prostate cancer risk too [22]. In the present study, we updated the data and used the current evidence to analyze whether MetS is associated with prostate cancer risk. We observed the same result as previous meta-analysis; no association could be detected between Mets and prostate cancer.
We believe the result is reliable for two reasons. Firstly, only longitudinal cohort studies were included in this analysis, imparting strong evidence for our conclusions. In addition, the association between MetS and prostate cancer may be affected by several factors, including heterogeneity among the individual studies. The heterogeneity may arise from differences in age, race, the definition of MetS [22], and geographic factors [26]. Further, MetS is a syndrome composed of at least 3 components, and the individual component may exert antagonistic functions on one another Thus the syndrome may represent an integrated outcome that combines neutralizing positive and negative functions. For example, a meta-analysis revealed that diabetes mellitus was significantly negatively associated with prostate cancer risk in population-based studies (RR = 0.72, 95% CI: 0.64-0.81) and cohort studies conducted in the USA (RR = 0.79, 95% CI: 0.73, 0.86) [38]. Furthermore, several genome-wide association studies suggest that diabetes mellitus and prostate cancer share certain genetic factors, including the HNF1β and JAZF1 genes, and a previous study suggested that JAZF1 might represent a potential target against diabetes and obesity [39]. Although hypertension was found to be positively associated with prostate cancer risk [33, 40–42], Obesity is negatively with localized prostate cancer (0.94, 95% CI, 0.91-0.97) and positively associated with advanced prostate cancer risk (1.07, 95% CI 1.01-1.13) [43].
However, after analyses of several parameters of PCa aggressiveness and progression, we found MetS to be significantly associated with an increased risk of prostate cancer with a high-Gleason score or advanced clinical stage, with biochemical recurrence after primary treatment and with prostate cancer-specific mortality. If confirmed by more investigations, this finding may open a new research field on PCa development and progression, potentially leading to new strategies or methods for PCa treatment. MetS is a major public health problem and prostate cancer is the most prevalent solid organ tumor, accounts for 29% of all cancer cases and the second most common cause of death by cancer among men in the USA [44]. Therefore we believe that there is a compelling need to investigate this association between MetS and prostate cancer although the association is not strong.
Nevertheless, the reliability of these results is limited. First, Gleason score and clinical stage data were extracted from cross-sectional studies not longitudinal cohort studies. Second, there exists a small difference among studies on the definition of high-grade Gleason PCa, some authors defined a high Gleason score ≥ 7 whereas others defined a high score as >7. Third, the pathological stage data in some studies were from biopsy not radical prostatectomy specimens. Last but not least, to date there remains limited studies focusing on this association, although many of the available studies are well designed case-control or longitudinal cohort studies.
In addition to the limitations listed above, another limitation for the analyses of the association between MetS and prostate cancer risk or prostate cancer parameters is that we did not perform a meta-regression to attempt to explain the heterogeneity of the study because of the varying adjustments in the individual studies. The result of a recent meta-analysis on 9 cross-sectional studies of metabolic syndrome in adult cancer survivors increases the weight of this suspicion, as it revealed that no significant association was found for non-hematologic malignancies, including testicular tumor, prostate cancer, sarcoma, and epithelial ovarian [45]. Therefore, there is an urgent future need to confirm this association and to find potential mechanisms to explain how metabolic factors affect the development or progression of PCa.
Conclusions
Based on the current findings, MetS is not associated with prostate cancer risk, but preliminary evidences demonstrates that men with MetS more frequently suffer high-grade prostate cancer, more advanced disease and are at greater risk of progression after radical prostatectomy and prostate cancer-specific death. Together, these findings indicate that MetS may be associated with the progression of prostate cancer and adverse clinical outcomes. Further studies with adjustment for appropriate confounders and larger, prospective, multicenter investigations are required in the future.
Abbreviations
- PCa:
-
Prostate cancer
- MetS:
-
Metabolic syndrome
- RR:
-
Relative risk
- OR:
-
Odd ratio
- HR:
-
Hazard ratio
- CIs:
-
Confidence intervals.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D: Global cancer statistics. CA Cancer J Clin. 2011, 61 (2): 69-90. 10.3322/caac.20107.
Siegel R, Ward E, Brawley O, Jemal A: Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011, 61 (4): 212-236. 10.3322/caac.20121.
Nelson WG, De Marzo AM, Isaacs WB: Prostate cancer. N Engl J Med. 2003, 349 (4): 366-381. 10.1056/NEJMra021562.
Reaven GM: Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988, 37 (12): 1595-1607. 10.2337/diabetes.37.12.1595.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC: Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009, 120 (16): 1640-1645. 10.1161/CIRCULATIONAHA.109.192644.
Pothiwala P, Jain SK, Yaturu S: Metabolic syndrome and cancer. Metab Syndr Relat Disord. 2009, 7 (4): 279-288. 10.1089/met.2008.0065.
Rosato V, Zucchetto A, Bosetti C, Dal Maso L, Montella M, Pelucchi C, Negri E, Franceschi S, La Vecchia C: Metabolic syndrome and endometrial cancer risk. Ann Oncol. 2011, 22 (4): 884-889. 10.1093/annonc/mdq464.
Pelucchi C, Negri E, Talamini R, Levi F, Giacosa A, Crispo A, Bidoli E, Montella M, Franceschi S, La Vecchia C: Metabolic syndrome is associated with colorectal cancer in men. Eur J Cancer. 2010, 46 (10): 1866-1872. 10.1016/j.ejca.2010.03.010.
Rosato V, Tavani A, Bosetti C, Pelucchi C, Talamini R, Polesel J, Serraino D, Negri E, La Vecchia C: Metabolic syndrome and pancreatic cancer risk: a case-control study in Italy and meta-analysis. Metabolism. 2011, 60 (10): 1372-1378. 10.1016/j.metabol.2011.03.005.
Zhou JR, Blackburn GL, Walker WA: Symposium introduction: metabolic syndrome and the onset of cancer. Am J Clin Nutr. 2007, 86 (3): s817-s819.
Laukkanen JA, Laaksonen DE, Niskanen L, Pukkala E, Hakkarainen A, Salonen JT: Metabolic syndrome and the risk of prostate cancer in Finnish men: a population-based study. Cancer Epidemiol Biomarkers Prev. 2004, 13 (10): 1646-1650.
Tande AJ, Platz EA, Folsom AR: The metabolic syndrome is associated with reduced risk of prostate cancer. Am J Epidemiol. 2006, 164 (11): 1094-1102. 10.1093/aje/kwj320.
Russo A, Autelitano M, Bisanti L: Metabolic syndrome and cancer risk. Eur J Cancer. 2008, 44 (2): 293-297. 10.1016/j.ejca.2007.11.005.
Martin RM, Vatten L, Gunnell D, Romundstad P, Nilsen TI: Components of the metabolic syndrome and risk of prostate cancer: the HUNT 2 cohort, Norway. Cancer Causes Control. 2009, 20 (7): 1181-1192. 10.1007/s10552-009-9319-x.
Inoue M, Noda M, Kurahashi N, Iwasaki M, Sasazuki S, Iso H, Tsugane S: Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev. 2009, 18 (3): 240-247. 10.1097/CEJ.0b013e3283240460.
Grundmark B, Garmo H, Loda M, Busch C, Holmberg L, Zethelius B: The metabolic syndrome and the risk of prostate cancer under competing risks of death from other causes. Cancer Epidemiol Biomarkers Prev. 2010, 19 (8): 2088-2096. 10.1158/1055-9965.EPI-10-0112.
Wallner LP, Morgenstern H, McGree ME, Jacobson DJ, St Sauver JL, Jacobsen SJ, Sarma AV: The effects of metabolic conditions on prostate cancer incidence over 15 years of follow-up: Results from the Olmsted County Study. BJU Int. 2011, 107 (6): 929-935. 10.1111/j.1464-410X.2010.09703.x.
Osaki Y, Taniguchi S, Tahara A, Okamoto M, Kishimoto T: Metabolic syndrome and incidence of liver and breast cancers in Japan. Cancer Epidemiol. 2012, 36 (2): 141-147. 10.1016/j.canep.2011.03.007.
Haggstrom C, Stocks T, Ulmert D, Bjorge T, Ulmer H, Hallmans G, Manjer J, Engeland A, Nagel G, Almqvist M, et al.: Prospective study on metabolic factors and risk of prostate cancer. Cancer. 2012, 118 (24): 6199-6206. 10.1002/cncr.27677.
Beebe-Dimmer JL, Nock NL, Neslund-Dudas C, Rundle A, Bock CH, Tang D, Jankowski M, Rybicki BA: Racial differences in risk of prostate cancer associated with metabolic syndrome. Urology. 2009, 74 (1): 185-190. 10.1016/j.urology.2009.03.013.
Pelucchi C, Serraino D, Negri E, Montella M, Dellanoce C, Talamini R, La Vecchia C: The metabolic syndrome and risk of prostate cancer in Italy. Ann Epidemiol. 2011, 21 (11): 835-841. 10.1016/j.annepidem.2011.07.007.
Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D: Metabolic Syndrome and Risk of Cancer: A systematic review and meta-analysis. Diabetes Care. 2012, 35 (11): 2402-2411. 10.2337/dc12-0336.
Castillejos-Molina R, Rodriguez-Covarrubias F, Sotomayor M, Gomez-Alvarado MO, Villalobos-Gollas M, Gabilondo F, Feria-Bernal G: Impact of metabolic syndrome on biochemical recurrence of prostate cancer after radical prostatectomy. Urol Int. 2011, 87 (3): 270-275. 10.1159/000329280.
Kheterpal E, Sammon JD, Diaz M, Bhandari A, Trinh QD, Pokala N, Sharma P, Menon M, Agarwal PK: Effect of metabolic syndrome on pathologic features of prostate cancer. Urol Oncol. 2012, Epub ahead of print
De Nunzio C, Freedland SJ, Miano R, Trucchi A, Cantiani A, Carluccini A, Tubaro A: Metabolic syndrome is associated with high grade gleason score when prostate cancer is diagnosed on biopsy. Prostate. 2011, Epub ahead of print
Morote J, Ropero J, Planas J, Bastaros JM, Delgado G, Placer J, Celma A, de Torres IM, Carles J, Reventos J, et al.: Metabolic syndrome increases the risk of aggressive prostate cancer detection. BJU Int. 2012, Epub ahead of print
Post JM, Beebe-Dimmer JL, Morgenstern H, Neslund-Dudas C, Bock CH, Nock N, Rundle A, Jankowski M, Rybicki BA: The Metabolic Syndrome and Biochemical Recurrence following Radical Prostatectomy. Prostate Cancer. 2011, 2011: 245642-
Jeon KP, Jeong TY, Lee SY, Hwang SW, Shin JH, Kim DS: Prostate cancer in patients with metabolic syndrome is associated with low grade Gleason score when diagnosed on biopsy. Korean J Urol. 2012, 53 (9): 593-597. 10.4111/kju.2012.53.9.593.
B.K H: The characteristics of prostate cancer with metabolic syndrome in Korean men. Korean J Urol. 2007, 48 (6): 585-591. 10.4111/kju.2007.48.6.585.
Jaggers JR, Sui X, Hooker SP, LaMonte MJ, Matthews CE, Hand GA, Blair SN: Metabolic syndrome and risk of cancer mortality in men. Eur J Cancer. 2009, 45 (10): 1831-1838. 10.1016/j.ejca.2009.01.031.
Antonio C, Francesco C, Cosimo DN, Andrea T, Rocco D: Patients with metabolic syndrome and widespread high grade prostatic intraepithelial neoplasia are at a higher risk factor of prostate cancer on re-biopsy: a prospective single cohort study. Urol Oncol. 2012, Epub ahead of print
Hammarsten J, Hogstedt B: Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur J Cancer. 2005, 41 (18): 2887-2895. 10.1016/j.ejca.2005.09.003.
Asmar R, Beebe-Dimmer JL, Korgavkar K, Keele GR, Cooney KA: Hypertension, obesity and prostate cancer biochemical recurrence after radical prostatectomy. Prostate Cancer Prostatic Dis. 2012, Epub ahead of print
Lund Haheim L, Wisloff TF, Holme I, Nafstad P: Metabolic syndrome predicts prostate cancer in a cohort of middle-aged Norwegian men followed for 27 years. Am J Epidemiol. 2006, 164 (8): 769-774. 10.1093/aje/kwj284.
Beebe-Dimmer JL, Dunn RL, Sarma AV, Montie JE, Cooney KA: Features of the metabolic syndrome and prostate cancer in African-American men. Cancer. 2007, 109 (5): 875-881. 10.1002/cncr.22461.
Higgins JP, Thompson SG: Quantifying heterogeneity in a meta-analysis. Stat Med. 2002, 21 (11): 1539-1558. 10.1002/sim.1186.
Hsing AW, Sakoda LC, Chua S: Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr. 2007, 86 (3): s843-857.
Zhang F, Yang Y, Skrip L, Hu D, Wang Y, Wong C, Qiu J, Lei H: Diabetes mellitus and risk of prostate cancer: an updated meta-analysis based on 12 case-control and 25 cohort studies. Acta Diabetol. 2012, Epub ahead of print
Li L, Yang Y, Yang G, Lu C, Yang M, Liu H, Zong H: The role of JAZF1 on lipid metabolism and related genes in vitro. Metabolism. 2011, 60 (4): 523-530. 10.1016/j.metabol.2010.04.021.
Fitzpatrick AL, Daling JR, Furberg CD, Kronmal RA, Weissfeld JL: Hypertension, heart rate, use of antihypertensives, and incident prostate cancer. Ann Epidemiol. 2001, 11 (8): 534-542. 10.1016/S1047-2797(01)00246-0.
Ganesh B, Saoba SL, Sarade MN, Pinjari SV: Risk factors for prostate cancer: An hospital-based case-control study from Mumbai, India. Indian J Urol. 2011, 27 (3): 345-350. 10.4103/0970-1591.85438.
Martin RM, Vatten L, Gunnell D, Romundstad P: Blood pressure and risk of prostate cancer: Cohort Norway (CONOR). Cancer Causes Control. 2010, 21 (3): 463-472. 10.1007/s10552-009-9477-x.
Discacciati A, Orsini N, Wolk A: Body mass index and incidence of localized and advanced prostate cancer–a dose-response meta-analysis of prospective studies. Ann Oncol. 2012, 23 (7): 1665-1671. 10.1093/annonc/mdr603.
Siegel R, Naishadham D, Jemal A: CA Cancer J Clin. 2012, 62 (1): 10-29. 10.3322/caac.20138.
Jung HS, Myung SK, Kim BS, Seo HG: Metabolic syndrome in adult cancer survivors: a meta-analysis. Diabetes Res Clin Pract. 2012, 95 (2): 275-282. 10.1016/j.diabres.2011.08.029.
Acknowledgments
The authors thank Dina A Yousif from department of Medcine, Vanderbilt University, USA for checking the English language of the manuscript.
Funding
This study was supported by China Scholarship Council (NO: 2009622110) and National Science Fund for Distinguished Young Scholars (NO: 81202016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
No potential conflicts of interest were disclosed.
Authors’ contributions
This study was designed and supervised by XJ. Literature search, selection and data extraction was by YX and HX, and data analyses were performed by YX, HX, ZC, SJ, QX, YZ and GL. Data interpretation and manuscript writing received contributions from all authors. All authors read and approved the final manuscript.
Yu-zhu Xiang, Hui Xiong contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Xiang, Yz., Xiong, H., Cui, Zl. et al. The association between metabolic syndrome and the risk of prostate cancer, high-grade prostate cancer, advanced prostate cancer, prostate cancer-specific mortality and biochemical recurrence. J Exp Clin Cancer Res 32, 9 (2013). https://doi.org/10.1186/1756-9966-32-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-9966-32-9