Abstract
Objectives
To explore the function of p38MAPK and caspase-3 in DADS-induced apoptosis in human HepG2 cells, and discuss the signal transduetion mechanism of HepG2 cells in the apoptosis process induced by DADS by using the inhibitors of p38MAPK (SB203580) and caspase-3 (Z-DEVD-FMK).
Methods
After the human HepG2 cells had been treated with the DADS and inhibitors for 24 h, cell viability was determined by the MTT method, apoptosis was evaluated by flow cytometry (FCM) and the expressions of p38MAPK and caspase-3 were measured by western-blot.
Results
Our results indicated that DADS activities the p38MAPK and caspase-3, but the inhibitors, SB203580 and Z-DEVD-FMK (for p38MAPKand for caspase-3, respectively), both have the effect of inhibitory activity on P38MAPK and caspase-3. Furthermore, a combination treatment with both DADS and inhibitor (SB203580 or Z-DEVD-FMK) decreases the inhibitory and apoptotic activity of HepG2 cells increased compared with DADS-treated.
Conclusions
Our data indicate that p38MAPK and caspase-3 are involved in the process of DADS-induced apoptosis in human HepG2 cells and interact with each other.
Similar content being viewed by others
Background
The MAPK (mitogen-activated protein kinase) system is a cluster of serine/threonine protein kinases in the cells, and the activitied MAPKs participate in a variety of cellular responses including genetic transcription, inducing cell apoptosis, maintaining cell and regulating cell cycle, and so on [1–3]. The p38MAPK is the key member of the MAPK family and more commonly activated in response to cytokines, stress and cellular damage [4, 5]. A large number of studies have shown that the activity of p38MAPK is necessary in the apoptosis process induced by various anti-cancer drugs. Caspase enzymes play a very important role when cells started apoptosis as the central effector of apoptosis. Caspase-3, is the ultimate enforcer of apoptotic death, which can cleavage many proteins of important structure and function directly[6].
Diallyl disulfide (DADS) is one kind of oil-soluble sulfur organic compounds, it is a potential broad-spectrum anti-cancer drug. Studies have shown that DADS can inhibit human tumor cells grow including those of colon, lung, skin, breast, liver origins and prostate [7–10]. There are also lots of reports about the caspase-3 involvement during apoptosis process with DADS induction, such as The DADS induced apoptosis by the activation of caspase-3 in human leukemia HL-60 cells in a dosedependent manner, DADS promoted caspase-3 activity and increased cyclin E and decreased CDK2 gene expression which may lead to the G2/M arrest of T24 cells, Effects of diallyl disulfide (DADS) on expression of apoptosis associated proteins in androgen independent human prostate cancer cells (PC-3) [11, 12], and so on. Our previous studies have shown that the activated p38MAPK appears to play a cytoprotective role, and the MAPK specific inhibitors enhance apoptotic effects in HepG2 hepatoma cells with DADS treatment[13]. In this report we used the inhibitors of p38MAPK (SB203580) and caspase-3 (Z-DEVD-FMK) to detect the relation of p38MAPK and caspase-3 in the apoptosis process induced by DADS, we found that p38MAPK and caspase-3 are involved in the process of DADS-induced apoptosis in human HepG2 cells and interact with eachother.
Materials and methods
Major reagents
DADS (80% purity) was purchased from Fluka Co., Dulbecco's modified Eagle medium (DMEM) medium, BSA and SB203580 were purchased from Sigma. Z-DEVD-FMK was purchased from CALBIOCHEM (USA), goat horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody were purchased from Santa Cruz Biotech. Antibodies to p38, phospho-p38 (p-p38), caspase-3 were purchased from Cell Signaling.
Cell culture
HepG2 (the human hepatoma cell line) were provided by the Xiangya school of medicine and cultured in DMEM with 10% heat-inactivated fetal bovine serum (FBS), benzylpenicillin (100 kU/L) and streptomycin (100 mg/L) at 37°C in an incubator containing humidified air with 5% CO2.
Cell viability assay
Cells were seeded into 96-well plates at 1 × 104 cells per well 24 h before treatment. The cultures were then rinsed in phenol-free DMEM medium and incubated with respective test substances in phenol-free and serumfree DMEM for 24 h. In the inhibition test, Cells were treated with DADS after being treated with inhibitors 30 min. At the end of this time interval, 20 μl (5 mg/ml) MTT [3-(4,5dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added to each well, and after incubation at 37°C for 4 h the MTT solution was removed and 200 μl of dimethylsulfoxide (DMSO) was added to dissolve the crystals. The absorbance of each well at 570 nm was measured.
Flow cytometry analysis
Cells were seeded into 100 ml cell culture bottles at 12 × 106 cells 24 h before treatment. Then cells were treated according to the aforementioned method and incubated for 24 h. Afterwards, cells were collected, made into single cell suspension and centrifuged at 800 g for 5 min. Discard the supernatant, washed cells three times with the cool PBS and fixed them 24 h with cool alcohol at 4°C. Taked 1 ml cell suspension (106/ml), washed it three times with the cool PBS, treated it with RNase for 30 min at 37°C, and stained it with PI for 30 min at 37°C in a dark environment. Then the flow cytometry analysis can be carried out.
Western-blotting
Taked the cells in the logarithmic growth phase, treated them according to the aforementioned method and incubated for 24 h. After fragmentation on ice for 20 min, the lysates were centrifuged at 15,000 g for 10 min at 4°C, collected the protein and quantitated it with the BCA method, electrophoresed and isolated protein by the SDS-PAGE (10%), used the electrotransfer method, carried out the blocking and hybridization on the cellulose nitrate film, detected the protein expression of cells using the ECL western blotting method. The densities of protein bands were calculated using the Quantyone software.
Statistics
Data are expressed as mean ± S.D of three independent experiments and evaluated by one-way analysis of variance (ANOVA). Significant differences were established at P < 0.05.
Results
Changes of cell activity
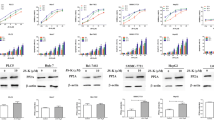
Cell viability was determined by the MTT assay. As shown in Figure 1. After treatment and incubated for 24 h, the inhibition ratio of treated with 10 μmol/L SB203580 and 100 μmol/L DADS was 19.45% at 24 h, and the inhibition ratio of treated with 10 μmol/L Z-DEVD-FMK and 100 μmol/L DADS was 17.64% at 24 h, both of them were lower than the inhibition ratio of treated with 100 μmol/L DADS at 24 h, but they were both higher than the inhibition ratio of treated with 10 μmol/L SB203580 and 10 μmol/L Z-DEVD-FMK respectively (9.73% and 6.77%).
Results of the MTT assay. Lane 1: control (untreated), lane 2: Z-DEVD-FMK (10 μmol/L), lane 3: SB203580 (10 μmol/L), lane 4: treated with DADS (100 μmol/L) after being treated with SB203580 (10 μmol/L) for 30 min lane 5: treated with DADS (100 μmol/L) after being treated with Z-DEVD-FMK (10 μmol/L) for 30 min, lane6: DADS (100 μmol/L). Cells viability was determined by MTT assay as described in Materials and Methods. Data are expressed as mean ± S.D and evaluated by one-way analysis of variance (ANOVA). Results are representative of three replicates (P < 0.01).
Flow-cytometric analysis of apoptosis
The results of flow cytometry analysis showed, the rate of SB203580-DADS group and SB203580-Z-DEVD-FMK group was 18.98% and 17.45% respectively, 1.86% of control group, 8.50% when treated with SB203580 (10 μmol/L), 6.02% when treated with Z-DEVD-FMK (10 μmol/L), and 25.23% when treated with DADS (Figure 2). These results suggested that inhibitors of P38MAPK and caspase-3 both had obvious effect of inhibiting apoptosis (Figure 3).
Effects of each group on apoptosis in in human HepG2 cells. A. Control (untreated), B. Z-DEVD-FMK (10 μmol/L), C. SB203580 (10 μmol/L), D. treated with DADS (100 μmol/L) after being treated with SB203580 (10 μmol/L) for 30 min, E. treated with DADS (100 μmol/L) after being treated with Z-DEVD-FMK (10 μmol/L) for 30 min, F. DADS (100 umol/L). Results are representative of three replicates (P < 0.01).
Western-blot analysis
After various treatment for 24 h, the zymogen bands of caspase-3 treated with DADS (100 μmol/L) became thinner significantly compared with the control gtoup, proving that DADS could advance the activity of caspase-3; after treated with SB203580 (10 μmol/L) and Z-DEVD-FMK (10 μmol/L) respectively, the zymogen bands of caspase-3 became thicker significantly compared with treated with DADS (100 μmol/L), but compared with the DADS (100 μmol/L) group that 30 minutes ahead of schedule by adding inhibitor, the band is only slightly thinner (Figure 4).
Effects of each group on the protein expressions by Western blot. Lane 1: control (untreated), lane 2: treated with DADS (100 μmol/L) after being treated with SB203580 (10 μmol/L) for 30 min, lane 3: SB203580 (10 μmol/L), lane 4: Z-DEVD-FMK (10 μmol/L), lane 5: treated with DADS (100 μmol/L) after being treated with Z-DEVD-FMK (10 μmol/L) for 30 min, lane6: DADS (100 μmol/L). The results are representative of three independent experiment.
Similarly, SB203580 (10 μmol/L) and Z-DEVD-FMK (10 μmol/L) had inhibition effect on the P-p38 MAPK, When SB203580(or Z-DEVD-FMK) was added to HepG2 hepatoma cells for 30 min before DADS treatment or only added SB203580 (or Z-DEVD-FMK) to HepG2 hepatoma cells, P-p38 MAPK was markedly decreased, but DADS induced activations of P-p38 MAPK, compared to DADS-treatment alone or no treatment (Figure 4).
These results confirm that SB203580 and Z-DEVD-FMK could inhibit the activity of P-p38 MAPK and caspase-3. But the inhibition of SB203580 was stronger than Z-DEVD-FMK, comparatively (Figure 5).
Discussion
Apoptosis is a very complex process with the complexity and diversity, different cells in different stress have different signal transduction pathways. Extracellular signals how pass to cells and cause cells to the corresponding reaction is very important to the occurrence of apoptosis in the process of cell apoptosis.
The MAPK (mitogen-activated protein kinase) system is a cluster of intracellular serine/threonine protein kinases, playing an important role in a variety of signal transduction pathways of the mammalian cells. In recent years, many research report that apoptosis signal transduction and activation of caspase have a closely relationship, and have found 16 members of caspase family in mammalian cells [14–16]. All the Caspase exit in the form of inactive zymogen, can lead to caspase cascade reaction after be activitied, and eventually induce apoptosis. Undynamic caspase-3 will trigger apoptosis when it is activitied, and play a very important role when cells started apoptosis as the central effector of apoptosis [17–20].
Our previous work has demonstrated that DADS transiently activates both p38MAPK and p42/44MAPK while it induces apoptosis in a time and dose dependent manner in human HepG2 hepatoma cells[13]. The present study focuses on the role of p38MAPK and caspase-3 in cell apoptosis and DADS-induced apoptosis. To test the relation of p38MAPK and caspase-3 in the apoptosis process of human HepG2 cells induced by DADS, we used the inhibitors of p38MAPK (SB203580) and caspase-3 (Z-DEVD-FMK), the methods of MTT, flow cytometry analysis and western blot, The results presented in this study established a potential role for inhibitors of p38MAPK and caspase-3 in DADS-induced apoptosis. First, inhibitor (SB203580 or Z-DEVD-FMK) have the effect of inhibitory activity on p38MAPK and caspase-3. Second, a combination treatment with both DADS and inhibitor (SB203580 or Z-DEVD-FMK) decreases the inhibitory and apoptotic activity of HepG2 cells increased compared with DADS-treated (Figure 1, Figure 3, Figure 4 and Figure 5). The combined effect suggests a co-chemocytotoxic value in human HepG2 cells. In conclusion, our results show that p38MAPK and caspase-3 are involved in the process of DADS-induced apoptosis in human HepG2 cells and interact with each other.
At present, there have made some progress on the effect of MAPK signaling pathway in cellular apoptosis, but need in-depth study to fully reveal its mechanisms of action. Our results show that p38MAPK and caspase-3 are involved in the process of DADS-induced apoptosis in human HepG2 cells, enhance DADS-induced apoptosis and interact with each other, but its mechanism remains to be further discussed [21–24]. Further study the relationship of MAPK signal transduction pathway and caspase in the cellular apoptosis process, will have important significance for studying anti-tumor mechanisms of DADS and designing new drugs.
References
Tian W, Zhang Z, Cohen DM: MAPK signaling and the kidney. Am J Physiol Renal Physiol. 2000, 279: 593-604.
Widmann C, Gibson S, Jarpe MB: Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999, 79: 143-180.
Tortora G, Bianco R, Daniele G: Overoming resistance to molecularly targeted anticancer therapies: Rational drug combinations based on EGFR and MAPK inhibition for solid tumours and haematologic malignancies. Drug Resist Updat. 2007, 10: 81-100. 10.1016/j.drup.2007.03.003.
Mendelson KG, Contois LR, Tevosian SG, Davis RJ, Paulson KE: Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc Natl Acad Sci USA. 1996, 93: 12908-13. 10.1073/pnas.93.23.12908.
Niisato N, Post M, van Driessche W, Marunaka Y: Cell swelling activates stress-activated protein kinases, p38MAP kinase and JNK, in renal epithelial A6 cells. Biochem Biophys Res Commun. 1999, 266: 547-50. 10.1006/bbrc.1999.1843.
Ronit H, Liola L, Hongein Li, Junying Yuan, Reuven S: Need for caspases in apoptosis of trophic factor-deprived PC12 cells. J Neuronsci Res. 1997, 50: 69-80. 10.1002/(SICI)1097-4547(19971001)50:1<69::AID-JNR8>3.0.CO;2-J.
Park EK, Kwon KB, Park KI: Role of Ca2+ in diallyl diaulfide-induced apoptotic cell death of HCT-15 cells. Exp Mol Med. 2002, 34: 250-257.
Hong YS, Ham YA, Choi JH: Effects of diallyl disulfur compounds and garlic extract on the expression of Bcl-2, Bax, and p53 in non-small lung cancer cell lines. Exp Mol Med. 2000, 32: 127-134.
Nakagawa H, Tsuta K, Kiuchui K: Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis. 2001, 22: 891-897. 10.1093/carcin/22.6.891.
Nagathihalli SN, Kandangath RA, Om VS: Diallyl disulfide causes caspase-dependent apoptosis in human cancer cells through a Bax-triggered mitochondrial pathway. J Nutritional Biochem. 2010, 21: 405-412. 10.1016/j.jnutbio.2009.01.015.
Kwon KB, Yoo SJ, Ryu DG, Yang JY, Rho HW, Kim JS: Induction of apoptosis by diallyl disulfide through activation of caspase-3 in human leukemia HL-60 cells. Biochem Pharmacol. 2002, 63: 41-47. 10.1016/S0006-2952(01)00860-7.
Gayathri R, Gunadharini DN, Arunkumar A: Effects of diallyl disulfide (DADS) on expression of apoptosis associated proteins in androgen independent human prostate cancer cells (PC-3). Molecular and Cellular Biochemistry. 2009, 320: 197-203. 10.1007/s11010-008-9903-5.
Wen J, Zhang YW, Xu M: Enhancement of diallyl disulfide-induced apoptosis by inhibitors of MAPKs in human HepG2 hepatoma cells. Biochem Pharmacol. 2004, 68: 323-331. 10.1016/j.bcp.2004.03.027.
Lu HF, Sue CC, Yu CS: Diallyl disulfide(DADS) induced apoptosis undergo capase-3 activity in human bladder cancer T24 cells. Food Chem Toxicol. 2004, 42: 1543-1552. 10.1016/j.fct.2003.06.001.
Marks N, Berg MJ: Recent advances on neuronal caspases in development and neurodegeneration. Neuroehem Int. 1999, 35: 195-220. 10.1016/S0197-0186(99)00061-3.
Henkels KM, Turchi JJ: Cisplatin-induced apoptosis proceeds by caspases-3 dependent and independent path ways in cisplatin resistant and sensitive human ovarian cancer cell lines. Cancer Res. 1999, 59: 3077-3083.
Chen YC, Shen SC: Emodin induces apoptosis in human promyeloeukemic HL-60 cells accompained by activation of caspase-3 cascade but independent of reactive oxygen species production. Biochem Pharmacol. 2002, 64: l7l3-1724.
Holmanova J, Vaeulova A, Kozubik A: Polvunsaturated fattyacids sensitize human colon adenocarcinoma HT-29 cells to death receptor-mediatedapoptosis. Cancer Lett. 2005, 218: 33-41. 10.1016/j.canlet.2004.07.038.
Kwon KB, Yoo SJ, Ryu DG: Induction ofapoptosis by dia11yl disulfide through activation of Caspase-3 in human leukemia HL-60 cells. Biochem Pharmaeol. 2002, 63: 41-47. 10.1016/S0006-2952(01)00860-7.
Zhu XF, Liu ZC, Xie BF: Involvement of caspase-3 activation in squamocin-induced apoptosis in leukemia cell line HL-60. Life Sci. 2002, 70: l259-1269. 10.1016/S0024-3205(01)01501-6.
Wen Jun, Wang Chun Xiao: Mitogen-activated protein kinase inhibitors induce apoptosis and enhance the diallyl disulfide-induced apoptotic effect in human CNE2 cells. Journal of Health Science. 2008, 54: 129-136. 10.1248/jhs.54.129.
Xiao Dong, Choi Sunga: Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation ofBcl-2. Oncogene. 2004, 23: 5594-5606. 10.1038/sj.onc.1207747.
Fan Yumei, Chen Hui: Opposing effects of ERK and P38 MAP kinases on Hela cell apoptosis induced by dipyrithione. Molecules and Cells. 2007, 23: 30-38.
Wu June, Hong Li-Chun: Mitogen-activated protein kinase(MAPK) signalling pathways in HepG2 cells infected with a virulent strain of klebsiella pneumoniae. Cellular Microbiology. 2006, 8: 1467-1474. 10.1111/j.1462-5822.2006.00725.x.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
FR, MX and CJ designed the experiments. CJ carried out most of experiments and drafted the manuscript. HM carried out partial experiments. All authors read and approved the final manuscript.
Chunxiao Ji contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ji, C., Ren, F., Ma, H. et al. The roles of p38MAPK and caspase-3 in DADS-induced apoptosis in human HepG2 cells. J Exp Clin Cancer Res 29, 50 (2010). https://doi.org/10.1186/1756-9966-29-50
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-9966-29-50