Abstract
Background
bFGF is an important growth factor for glioma cell proliferation and invasion, while connexin 43 is implicated in the suppression of glioma growth. Correspondingly, gliomas have been shown to have reduced, or compromised, connexin 43 expression.
Methods
In this study, a bFGF-targeted siRNA was delivered to the glioma cell line, U251, using adenovirus (Ad-bFGF-siRNA) and the expression of connexin 43 and its phosphorylation state were evaluated. U251 cells were infected with Ad-bFGF-siRNA (100, 50, or 25 MOI), and infection with adenovirus expressing green fluorescent protein (Ad-GFP) at 100 MOI served as a control. Western blotting and immunofluorescence were used to detect the expression levels, phosphorylation, and localization of connexin 43 in U251 cells infected, and not infected, with Ad-bFGF-siRNA.
Results
Significantly higher levels of connexin 43 were detected in U251 cells infected with Ad-bFGF-siRNA at 100 and 50 MOI than in cells infected with Ad-GFP, and the same amount of connexin 43 was detected in Ad-GFP-infected and uninfected U251 cells. Connexin 43 phosphorylation did not differ between Ad-bFGF-siRNA-infected and uninfected U251 cells. However, the ratio of phosphorylated to unphosphorylated connexin 43 in Ad-bFGF-siRNA cells was lower, and connexin 43 was predominantly localized to the cytoplasm. Using a scrape loading dye transfer assay, more Lucifer Yellow was transferred to neighboring cells in the Ad-bFGF-siRNA treated group than in the control group.
Conclusion
To our knowledge, this is the first description of a role for connexin 43 in the inhibition of U251 growth using Ad-bFGF-siRNA.
Similar content being viewed by others
Background
Basic fibroblast growth factor (bFGF) is a heparin-binding growth factor that is secreted as a pleiotropic protein and can act on various cell types, including tumor cells. bFGF is hypothesized to have a critical role in the development of the nervous system [1], and for gliomas, the level of bFGF present has been shown to correlate with tumor grade and clinical outcome [2], bFGF has also been shown to be up-regulated in transformed glial cells and to be overexpressed in malignant gliomas [3]. bFGF exerts its cellular functions through the binding of four FGF receptors (FGFRs), all of which are receptor tyrosine kinases (RTKs). The binding of bFGF by FGFRs recruits and activates several signaling pathways [4]. Accordingly, down-regulation of bFGF using antibodies or antisense sequences has been shown to inhibit tumor cell tumorigenicity and metastasis [3, 5, 6]. A study by De Vuyst et al. also demonstrated a role for bFGF in the inhibition of gap junction (GJ) communication in the glioma cell line, C6, following exogenous expression of connexin 43 [7].
Connexin 43 (Cx43) is the predominant component of GJs which are composed of six connexin proteins and are differentially expressed in various cell types [8]. Several studies have demonstrated that Cx43 is one of the major GJ proteins expressed by astrocytes and glial cells [9], and in high-grade human gliomas, its expression is significantly reduced. Decreased expression of Cx43 observed in a variety of tumor types, including tumors of the central nervous system, can also affect GJ intercellular communication (GJIC) [10, 11]. Restoration of GJIC by exogenous expression of Cx43 has reversed the transformed phenotype of certain tumor cells, including high-grade human gliomas [12, 13]. In addition, susceptibility of the transfected glioma cells to apoptosis was enhanced in response to chemotherapeutic agents [14]. While it has been found that expression of Cx43 is inversely related to glioma cell proliferation and tumor grade [12, 15, 16], the specific regulatory mechanisms involving Cx43 in gliomas remains unclear.
In the present study, down-regulation of bFGF expression by a siRNA specifically targeted to bFGF is shown to significantly increase the expression of Cx43 without effecting the phosphorylation of Cx43 at S368 in the glioma cell line, U251.
Methods
Adenoviral vector construction
From four siRNA sequences that were designed for targeting bFGF, an optimal target sequence (5'-CGAACTGGGCAGTATAAACTT-3') was selected [17] and cloned into the plasmid vector, pGenesil-1. The siRNA expression cassette was subsequently excised from pGenesil-1 using EcoR I and HindIII and ligated into the linearized adenoviral shuttle vector, pGStrack-CMV. pGStrack-CMV-bFGF-siRNA was then co-transfected with the pAd vector backbone into DH5α bacteria for the recombinant generation of Ad-bFGF-siRNA, which was further amplified in HEK293 cells. Viral particles were purified using cesium chloride density gradient centrifugation.
Cell culture and adenovirus infection
The human glioma cell line, U251, was maintained in Dulbcco's modified Eagle medium (DMEM) supplemented with 10% heat inactivated fetal bovine serum (FBS), 100 U/ml of penicillin, and 100 μg/ml of streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. All media and serum were purchased from Gibcol.
U251 cells (1 × 105) in serum-free DMEM were infected with Ad-bFGF-siRNA at 100, 50, and 25 MOI (MOI is calculate as PFU/cell numbers) in a humidified atmosphere containing 5% CO2 at 37°C. Infection with Ad-GFP at 100 MOI served as a control. Virus-containing medium was removed 8 h later and replaced with fresh DMEM medium containing 10% FBS. Cells were incubated for another 72 h, then mRNA or protein was extracted.
MTT assay for cell proliferation
Cell proliferation was measured using MTT assay. 5 × 103 cells/well were seeded into 96 wells plate. After the adhesion of the cells, they were infected with Ad-bFGF-siRNA, meanwhile untreated cells and cells infected with Ad-GFP served as control and mock control. During consecutive seven days, 20 μl MTT solution (5 mg/ml) in PBS was added to each well for 4 h. After the culture medium was drained out, 150 μl of DMSO was added into each well. Absorbance of each well was measured on a microplate reader. Three duplicate wells were set up for each group.
RT-PCR
Total RNA was extracted from cultured cells using TRizol reagents (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's directions. First-strand cDNA was synthesized from total RNA(1 μg) using AMV reverse transcriptase (TaKaRa) with oligo(dT) primer at 42°C for 1 h in a 25 μl volume. RT product (2 μl) with cDNAs was mixed with bFGF or β-actin specific primers in a PCR buffer containing 2.5 mM dNTP, 2.5 mM MgCl2 and 1 U Taq polymerase (TaKaRa). PCR amplification was performed over 31 cycles (45 sec at 94°C, 60 sec at 60°C, and 45 sec at 72°C) to amplify bFGF, and over 25 cycles (30 sec at 94°C, 30 sec at 57°C, and 90 sec at 72°C) to amplify β-actin. Primers used for amplifying bFGF included: forward-5'-CACCATGGCAGCCGGCAGCATCA-3' and reverse-5'-TCAGCTCTTAGCAGACATTGG-3'. Primers used to amplify β-actin included: forward-5'-CCTCGCCTTTGCCGATC-3' and reverse-5'-GGATCTTCATGAGGTAGTCAGTC-3'. Amplified DNA fragments were separated in 2% agarose gels and visualized using ethidium bromide staining.
Western blotting
Western blot analysis was performed on whole cell extracts obtained by direct dissolution of cells in culture flasks using a whole cell protein extract reagent according to the manufacturer's directions (PIERCE). Protein concentrations were determined using a bicinchoninic acid (BCA) protein assay kit with bovine serum albumin as a standard. Proteins (40 μg/lane) were separated on 12% SDS-PAGE gels and transferred onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 3% fat-free milk in PBST (0.2% Tween-20 in PBS, pH 7.6) then incubated with primary antibody for 18-24 h at 4°C. Membranes were subsequently incubated with secondary antibodies conjugated to horseradish peroxidase (1:5000) for 1 h at RT. Bound antibody was visualized using an Enhanced Chemiluminescence (ECL) western blot detection kit (Amersham Pharmacia Biotech). Primary antibodies used included: anti-bFGF (rabbit polyclonal, 1:1000, Santa Cruz), anti-Cx43 (rabbit polyclonal, 1:1000, Cell Signaling), anti-pCx43 for S368 (rabbit polyclonal, 1:1000, Cell Signaling), and anti-β-actin (mouse monoclonal, 1:1000, Santa Cruz).
Immunofluorescence
U251 cells grown on cover slips were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.5% Triton X-100/PBS (Sigma-Aldrich) for 20 min. Cells were then washed twice with PBS and blocked in 3% bovine serum albumin (BSA) for 30 min prior to incubating the cells with primary antibodies recognizing Cx43 or p-Cx43 (S368) for 1 h in a humidified chamber. After several PBS washes, cells were incubated with tetraethyl rhodamine isothiocyanate(TRITC)-conjugated secondary antibodies for 1 h. After washing with PBS, cells were stained with Hoechst 33258 (Sigma-Aldrich) for 15 min and immunofluorescence was detected using a fluorescence microscope (Olympus).
Scrape loading and dye transfer (SL/DT)
Levels of GJIC in control and treated U251 cells were determined using the scrape loading and dye transfer (SL/DT) technique with the fluorescent dye, Lucifer Yellow (LY), as a readout (Sigma). Briefly, U251 cells were seeded in 6-well plates and grown to confluency. After rinsing with PBS, cells were incubated with 0.05% (w/v) Lucifer Yellow in PBS. Scrape loading was performed using a surgical scalpel to draw several clear straight lines on the cell monolayer. After 5 min, the Lucifer Yellow solution was removed, cells were washed 4 times with PBS, and transfer of Lucifer Yellow was detected using an inverted fluorescence microscope.
Statistical Analysis
All data were analyzed using SPSS 13.0 software. Significant differences were determined using either one-way analysis of variance (ANOVA) or a two-tailed Student t-test. A p-value < 0.05 was considered significant.
Results
Down-regulation of bFGF mRNA and protein in U251 cells using bFGF-targeted siRNA
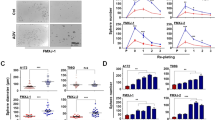
To examine changes in bFGF gene expression induced by adenoviral infection of bFGF-targeted siRNA, RT-PCR and western blot were performed. Both mRNA and protein levels of bFGF in Ad-bFGF-siRNA-infected U251 cells were dramatically reduced compared to bFGF levels in U251 infected with Ad-GFP or uninfected U251 (Fig. 1A, B). These results indicate that bFGF siRNA delivered by adenoviral infection can specifically suppress the expression of bFGF in U251 cells.Meanwhile, U251 cells, which were inhibited expression of bFGF using Ad-bFGF-siRNA, showed decrease of proliferation and survival rate compared to untread U251 cells and Ad-GFP treatment detected by MTT assay(Fig. 2A, B).
Infection with Ad-bFGF-siRNA decreased the expression of bFGF mRNA and protein in U251 cells in a dose-dependent manner. The level of bFGF mRNA (A) and protein (B) in control, Ad-GFP, and Ad-bFGF-siRNA-infected U251 cells as measured by RT-PCR and western blot. The upper panels include representative RT-PCR and western blot results, while the lower panels provide the relative band density ratios for bFGF mRNA and protein relative to β-actin (mean ± SD, n = 3) (*p < 0.05 vs. control).
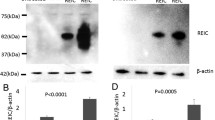
The effect of bFGF-targeted siRNA on connexin 43 and phosphorylation of connexin 43 at PKC target site S368 in U251 cells
Expression of Cx43 and phosphorylation of Cx43 at PKC target site S368 was detected in both bFGF-inhibited and uninhibited U251 cells using western blotting. In addition, the subcellular distribution of them in U251 cells was examined using indirect immunofluorescence. Western blotting revealed that inhibition of bFGF correlated with significantly higher levels of an immunoreactive 43 kDa band detected by a polyclonal Cx43 antibody relative to untreated U251 cells (Fig. 3A, B). While, down-regulation of bFGF did not affect phosphorylation of Cx43 at S368(Fig. 3A, C). Immunofluorescence studies identified Cx43 and p-Cx43 to be predominantly localized to the cytoplasm (Fig. 4A, B).
Ad-bFGF-siRNA in U251 cells increases connexin 43 protein levels and no affect the level of p-connexin 43 at S368 site. A) Expression of connexin 43 and p-connexin 43 at S368 site U251 cells infected with Ad-bFGF-siRNA and untreated U251 cells. A representative western blot is shown. B) Relative density values of Cx43 compared to β-actin from western blot analysis are provided. C) Relative density values of p-Cx43 compared to Cx43 from western blot analysis are provided. (mean ± SD, n = 3) (*p < 0.05 vs. control).
Subcellular localization of Cx43 and p-Cx43 (S368) in Ad-bFGF-siRNA infected U251 cells. A) Subcellular localization of Cx43 in U251 cells stained with anti-Cx43 antibody and with Hoechst 33258 staining to identify nuclei. B) Subcellular localization of p-Cx43(S368) in U251 cells stained with an anti-p-Cx43 antibody and Hoechst 33258 staining to identify nuclei.
Infection with Ad-bFGF-siRNA improves intercellular communication
Scrape loading and dye transfer (SL/DT) assays were used to evaluate the permeability of GJs in U251 cells infected with Ad-bFGF-siRNA. Detection of the fluorescent dye, Lucifer Yellow (LY), showed a higher number of Ad-bFGF-siRNA-infected cells exhibited fluorescence than untreated U251 cells (Fig. 5). These results indicate that down-regulation of bFGF increased the GJIC between U251 cells.
Ad-bFGF-siRNA improves GJIC between U251 cells. GJIC was assessed in U251 cells infected with Ad-bFGF-siRNA (100 MOI) for 48 h compared to untreated U251 cells using scrape loading dye transfer assays. A) In untreated cells, Lucifer Yellow was restricted to the cells at the border of the scraped line with only minimal transfer of Lucifer Yellow to neighboring cells. B) In Ad-bFGF-siRNA U251 cells, an increase in the transfer of Lucifer Yellow between cells was detected.
Discussion
The autocrine and paracrine signaling of bFGF makes it one of the most potent mitogenic factors for glial cell growth and differentiation. High levels of bFGF expression have also been associated with malignant grades of glioma, and in neoplastic astrocytes, bFGF stimulates the proliferation of astrocytoma cells. Conversely, inhibition of bFGF expression, or receptor binding of bFGF, has been demonstrated to inhibit glioma proliferation both in vitro and in vivo[18]. In the present study, infection with Ad-bFGF-siRNA down-regulated expression of bFGF in U251 cells, inhibited cell proliferation, and increased expression of Cx43. Huang have reported that Cx43 may suppress glioma proliferation by dowregulation of monocyte chemotactic protein 1(MCP-1)[19], the inhibitory effect of bFGF siRNA on U251 cell proliferation is at least partially due to the increased expression of Cx43, which may affect expression of other growth factors, such as down regulating MCP-1. However the correlation between downregulation of bFGF and inducion of Cx43 is still unclear, Ueki'study may provided some implicant, Ueki demonstrated in cortical astrocytes that epidermal growth factor (EGF) results in a decrease in the expression of Cx43 mRNA and protein and the decrease is associated with the receptor tyrosine kinase pathway, meanwhile the MEK inhibitor prevents EGF-stimulated down-regulation of Cx43 expression[20].
Immunofluorecence studies further demonstrated that increased expression of Cx43 localized primarily to the cytoplasm, with fewer molecules localizing to the perinucleus and sporadic plaques detected at the plasma membrane. In addition, dye transfer assays demonstrated that intercellular communication was improved for U251 cells infected with Ad-bFGF-siRNA. Consistent with data from other studies [21, 22], it was observed that although localization of Cx43 was predominant at cytoplasm, the functions of GJIC mediated by Cx43 were normal.
Lack of Cx43 expression and aberrant localization of Cx43 have been associated with a lack of GJIC between tumor cells [23]. While gene mutations may play a role in deficient Cx43 expression, the precise mechanisms involved in decreased expression of Cx43 in tumor cells is still unclear. An increasing number of studies have shown that Cx43 can abnormally localize and accumulate in the cytoplasm in some cancer cell lines, including glioma cell lines. However, nuclear localization of connexin 43 has been reported in both src and neu oncogene-transformed rat liver epithelial cells [23]. Aberrant localization of Cx43 may also be associated with intact function of cytoskeletal elements [24].
Several studies have reported a role for Cx43 in both physiological and pathological conditions, although with contrasting results [25–27]. There are two mechanisms that have been postulated to explain the observed discrepancies. For example, Cx43 may directly mediate intercellular communication to permit the transport of factors that inhibit or enhance cell growth, or alternatively, Cx43 may affect GJs directly [28, 29]. Based on studies in a rat glioma cell line, regulation of glioma growth is proposed to be more dependent on the behavior of connexins than the activity of GJIC [30]. Therefore, it is possible that Cx43 may effect tumor growth independently of GJ formation. Despite these insights, further studies are necessary to define the precise role of Cx43 in glioma cell communication and growth.
References
Eckenstein FP: Fibroblast growth factors in the nervous system. J Neurobiol. 1994, 25: 1467-1480. 10.1002/neu.480251112.
Fukui S, Nawashiro H, Otani N, Ooigawa H, Nomura N, Yano A, Miyazawa T, Ohnuki A, Tsuzuki N, Katoh H, Ishihara S, Shima K: Nuclear accumulation of basic fibroblast growth factor in human astrocytic tumors. Cancer. 2003, 97: 3061-3067. 10.1002/cncr.11450.
Baguma-Nibasheka M, Li AW, Murphy PR: The fibroblast growth factor-2 antisense gene inhibits nuclear accumulation of FGF-2 and delays cell cycle progression in C6 glioma cells. Mol Cell Endocrinol. 2007, 267: 127-136. 10.1016/j.mce.2007.01.008.
Bikfalvi A, Klein S, Pintucci G, Rifkin DB: Biological roles of fibroblast growth factor-2. Endocr Rev. 1997, 18: 26-45. 10.1210/er.18.1.26.
Takahashi JA, Fukumoto M, Kozai Y, Ito N, Oda Y, Kikuchi H, Hatanaka M: Inhibition of cell growth and tumorigenesis of human glioblastoma cells by a neutralizing antibody against human basic fibroblast growth factor. FEBS Lett. 1991, 288: 65-71. 10.1016/0014-5793(91)81004-R.
Aoki T, Kato S, Fox JC, Okamoto K, Sakata K, Morimatsu M, Shigemori M: Inhibition of autocrine fibroblast growth factor signaling by the adenovirus-mediated expression of an antisense transgene or a dominant negative receptor in human glioma cells in vitro. Int J Oncol. 2002, 21: 629-636.
De Vuyst E, Decrock E, De Bock M, Yamasaki H, Naus CC, Evans WH, Leybaert L: Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell. 2007, 18: 34-46. 10.1091/mbc.E06-03-0182.
Laird DW: Life cycle of connexins in health and disease. Biochem J. 2006, 394: 527-543. 10.1042/BJ20051922.
Giaume C, Fromaget C, el Aoumari A, Cordier J, Glowinski J, Gros D: Gap junctions in cultured astrocytes: single-channel currents and characterization of channel-forming protein. Neuron. 1991, 6: 133-143. 10.1016/0896-6273(91)90128-M.
Kardami E, Dang X, Iacobas DA, Nickel BE, Jeyaraman M, Srisakuldee W, Makazan J, Tanguy S, Spray DC: The role of connexins in controlling cell growth and gene expression. Prog Biophys Mol Biol. 2007, 94: 245-264. 10.1016/j.pbiomolbio.2007.03.009.
Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G: Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002, 383: 725-737. 10.1515/BC.2002.076.
Soroceanu L, Manning TJ, Sontheimer H: Reduced expression of connexin-43 and functional gap junction coupling in human gliomas. Glia. 2001, 33: 107-117. 10.1002/1098-1136(200102)33:2<107::AID-GLIA1010>3.0.CO;2-4.
Huang RP, Fan Y, Hossain MZ, Peng A, Zeng ZL, Boynton AL: Reversion of the neoplastic phenotype of human glioblastoma cells by connexin 43 (cx43). Cancer Res. 1998, 58: 5089-5096.
Huang RP, Hossain MZ, Huang R, Gano J, Fan Y, Boynton AL: Connexin 43 (cx43) enhances chemotherapy-induced apoptosis in human glioblastoma cells. Int J Cancer. 2001, 92: 130-138. 10.1002/1097-0215(200102)9999:9999<::AID-IJC1165>3.0.CO;2-G.
Huang RP, Hossain MZ, Sehgal A, Boynton AL: Reduced connexin43 expression in high-grade human brain glioma cells. J Surg Oncol. 1999, 70: 21-24. 10.1002/(SICI)1096-9098(199901)70:1<21::AID-JSO4>3.0.CO;2-0.
Pu P, Xia Z, Yu S, Huang Q: Altered expression of Cx43 in astrocytic tumors. Clin Neurol Neurosurg. 2004, 107: 49-54. 10.1016/j.clineuro.2004.03.006.
Wang SJ, Wang JH, Zhang YW, Xu XN, Liu HS: [Effects of small interfering RNA targeting basic fibroblast growth factor on proliferation and apoptosis of glioma cell line U251]. Ai Zheng. 2008, 27: 905-909.
Auguste P, Gursel DB, Lemiere S, Reimers D, Cuevas P, Carceller F, Di Santo JP, Bikfalvi A: Inhibition of fibroblast growth factor/fibroblast growth factor receptor activity in glioma cells impedes tumor growth by both angiogenesis-dependent and -independent mechanisms. Cancer Res. 2001, 61: 1717-1726.
Huang R, Lin Y, Wang CC, Gano J, Lin B, Shi Q, Boynton A, Burke J, Huang RP: Connexin 43 suppresses human glioblastoma cell growth by down-regulation of monocyte chemotactic protein 1, as discovered using protein array technology. Cancer Res. 2002, 62: 2806-2812.
Ueki T, Fujita M, Sato K, Asai K, Yamada K, Kato T: Epidermal growth factor down-regulates connexin-43 expression in cultured rat cortical astrocytes. Neurosci Lett. 2001, 313: 53-56. 10.1016/S0304-3940(01)02249-2.
Cottin S, Ghani K, Caruso M: Bystander effect in glioblastoma cells with a predominant cytoplasmic localization of connexin43. Cancer Gene Ther. 2008, 15: 823-831. 10.1038/cgt.2008.49.
Sanson M, Marcaud V, Robin E, Valery C, Sturtz F, Zalc B: Connexin 43-mediated bystander effect in two rat glioma cell models. Cancer Gene Ther. 2002, 9: 149-155. 10.1038/sj.cgt.7700411.
Mesnil M, Crespin S, Avanzo JL, Zaidan-Dagli ML: Defective gap junctional intercellular communication in the carcinogenic process. Biochim Biophys Acta. 2005, 1719: 125-145. 10.1016/j.bbamem.2005.11.004.
Thomas T, Jordan K, Laird DW: Role of cytoskeletal elements in the recruitment of Cx43-GFP and Cx26-YFP into gap junctions. Cell Commun Adhes. 2001, 8: 231-236. 10.3109/15419060109080729.
Shao Q, Wang H, McLachlan E, Veitch GI, Laird DW: Down-regulation of Cx43 by retroviral delivery of small interfering RNA promotes an aggressive breast cancer cell phenotype. Cancer Res. 2005, 65: 2705-2711. 10.1158/0008-5472.CAN-04-2367.
Xu X, Francis R, Wei CJ, Linask KL, Lo CW: Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development. 2006, 133: 3629-3639. 10.1242/dev.02543.
Bates DC, Sin WC, Aftab Q, Naus CC: Connexin43 enhances glioma invasion by a mechanism involving the carboxy terminus. Glia. 2007, 55: 1554-1564. 10.1002/glia.20569.
Goodenough DA, Paul DL: Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003, 4: 285-294. 10.1038/nrm1072.
Lin JH, Yang J, Liu S, Takano T, Wang X, Gao Q, Willecke K, Nedergaard M: Connexin mediates gap junction-independent resistance to cellular injury. J Neurosci. 2003, 23: 430-441.
Mennecier G, Derangeon M, Coronas V, Herve JC, Mesnil M: Aberrant expression and localization of connexin43 and connexin30 in a rat glioma cell line. Mol Carcinog. 2008, 47: 391-401. 10.1002/mc.20393.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30672158).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
BZ participated in study design, performed experiments and and drafted the manuscript. XF carried out experiments. JW participated in study design and revised manuscript. XX participated in study design and helped to draft the manuscript. HLcarried out statistical analyses NL performed experiments and helped to draft the manuscript. All authors approved the final manuscript.
Biao Zhang, Xuequan Feng contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zhang, B., Feng, X., Wang, J. et al. Adenovirus-mediated delivery of bFGF small interfering RNA increases levels of connexin 43 in the glioma cell line, U251. J Exp Clin Cancer Res 29, 3 (2010). https://doi.org/10.1186/1756-9966-29-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-9966-29-3