Abstract
Background
To determine the efficacy and safety of heparin (unfractionated heparin (UFH) or low-molecular-weight-heparin (LMWH)) and fondaparinux in improving the survival of patients with cancer.
Methods
We conducted in January 2007 a comprehensive search for relevant randomized clinical trials (RCTs). We used a standardized form to extract in duplicate data on methodological quality, participants, interventions and outcomes of interest including all cause mortality, thromboembolic events, and bleeding events. We assessed the methodological quality for each outcome by grading the quality of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology
Results
Of 3986 identified citations, we included 5 RCTs, none of which evaluated fondaparinux. The quality of evidence was moderate for survival, low for major and minor bleeding, and very low for DVT. Heparin therapy was associated with a statistically and clinically significant survival benefit (hazard ratio (HR) = 0.77; 95%CI = 0.65–0.91). In subgroup analyses, patients with limited small cell lung cancer experienced a clear survival benefit (HR = 0.56; 95%CI = 0.38–0.83). The survival benefit was not statistically significant for either patients with extensive small cell lung cancer (HR = 0.80; 95%CI = 0.60–1.06) or patients with advanced cancer (HR = 0.84; 95%CI = 0.68–1.03). The increased risk of bleeding with heparin was not statistically significant (relative risk (RR) = 1.78; 95%CI = 0.73–4.38).
Conclusion
This review suggests a survival benefit of heparin in cancer patients in general, and in patients with limited small cell lung cancer in particular.
Similar content being viewed by others
Background
Researchers have hypothesized that heparin improves outcomes in cancer patients through an antitumor effect in addition to its antithrombotic effect [1]. In a 1992 trial comparing nadroparin, a low-molecular-weight-heparin (LMWH), to unfractionated heparin (UFH) in patients with deep vein thrombosis (DVT), nadroparin unexpectedly reduced mortality in the subgroup of cancer patients [2]. At the same time, the risk of bleeding with anticoagulants is higher in patients with cancer compared to those without cancer [3]. Heparins are also known to cause thrombocytopenia [4].
A 1999 systematic review of the effects of UFH on survival in patients with malignancy found three trials of high methodological quality but with conflicting results. [5] Since then reports on several randomized controlled trials (RCTs) on this subject have been published [6, 7], including at least one study in patients with small cell lung cancer [8]. The purpose of this study was to determine the efficacy and safety of parenteral anticoagulation in improving survival of patients with cancer in general and lung cancer in particular.
Methods
Data Sources and Searches
The search was part of a comprehensive search for studies of anticoagulation in patients with cancer. We electronically searched in January 2007 the following databases from the date of their inception: The Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE and ISI the Web of Science (see Additional file 1). We also hand searched the conference proceedings of the American Society of Clinical Oncology and of the American Society of Hematology. We reviewed the reference lists of included papers and used the related article feature in PubMed. We applied no language restrictions.
Study Selection
Two reviewers independently screened the titles and abstracts for eligibility. We retrieved the full texts of articles judged as potentially eligible by at least one reviewer. Two reviewers then independently screened the full texts articles for eligibility and resolved their disagreements by discussion. We included abstracts only if authors supplied us with the necessary information about their methods and results.
We included only RCTs. Study participants had to have cancer but no indication for prophylactic or therapeutic anticoagulation. Interventions included one of the three classes of parenteral anticoagulants approved for clinical use: UFH, LMWH, and/or fondaparinux. The review outcomes were: survival (primary outcome), symptomatic DVT, symptomatic pulmonary embolism, major bleeding, minor bleeding, and thrombocytopenia. DVT and PE events had to be diagnosed using objective diagnostic tests.
Data Extraction and Quality Assessment
Two reviewers independently extracted data using a standardized form and resolved their disagreements by discussion. We contacted authors for incompletely reported data.
We extracted time to event data by abstracting the log(hazard ratio) and its variance from trial reports; if these were not reported, we digitised the published Kaplan-Meier survival curves and estimated the log(hazard ratio) and its variance using Parmar's methods [9]. We performed these calculations in Stata 9, using a specially written program, which yielded the reported log(HR) and variance when used on the data presented in Table V of Parmar 1998 [9].
We also extracted categorical data necessary to conduct intention-to-treat (ITT) analyses. We collected all cause mortality at one year (time point defined a priori) and at 2 years (time point defined post hoc based upon results reported in the individual RCTs).
We assessed the following methodological criteria: allocation concealment, blinding (patient, provider, outcome assessor, data analyst), whether the analysis followed the ITT principle, whether study was stopped early for benefit, and percentage of follow-up. We assessed the methodological quality for each outcome by grading the quality of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology [10].
Analysis
We calculated the agreement between the two reviewers for eligibility assessment using kappa statistic. We created an inverted funnel plot for the primary outcome to check for possible publication bias.
For time to event data, we pooled the log(HR)s using a random-effects model and the generic inverse variance facility of RevMan 4.2. For categorical data, we calculated the relative risk (RR) separately for each study for the incidence of outcomes by treatment arm. We then pooled the results of the different studies using a random-effects model.
We measured homogeneity across studies using the I2 statistics [11] and considered the following classification of heterogeneity based on the value of I2 (Higgins, personal communication): 0–50 = low; 30–80 = moderate and worthy of investigation; 60–100 = severe and worthy of understanding; 95–100 = aggregate with major caution. We planned to explore heterogeneity by conducting subgroup analyses based on the type of intervention and the characteristics of participants. We also planned for sensitivity analysis excluding poor quality trials.
Results
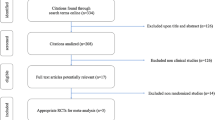
Figure 1 shows the trial flow. The search strategy identified 3986 citations, including 322 duplicates. The title and abstract screening of the 3664 unique citations identified 51 as potentially eligible. The full text screening of the 51 citations identified 5 eligible RCTs published as full reports [6, 7, 12–14]. We identified 4 earlier published abstracts for 3 of the 5 included RCTs [12, 15–17]. We also identified six eligible studies published as abstracts but we were unable to obtain the needed data from the authors [18–23]. Agreement between reviewers for eligibility was excellent (kappa = 0.94).
Included studies
The five included studies recruited 1189 participants and reported follow-up data on 1175 [6–8, 13, 14]. The intervention was UFH in one study [13] and LMWH in four [6–8, 14] and fondaparinux in none. Table 1 details the characteristics of these studies.
Methodological quality of included studies
Allocation was adequately concealed in four studies [6, 7, 13, 14] and it was unclear whether it was adequately concealed in the fifth study [8]. Two studies blinded participants, caregivers, and outcome assessors [7, 14], one study blinded patients and caregivers [6], one study blinded outcome assessors and data analysts [13], and one study blinded only outcome assessors [8]. The lowest percentage of follow up in the five studies was 97%. Only one study did not use ITT analysis [14]. One study was stopped early for insufficient accrual [14]. According to GRADE methodology, the quality of evidence was moderate for survival, low for major and minor bleeding, and very low for DVT (Table 2).
Quantitative results
There was low to moderate heterogeneity (I2 = 47.5%) for the survival outcome. The small number of trials permitted subgroup analyses only for the subgroups of patients with small cell lung cancer (SCLC) and with "advanced cancer" (as defined in individual studies). The inverted funnel plot for the primary outcome of mortality at 1 year did not suggest publication bias (Figure 2).
All cause mortality
Based on a pooled estimate from the 5 RCTs, heparin was associated with a statistically significant survival benefit (HR = 0.77; 95%CI = 0.65 – 0.91; I2 = 47%) (Figure 3). Excluding the study by Lebeau et al. [13] (the only study using UFH) then the study by Altinbas et al. [8] (in which the allocation was not clearly concealed) yielded estimates similar to the primary meta-analysis.
The categorical analysis confirmed those results with a statistically significant reduction of mortality at 12 months (RR = 0.87; 95%CI = 0.80–0.95) and at 24 months (RR = 0.92; 95%CI = 0.86 – 0.99).
Small cell lung cancer
In patients with limited SCLC, heparin was associated with a statistically significant survival benefit (HR = 0.56; 95%CI = 0.38 – 0.83), with no heterogeneity between trials (I2 = 0) (Figure 4). In the categorical analysis, heparin was associated with a statistically significant reduction of mortality at 12 months (RR = 0.60; 95%CI = 0.42–0.87) but not at 24 months (RR = 0.90; 95%CI = 0.71–1.14). Excluding the study by Altinbas et al. did not change the results in terms of statistical significance.
For extensive SCLC, heparin was associated with a non-statistically significant survival benefit (HR = 0.80; 95%CI = 0.60–1.06; I2 = 0) (Figure 4). The results were similarly non-statistically significant in the categorical analysis at 12 months (RR = 0.93; 95%CI = 0.76 = 1.15) and 24 months (RR = 0.88; 95%CI = 0.65–1.18).
Advanced cancer
Based on a pooled estimate from studies including patients with advanced cancer [6, 7], heparin was associated with a non-statistically significant survival benefit (HR = 0.84; 95%CI = 0.68–1.03) (Figure 3), with moderate heterogeneity between trials (I2 = 47%). The effect of heparin on mortality was borderline significant at 12 months (RR = 0.89; 95%CI = 0.80–1.00) and 24 months (RR = 0.92; 95%CI = 0.85–1.00).
Klerk et al [7] defined a priori two subgroups of patients with life expectancy less and greater than 6 months respectively. The HR for survival was 0.64 (95%CI = 0.45–0.90) for patients with longer life expectancy and 0.88 (95%CI = 0.62–1.25) for patients with shorter life expectancy)
Venous thromboembolism
Based on pooled estimates from two RCTs [6, 8], heparin therapy was associated with a non-statistically significant reduction in DVT (RR = 0.61; 95%CI = 0.08–4.91).
Major and minor bleeding
Pooled estimates showed that heparin therapy was associated with increased bleeding that was non-statistically significant for minor bleeding (RR = 2.07; 95%CI = 0.78–5.51), or major bleeding (RR = 1.50; 95%CI = 0.26–8.80) or any bleeding (RR = 1.78; 95%CI = 0.73–4.38) (Figure 5). After excluding the study by Altinbas et al. the results remained non-statistically significant.
Three studies assessed thrombocytopenia as an outcome but reported no events [7, 8, 13]. None of the studies reported participants withdrawing from treatment.
Discussion
Heparin therapy (with either UFH or LMWH) was associated with a statistically and patient important survival benefit in cancer patients who had no indication for parenteral anticoagulation. In subgroup analyses, patients with limited SCLC experienced a clear survival benefit. The survival benefit was not statistically significant for either patients with extensive SCLC or patients with advanced cancer. The increased risk of bleeding with heparin was not statistically significant. We did not identify any study using fondaparinux as the anticoagulant.
The strengths of this study include our systematic approach to searching, study selection and data extraction which has minimized the likelihood of missing relevant studies. The quality of evidence was high for survival; all included studies were RCTs with moderate percentages of follow-up and allocation was clearly adequate in all but one included study. This moderate quality of evidence for surival, and the low likelihood of publication bias increase the confidence in the internal validity of our findings. Furthermore, we conducted pooled survival analysis for the important outcomes.
There was a statistically significant reduction in mortality at 12 months but not at 24 months in patients with limited SCLC. This difference probably reflects a true difference of effect at different follow-up periods. Such a difference might be due to the relatively short overall survival of patients with SCLC enabling short term but not long term benefit. It might also be due to the initial contribution of the antithrombotic effect of LMWH. While this assumption does not contradict a concomitant antitumor effect of LMWH (see below), it acknowledges the antithrombotic role and its clinical importance in managing patients with limited SCLC.
The non-significant findings in this study may be due to the small number of RCTs, of participants and of events. For example, compared with the data at 12 months, the results at 6 months tended to be non-significant; the latter could be explained by a smaller number of events in the early follow up period. The interpretation of findings is also limited by not including data from the 7 trials published as abstracts only.
Interpretation of the findings of this review is somewhat limited by the moderate heterogeneity between the results of different trials, which was not completely explained by subgroup analyses based on type of cancer. The heterogeneity could be related to variety in the stages of cancers, and in the types, dosing, schedules and duration of heparin therapy. The relatively small number of studies and the inclusion of different types of cancer in the same study precluded us from conducting the necessary subgroup analyses to explore all of these factors.
The statistically significant survival benefit of heparin in the subgroup of patients with limited SCLC in this review and in the subgroup of patients with life expectancy greater than 6 months in the study by Klerk et al [7] suggest that less ill patients receive greater benefit from heparin. The CLOT trial [24] supports these findings indirectly; in that study, patients with solid tumors and an acute venous thromboembolic event had improved survival if they did not have a metastatic disease at the time of study entry.
Studies with shorter periods of heparin therapy (i.e. 5 and 6 weeks) [7, 13], appear to provide similar benefit as those with longer periods (i.e. 12 weeks) [6]. The clinical implication would be major if in fact, prolonging the duration of therapy does not provide additional benefit while increasing the risk of side effects (mainly bleeding events). However, none of the included studies was designed to address this question.
Lazo-Lannger et al. conducted a systematic review addressing the same question as this review [25]. Although that review had different inclusion criteria from our review (in particular, it excluded the trial of Lebeau) and obtained slightly different estimates of HRs using Parmar's methods, it reported similar results: a hazard rate comparing mortality in the heparin and control arms of 0.83 (95% CI: 0.70 to 0.99). This consistency of results from independent reviews confirms the robustness of the findings. However, Lazo-Lannger et al. did not report any subgroup analysis in patients with small cell lung cancer. While there are important pitfalls in subgroup analysis, they serve to generate hypotheses that should further be explored [26].
"The survival benefit in patients with cancer of anticoagulation is probably only in part mediated through an antithrombotic effect, i.e. through the prevention of fatal thromboembolic events. In fact, the survival curves of the included studies show consistent survival benefit beyond the duration of heparin therapy. Similarly, the meta-analysis shows a statistically significant survival benefit at 12 months while the duration of heparin therapy in 4 of the included studies was 5 weeks, 6 weeks, 18 weeks, and 12 months respectively. Experts in the field have attributed this phenomenon to an antitumor effect of anticoagulation [27, 28].
Basic research supports the hypothesis of an antitumor effect of anticoagulation. Studies have implicated the tumour-mediated activation of the haemostatic system in both the formation of tumour stroma and in tumour metastasis [29–31]. There is also evidence that heparin inhibits expression of oncogenes, the formation by cancer cells of thrombin and fibrin induced, and the intravascular arrest of cancer cells, and thus metastasis [32].
The antitumor effect does not appear to be the same across anticoagulant classes. In a systematic review of oral anticoagulation for prolonging survival in patients with cancer, warfarin improved early survival in patients with extensive SCLC but not in patients with limited SCLC [33]. In another systematic review of the initial treatment of VTE in patients with cancer, LMWH provided a survival benefit compared with UFH [34]. Finally, for long term treatment of VTE in this population, LMWH compared with oral anticoagulation reduced the incidence of venous thromboembolism although did not provide a survival benefit [35].
Conclusion
In conclusion, this systematic review suggests a survival benefit of heparin in cancer patients in general, and in patients with limited small cell lung cancer in particular. It also suggests a higher benefit in patients with limited cancer or a longer life expectancy. The decision for a patient with cancer to start heparin therapy for survival benefit should balance the benefits and downsides and integrate the patient's values and preferences [36]. Patients with a high preference for a short survival prolongation and limited aversion to bleeding who do not consider heparin therapy a burden may opt to use heparin, while those with aversion to bleeding may not.
Future research should investigate the effects anticoagulation in patients with different types and stages of cancers comparing different types, dosing and duration of therapy [37].
Abbreviations
- DVT:
-
Deep vein thrombosis
- HR:
-
Hazard ratio
- ITT:
-
Intention-to-treat
- LMWH:
-
low-molecular-weight-heparin
- RCT:
-
Randomized clinical trial
- RR:
-
Relative risk
- SCLC:
-
Small cell lung cancer
- UFH:
-
unfractionated heparin.
References
Thodiyil P, Kakkar AK: Can low-molecular-weight heparins improve outcomes in patients with cancer?. Cancer treatment Reviews. 2002, 28: 151-155. 10.1016/S0305-7372(02)00040-3
Prandoni P, Lensing AW, Buller HR, Carta M, Cogo A, Vigo M, Casara D, Ruol A, ten Cate JW: Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deep-vein thrombosis. Lancet. 1992, 339: 441-445. 10.1016/0140-6736(92)91054-C
Prandoni P, Lensing AWA, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, Girolami A: Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002, 100 (10): 3484-3488. 0.1182/blood-2002-01-0108
Girolami B, Girolami A: Heparin-induced thrombocytopenia: a review. Seminars in Thrombosis & Hemostasis. 2006, 32 (8): 803-809. 10.1055/s-2006-955463.
Smorenburg SM, Hettiarachchi RJ, Vink R, Buller HR: The effects of unfractionated heparin on survival in patients with malignancy-a systematic review. Thrombosis & Haemostasis. 1999, 82 (6): 1600-1604.
Kakkar AK, Levine MN, Kadziola Z, Lemoine NR, Low V, Patel HK, Rustin G, Thomas M, Quigley M, Williamson RC: Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). Journal of Clinical Oncology. 2004, 22 (10): 1944-1948. 10.1200/JCO.2004.10.002
Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, Prandoni P, Bos MM, Richel DJ, van Tienhoven G, Buller HR: The effect of low molecular weight heparin on survival in patients with advanced malignancy.[see comment]. Journal of Clinical Oncology. 2005, 23 (10): 2130-2135. 10.1200/JCO.2005.03.134
Altinbas M, Coskun HS, Er O, Ozkan M, Eser B, Unal A, Cetin M, Soyuer S: A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. Journal Of Thrombosis And Haemostasis. 2004, 2 (8): 1266-1271. 10.1111/j.1538-7836.2004.00871.x. 10.1111/j.1538-7836.2004.00871.x
Parmar MKB, Torri V, Stewart L: Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998, 17: 2815-2834. 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, for the GWG: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. 2008, 336 (7650): 924-926.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ. 2003, 327 (7414): 557-560. 10.1136/bmj.327.7414.557
Altinbas M, Coskun HS, Er O, Ozkan M, Eser B, Unal A, Cetin M: Prospective Randomized Study of Epirubicine Cyclophosphamide and Vincristine Combination Chemotherapy (CEV) ¦ Low Molecular Weight Heparin (LMWH) in Small Cell Lung Cancer (SCLC). Proc Am Soc Clin Oncol. 2001 , 20: abstr 1280-
Lebeau B, Chastang C, Brechot JM, Capron F, Dautzenberg B, Delaisements C, Mornet M, Brun J, Hurdebourcq JP, Lemarie E: Subcutaneous heparin treatment increases survival in small cell lung cancer. "Petites Cellules" Group. Cancer. 1994, 74 (1): 38-45. 10.1002/1097-0142(19940701)74:1<38::AID-CNCR2820740108>3.0.CO;2-E
Sideras K, Schaefer PL, Okuno SH, Sloan JA, Kutteh L, Fitch TR, Dakhil SR, Levitt R, Alberts SR, Morton RF, Rowland KM, Novotny PJ, Loprinzi CL: Low-molecular-weight heparin in patients with advanced cancer: a phase 3 clinical trial. Mayo Clin Proc. 2006, 81 (6): 758-767.
Altynbas M, Ozkan M, Coskun HS, Er O, Eser B, Unal A, Cetin M: Efficiency of cyclophosphamide, epirubicin, vincristine (CEV) +/- low molecular weight heparin (LMWH) in small cell lung cancer (SCLC): Preliminary results. Annals Of Oncology. 2000, 11: 117-117.
Kakkar AK, Kadziola Z, Williamson RCN, Levine MN, Low V, Lemoine NR: Low molecular weight heparin (LMWH) therapy and survival in advanced cancer. Blood. 2002, 100 (11): 148A-148A.
Sideras K, Schaefer PL, Okuno SH, Sloan JA, Kutteh L, Dakhil SR, Levitt R, Novotny PJ, Loprinzi CL: Phase III clinical trial evaluating low-molecular weight heparin (LMWH) in patients with advanced cancer: A North Central Cancer Treatment Group study. Journal Of Clinical Oncology. 2005, 23 (16): 775S-775S.
Barkagan ZS: The results of the use of low molecular weight heparin (LMWH) for prevention and treatment of thrombosis in cancer patients. Thrombosis And Haemostasis. 1997, 772-772.
Freund M, Kakkar AK, Haas S, Heilmann L, von Tempelhoff GF, Brom J, Weidinger G: A randomized trial of the low molecular weight heparin certoparin against placebo in the long-term prevention of venous thromboembolism in patients with metastatic breast cancer. Blood. 2003, 102 (11): 210A-210A.
Gatzemeier U, Freund M, Haas S, Kakkar A, Zatloukai P, Kelbel C, Tchibisov L, Shparyk Y, Ciuleanu T, Huisman M: Prevention of thromboembolic complications with the low-molecular-weight heparin certoparin in non-small-cell lung carcinoma (TOPIC-2). Lung Cancer. 2005, 49: S56-S56. 10.1016/S0169-5002(05)80297-1. 10.1016/S0169-5002(05)80297-1
Graf AH, Graf B, Traun H, Staudach A: [Risk and prevention of thromboembolism complications in gynecologic malignancies]. Gynakologisch-Geburtshilfliche Rundschau. 1996, 36 (1): 37-39.
Graf B, Graf AH, Traun H, Forstner K, Rettenbacher L, Sailer S, Staudach A: Prophylaxis of thromboembolism in radiotherapy for gynecologic malignancies: low molecular weight (LMW) heparin (fragmin (R)) vs coumarin (sintrom(R)). Haemostasis. 1994, 24 (Suppl 1): 315-Abstract No 316.
Salat C, Breitruck H, Reinhardt B, Hiller E: Thromboprophylaxis with low molecular weight heparin (LMWH) and conventional low dose heparin in patients with malignant diseases. Blut. 1990, 61 (2-3): 142-
Lee AYY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, Gent M, Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer I: Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer.[see comment]. N Engl J Med. 2003, 349 (2): 146-153. 10.1056/NEJMoa025313
Lazo-Langner A, Goss GD, Spaans JN, Rodger MA: The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. Journal of Thrombosis & Haemostasis. 2007, 5 (4): 729-737. 10.1111/j.1538-7836.2007.02427.x.
Oxman A, Guyatt G: When to believe a subgroup analysis. Users' guides to the medical literature: a manual for evidence-based clinical practice. Edited by: Guyatt G, Rennie D. 2002, 553-565. Chicago , AMA Press
Kakkar AK: Low-molecular-weight heparin and wurvival in patients with malignant disease. Cancer control. 2005, 12 (S1): 22-30.
Cosgrove RH, Zacharski LR, Racine E, Andersen JC: Improved cancer mortality with low-molecular-weight heparin treatment: a review of the evidence. Seminars in Thrombosis & Hemostasis. 2002, 28 (1): 79-87. 10.1055/s-2002-20566.
Dvorak HF: Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. New England Journal of Medicine. 1986, 315 (26): 1650-1659.
Francis JL, Biggerstaff J, Amirkhosravi A: Hemostasis and malignancy. Seminars in Thrombosis & Hemostasis. 1998, 24 (2): 93-109.
Levine MN, Lee AY, Kakkar AK: From Trousseau to targeted therapy: new insights and innovations in thrombosis and cancer. Journal of Thrombosis & Haemostasis. 2003, 1 (7): 1456-1463. 10.1046/j.1538-7836.2003.00275.x.
Smorenburg SM, Van Noorden CJ: The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacological Reviews. 2001, 53 (1): 93-105.
Akl EA, Kamath G, Kim SY, Yosuico V, Barba M, Terrenato I, Sperati F, Schunemann HJ: Oral anticoagulants may prolong the survival of a subgroup of patients with cancer: a systematic review. Journal of Experimental and Clinical Cancer Research. 2007, 26 (2): 461-470.
Akl EA, Rohilla S, Barba M, Sperati F, Terrenato I, Muti P, Schünemann HJ: Anticoagulation for the initial treatment of venous thromboembolism in cancer patients. The Cochrane Database of Systematic Reviews. 2007, 4:
Akl EA, Barba M, Rohilla S, Terrenato I, Sperati F, Muti P, Schünemann HJ: Anticoagulation for the long term treatment of venous thromboembolism in cancer patients. The Cochrane Database of Systematic Reviews. 2008, 2:
Haynes RB, Devereaux PJ, Guyatt GH: Clinical expertise in the era of evidence-based medicine and patient choice. ACP J Club. 2002, 136 (2): A11-4.
Alifano M, Benedetti G, Trisolini R: Can Low-Molecular-Weight Heparin Improve the Outcome of Patients With Operable Non-Small Cell Lung Cancer?: An Urgent Call for Research. Chest. 2004, 126 (2): 601-607.
Acknowledgements
This paper is based on a Cochrane Review to be first published in The Cochrane Library 2007, Issue 3. Cochrane reviews are regularly updated as new evidence emerges and in response to comments and criticisms, and The Cochrane Library should be consulted for the most recent version of the review. We thank Ms. Ann Grifasi for her administrative support. We thank Dr. Loprinzi and Dr. Paul Novotny of the Mayo Clinic for supplying unpublished data relating to the study of Sideras 2006. Holger Schünemann is supported by a EU Marie Curie Reintegration Grant (IGR 42192). Internal Funding from the State University of New York at Buffalo, NY, USA and the Italian National Cancer Institute Regina Elena, Rome, Italy. Funding bodies were not involved in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Schünemann: no personal payments from for-profit sponsors, but he received research grants and honoraria that were deposited into research accounts or received by a research group that he belongs to from AstraZeneca, Amgen, Chiesi Foundation, Lily, and Pfizer, Roche and UnitedBioSource for development or consulting regarding quality of life instruments for chronic respiratory diseases and as lecture fees related to the methodology of evidence based practice guideline development and research methodology. Institutions or organizations that he is affiliated with likely receive funding from for-profit sponsors that are supporting infrastructure and research that may serve his work.
Authors' contributions
EAA: protocol development, search for trials, screening, data extraction, data analysis, manuscript drafting, review coordination. MB: screening, data extraction. SR: screening, data extraction. IT: screening, data extraction. FS: screening, data extraction. PM: data analysis, methodological advice. HJS: protocol development, search for trials, data extraction, data analysis, methodological advice.
Electronic supplementary material
13046_2008_4_MOESM1_ESM.doc
Additional file 1: "Search strategies used for the electronic databases; parenteral anticoagulation to prolong survival systematic review". search strategy. (DOC 34 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Akl, E.A., van Doormaal, F.F., Barba, M. et al. Parenteral anticoagulation may prolong the survival of patients with limited small cell lung cancer: a Cochrane systematic review. J Exp Clin Cancer Res 27, 4 (2008). https://doi.org/10.1186/1756-9966-27-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-9966-27-4