Abstract
Background
The CyberKnife is an appealing delivery system for hypofractionated stereotactic body radiation therapy (SBRT) because of its ability to deliver highly conformal radiation therapy to moving targets. This conformity is achieved via 100s of non-coplanar radiation beams, which could potentially increase transitory testicular irradiation and result in post-therapy hypogonadism. We report on our early experience with CyberKnife SBRT for low- to intermediate-risk prostate cancer patients and assess the rate of inducing biochemical and clinical hypogonadism.
Methods
Twenty-six patients were treated with hypofractionated SBRT to a dose of 36.25 Gy in 5 fractions. All patients had histologically confirmed low- to intermediate-risk prostate adenocarcinoma (clinical stage ≤ T2b, Gleason score ≤ 7, PSA ≤ 20 ng/ml). PSA and total testosterone levels were obtained pre-treatment, 1 month post-treatment and every 3 months thereafter, for 1 year. Biochemical hypogonadism was defined as a total serum testosterone level below 8 nmol/L. Urinary and gastrointestinal toxicity was assessed using Common Toxicity Criteria v3; quality of life was assessed using the American Urological Association Symptom Score, Sexual Health Inventory for Men and Expanded Prostate Cancer Index Composite questionnaires.
Results
All 26 patients completed the treatment with a median 15 months (range, 13-19 months) follow-up. Median pre-treatment PSA was 5.75 ng/ml (range, 2.3-10.3 ng/ml), and a decrease to a median of 0.7 ng/ml (range, 0.2-1.8 ng/ml) was observed by one year post-treatment. The median pre-treatment total serum testosterone level was 13.81 nmol/L (range, 5.55 - 39.87 nmol/L). Post-treatment testosterone levels slowly decreased with the median value at one year follow-up of 10.53 nmol/L, significantly lower than the pre-treatment value (p < 0.013). The median absolute fall was 3.28 nmol/L and the median percent fall was 23.75%. There was no increase in biochemical hypogonadism at one year post-treatment. Average EPIC sexual and hormonal scores were not significantly changed by one year post-treatment.
Conclusions
Hypofractionated SBRT offers the radiobiological benefit of a large fraction size and is well-tolerated by men with low- to intermediate-risk prostate cancer. Early results are encouraging with an excellent biochemical response. The rate of new biochemical and clinical hypogonadism was low one year after treatment.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Recent analyses of clinical data suggest that large radiation fraction sizes are radiobiologically favorable compared to smaller fraction sizes in prostate cancer radiotherapy [1]. The CyberKnife (Accuray, Inc., Sunnyvale, CA) is an FDA-approved radiosurgical device that is ideal for accurately delivering hypofractionated stereotactic body radiation therapy (SBRT) [2]. Treatment is delivered by a linear accelerator mounted on a flexible robotic arm. A few hundred treatment beams are selected from a repertoire of greater than one thousand possible beam directions using inverse treatment planning. These beams are delivered in a non-isocentric, non-coplanar manner via circular collimators of varying sizes. Access to a large number of potential beam trajectories allows delivery of a highly conformal dose with steep dose gradients [3, 4]. Unlike standard radiation therapy delivery systems, the CyberKnife system incorporates a dynamic tracking system consisting of an orthogonal pair of diagnostic-quality x-ray imaging devices and software that can locate fiducials implanted within the prostate [5]. This provides updated position information in six dimensions (three translations combined with roll, pitch and yaw rotations) [6] to the robot, which adjusts the targeting of the therapeutic beam during treatment to correct for intra-fraction motion. These features allow for a reduction in the planning target volume (PTV) and potentially the dose to surrounding critical organs. These technical improvements should allow for dose escalation within the prostate while maintaining normal tissue tolerance.
The early efficacy and safety of CyberKnife hypofractionated dose-escalated SBRT have been documented for localized treatment of prostate cancer [7–9]. Stanford's phase II protocol delivered 36.25 Gy in 5 fractions of 7.25 Gy. This dose and fractionation were selected for radiobiologic dose escalation while keeping a constant predicted normal tissue late effect. In King et al.'s report on 41 "low-risk" patients, at a median of 33 months after treatment, the mean PSA was 0.44 ng/ml [7], suggesting a high rate of long-term control [10]. No patient experienced grade 4 toxicity, and only two patients experienced grade 3 late urinary morbidity. Similar results with similar regimens have been reported by others [8, 9].
Due to anatomic proximity, the testes are at risk for exposure to scattered radiation during prostate treatment. It has been suggested that the non-coplanar nature of CyberKnife SBRT may increase the risk of testicular irradiation during treatment [11]. The resulting decline in testosterone levels [12, 13] could be responsible for the low PSA nadirs [14] obtained with CyberKnife SBRT. If so, the post-treatment PSA response may not accurately reflect the likelihood of long-term tumor control with such treatment [10]. Equally important, the resulting endocrine changes may contribute to post-radiation hypogonadism with subsequent depression, cognitive decline, decreased libido and impotence [15]. Knowledge of the relative risks of hypogonadism due to available treatment options for prostate cancer could affect patients' treatment decisions. In this paper, we report on the use of CyberKnife SBRT as monotherapy for the treatment of 26 prostate cancer patients and show that the risk of new biochemical and clinical hypogonadism is low within the first year after treatment.
Methods
Patient Selection
Patients eligible for inclusion in this study had histologically-confirmed low- to intermediate-risk adenocarcinoma of the prostate (clinical stage ≤ T2b, Gleason score ≤ 7, PSA ≤ 20 ng/ml). Exclusion criteria included androgen deprivation therapy, clinically involved lymph nodes on imaging, distant metastases on bone scan, prior pelvic radiotherapy or prior radical prostate surgery. Institutional IRB approval was obtained for this retrospective review.
SBRT Treatment Planning and Delivery
Four gold fiducials were placed into the prostate prior to treatment planning: two at the apex and two at the base. To allow for fiducial stabilization, planning imaging was performed at least 7 days after fiducial placement. Patients underwent 1.5 T MR imaging followed shortly thereafter by a thin-cut (1.25 mm) CT scan. Both scans were performed with an empty bladder. Patients were advised to adhere to a low-fiber diet, starting at least five days prior to all treatment planning imaging and treatment delivery. They were restricted to nothing by mouth (NPO) the night before, and an enema was administered 1-2 hours prior to imaging and treatment.
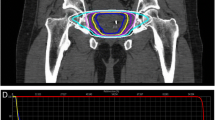
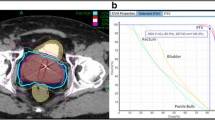
Fused CT and MR images were used for treatment planning (Figure 1). The gross target volume (GTV) was the prostate. The clinical target volume (CTV) included the prostate and the proximal seminal vesicles to the point where the left and right seminal vesicles separate. The PTV equaled the CTV expanded 3 mm posteriorly and 5 mm in all other dimensions. The prescription dose was 36.25 Gy to the PTV delivered in five fractions of 7.25 Gy over two weeks. The volume of the PTV receiving 36.25 Gy was at least 95%. The prescription isodose line was limited to ≥ 75%, which limited the maximum prostatic urethra dose to 133% of the prescription dose. The rectum, bladder, testes, penile bulb and membranous urethra were contoured structures and evaluated with dose-volume histogram analysis during treatment planning using Multiplan (Accuray Inc., Sunnyvale, CA) inverse treatment planning. Rectal volume receiving 36 Gy was limited to < 1 cc. The rectal dose-volume histogram (DVH) goals were < 50% rectal volume receiving 50% of the prescribed dose, < 20% receiving 80% of the dose, < 10% receiving 90% of the dose, and < 5% receiving 100% of the dose [7]. The empty bladder volume receiving 37 Gy was limited to < 10 cc [8]. Care was taken to avoid treatment beams that directly traversed the testes, and the scatter dose was kept to a minimum. Image-guidance was employed to minimize the required PTV treatment margins. Using computed tomography planning, target volume locations were related to the gold fiducial markers. Position verification was validated several times per minute during treatment using paired, orthogonal, and x-ray images.
Treatment planning axial (A) and sagittal (B) computed tomography images demonstrating the GTV (red), CTV and PTV expansion (dark blue), bladder (orange), rectum (green), bowel (yellow), membranous urethra (pink) and penile bulb (light blue). Isodose lines shown as follows: Blue 79% (prescription), white 70% and purple 50%.
Follow-up
PSA and total testosterone levels were obtained before treatment, one month after the completion of radiation, and during routine follow-up visits every 3 months for the first year. Samples were obtained in the morning and early afternoon to limit the effects of circadian variation [16]. Biochemical hypogonadism was defined as total serum testosterone level below 8 nmol/L [17]. Toxicity was assessed pre-treatment and at 1, 3, 6, 9 and 12 months post-treatment using the National Cancer Institute (NCI) Common Toxicity Criteria (CTC) version 3.0 [18] and the American Urological Association (AUA) symptom score (also known as International Prostate Symptom Score) [19]. Quality of life (QoL) was assessed pre-treatment and at follow-up visits using the Short Form-12 Health Survey (SF-12), the Expanded Prostate Cancer Index Composite (EPIC) [20] and the Sexual Health Inventory for Men (SHIM) [21].
Statistical Analysis
Skewed continuous variables, e.g., testosterone and PSA, were described as the sample median and range. Categorical variables were described as frequency and percentage. Obtaining PSA, total testosterone, and quality of life measurements sequentially in each patient constitutes a natural control for potentially wide baseline variation across patients. Therefore responses to radiotherapy were assessed using non-parametric pairwise Wilcoxon rank-sum testing [22].
Results
From January 2009 to June 2009, 26 prostate cancer patients were treated per our institutional protocol. Their median age was 69 years (range, 48-79 years). Similar numbers of Caucasians and African-Americans were enrolled reflecting the distribution of our patient population. Fourteen patients were low-risk, and 12 patients were intermediate-risk per the D'Amico Risk Classification [23]. Table 1 provides detailed patient characteristics.
At a median follow-up of 15 months (range, 13-19 months), the initial PSA response has been favorable, with decreased PSA levels in all patients. The median pre-treatment PSA was 5.75 ng/ml (range, 2.3-10.3 ng/ml); it decreased to a median of 0.7 ng/ml (range, 0.2-1.8 ng/ml) by one year post-treatment (Figure 2A), suggesting a high rate of long term disease control using this treatment regimen [24].
Consistent with our elderly patient population, pre-treatment total serum testosterone levels were low, ranging from 5.55 nmol/L to 39.87 nmol/L with a median value of 13.81 nmol/L[25]. The median testicular scatter dose was 2.1 Gy (range, 1.1-5.8 Gy). Post-treatment total serum testosterone levels fell in 18 patients (69%) and increased in 8 patients (31%). At one year the median serum testosterone value of 10.53 nmol/L (range, 5.79-22.38 nmol/L) was significantly lower than the pre-treatment value (p < 0.013) (Figure 2B). The median absolute fall was small (3.28 nmol/L) and the median percent fall was 23.75%. Pre- and post-treatment median total testosterone levels are shown in Figure 2B. In contrast to the total serum testosterone levels, the PSA to testosterone ratio decreased in all the patients, suggesting that the PSA decrease was not due solely to the drop in testosterone (Figure 2C). Based on the International Society for the Study of the Aging Male (ISSAM) definition (< 8 nmol/L) [18], the pre-treatment and 1-year biochemical hypogonadism rates were identical (Figure 3).
Toxicity has been minimal with no Grade 3 or higher gastrointestinal (GI) or gastrourinary (GU) toxicities (Table 2). Grade 1 and 2 acute toxicities included urinary symptoms requiring alpha blockers and bowel frequency/spasms requiring antidiarrheals. At one year post-treatment, the patients' perceptions of their physical (Figure 4A) and mental health (Figure 4B) were unchanged (Table 3). At one month post-treatment the mean AUA toxicity increased to 10.8 from a baseline of 6.8 (p = 0.0001), and the mean EPIC urinary score decreased to 82.7 from a baseline 90.5 (p = 0.0001), see Figures 5A and 5B and Table 3. Both mean AUA and EPIC urinary scores returned to baseline by one year after treatment. At one month post-treatment, the mean EPIC bowel score declined to 91.7 from a baseline of 95.7 (p = 0.042) (see Figure 5C and Table 3) and returned to baseline by one year after treatment.
Short Form-12 (SF-12) Health Survey quality of life: (A) SF-12 physical component score (PCS) and (B) SF-12 mental component score (MCS). The graphs show unadjusted changes in average scores over time. The scores range from 0 - 100 with higher values representing improved health status. Numbers above each time point indicate the number of observations contributing to the average.
Urinary and bowel quality of life using the American Urology Association (AUA) score and the Expanded Prostate Cancer Index Composite (EPIC): (A) AUA score, (B) EPIC urinary and (C) EPIC bowel. The graphs show unadjusted changes in average scores over time for each domain. AUA scores range from 0 - 35 with higher values representing worsening urinary symptoms. EPIC scores range from 0 - 100 with higher values representing a more favorable health-related QOL. Numbers above each time point indicate the number of observations contributing to the average. Error bars indicate 95% confidence intervals.
Sexual dysfunction is a major criterion for the clinical diagnosis of hypogonadism [26]. At one year post-treatment, the mean SHIM decreased to 14.3 from a baseline of 17.2, and the mean EPIC sexual scores decreased to 60.1 from a baseline of 66.7 (Figures 6A and 6B, Table 3). However, these changes were small and not statistically (p = 0.126 and p = 0.341, respectively) or clinically significant [27]. At one month post-treatment, the mean EPIC hormone score declined to 90.9 from a baseline of 94.2 (p = 0.039); it returned to baseline by one year post-treatment (Figure 6C and Table 3).
Sexual quality of life using the Health Inventory for Men (SHIM) and Expanded Prostate Cancer Index Composite (EPIC): (A) SHIM, (B) EPIC sexual and (C) EPIC hormonal scores. The graphs show unadjusted changes in average scores over time for each domain. SHIM scores range from 0 - 25 with lower values representing worsening sexual symptoms. EPIC scores range from 0 - 100 with higher values representing a more favorable health-related QOL. The graphs show unadjusted changes in average toxicity and QOL scores over time. Numbers above each time point indicate the number of observations contributing to the average.
Discussion
Pelvic irradiation causes a dose-dependent reduction in serum testosterone levels that increases with larger field sizes and higher testicular doses [28]. For conventional pelvic radiation therapy, the drop is approximately 10-30%; this reaches a nadir, on average, several months post-treatment and can persist for years thereafter [28–33]. In addition to precipitating clinical hypogonadism, with its adverse effects [15], this testosterone decline may undermine the utility of PSA as a tumor response marker [10]. Radiation dose escalation, hypofractionation, and the increased total body radiation with multi-field treatments [34] and image guidance [35] could enhance this testosterone decline. Thus, this study was aimed to assess the risk of biochemical and clinical hypogonadism following CyberKnife SBRT monotherapy for clinically localized prostate cancer.
In our study, we observed a small decline (23.75%) in total testosterone levels after SBRT treatment consistent with that reported by others [36] and similar to that seen with conventional prostate radiation therapy [30]. This decline in testosterone was unlikely responsible for a promising 12-month PSA nadir as variations in serum testosterone do not greatly affect PSA levels in eugonadal men [37, 38]. It remains to be determined whether testosterone decreases are temporary or permanent as these levels can take years to normalize [28]. Future studies will determine if testosterone levels fully recover to age-appropriate levels in our patient population.
The cause of this testosterone decline is unknown. Leydig cell dysfunction due to testicular scatter irradiation (mean dose of 2-4 Gy) in older men has been proposed as the major causative factor [12, 29, 31–33]. However, normal age-related testosterone decline [25] and treatment related stress [39] may also contribute. To determine if emotional and physiological stress could be responsible for our small decline in total testosterone, we examined acute toxicity and quality of life indicators. Acute Grade 2 GU and GI toxicities were observed in 27% and 0% of patients, respectively (Table 2). There were no Grade 3 or higher acute toxicities. These results appear comparable to other published external beam radiation therapy series [19, 40, 41]. In the opinion of the authors, it is unlikely that these minimal toxicities were responsible for the observed decline in serum testosterone. Consistent with findings of others, the small decline in total testosterone had minimal effects on quality of life [42]. Our AUA, SHIM and EPIC scores returned to baseline by one year after treatment (Table 3 and Figures 5 and 6). This is not unexpected as a total testosterone of 8 nmol/L is likely adequate for normal physiologic and sexual functioning [18]. Whatever the cause, the small decline in total testosterone does not appear to be clinically significant as it did not adversely affect the utility of the PSA as a measure of tumor response or induced clinical hypogonadism.
Conclusions
Hypofractionated SBRT is a promising new treatment option for men with low- and intermediate-risk prostate cancer. Early results suggest encouraging biochemical response with low toxicity and a low rate of new biochemical and clinical hypogonadism one year after treatment Investigation of more patients with longer follow-up is required to validate these conclusions.
Abbreviations
- AUA:
-
American Urological Association
- CTC:
-
Common Toxicity Criteria
- CTV:
-
clinical target volume
- DVH:
-
dose-volume histogram
- EPIC:
-
Expanded Prostate Cancer Index Composite
- GI:
-
gastrointestinal
- GU:
-
genitourinary
- GTV:
-
gross target volume
- ISSAM:
-
International Society for the Study of the Aging Male
- NCI:
-
National Cancer Institute
- NPO:
-
nothing by mouth
- PTV:
-
planning target volume
- QoL:
-
quality of life
- SHIM:
-
Sexual Health Inventory for Men
- SF-12:
-
Short Form-12
- and SBRT:
-
stereotactic body radiation therapy.
References
Fowler JF: The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005, 44: 265-276. 10.1080/02841860410002824.
Kilby W, Dooley J, Kuduvalli G, Sayeh S, Maurer CRJ: The CyberKnife Robotic Radiosurgery System in 2010. Technol Cancer Res Treat. 2010, 9: 433-452.
Webb S: Conformal intensity-modulated radiotherapy (IMRT) delivered by robotic linac--testing IMRT to the limit?. Phys Med Biol. 1999, 44: 1639-1654. 10.1088/0031-9155/44/7/305.
Hossain S, Xia P, Huang K, Descovich M, Chuang C, Gottschalk AR, Roach M, Ma L: Dose Gradient Near Target-Normal Structure Interface for Nonisocentric CyberKnife and Isocentric Intensity-Modulated Body Radiotherapy for Prostate Cancer. Int J Radiat Oncol Biol Phys.
Xie Y, Djajaputra D, King CR, Hossain S, Ma L, Xing L: Intrafractional motion of the prostate during hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2008, 72: 236-246. 10.1016/j.ijrobp.2008.04.051.
Kaiser A, Schultheiss TE, Wong JY, Smith DD, Han C, Vora NL, Pezner RD, Chen YJ, Radany EH: Pitch, roll, and yaw variations in patient positioning. Int J Radiat Oncol Biol Phys. 2006, 66: 949-955. 10.1016/j.ijrobp.2006.05.055.
King CR, Brooks JD, Gill H, Pawlicki T, Cotrutz C, Presti JC: Stereotactic Body Radiotherapy for Localized Prostate Cancer: Interim Results of a Prospective Phase II Clinical Trial. Int J Radiat Oncol Biol Phys. 2009, 73: 1043-1048. 10.1016/j.ijrobp.2008.05.059.
Friedland JL, Freeman DE, Masterson-McGary ME, Spellberg DM: Stereotactic body radiotherapy: an emerging treatment approach for localized prostate cancer. Technol Cancer Res Treat. 2009, 8: 387-392.
Katz AJ, Santoro M, Ashley R, Diblasio F, Witten M: Stereotactic body radiotherapy for organ-confined prostate cancer. BMC Urol. 2010, 10: 1-10.1186/1471-2490-10-1.
Ray ME, Thames HD, Levy LB, Horwitz EM, Kupelian PA, Martinez AA, Michalski JM, Pisansky TM, Shipley WU, Zelefsky MJ: PSA nadir predicts biochemical and distant failures after external beam radiotherapy for prostate cancer: a multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2006, 64: 1140-1150. 10.1016/j.ijrobp.2005.07.006.
King CR, Lo A, Kapp DS: Testicular dose from prostate cyberknife: a cautionary note. Int J Radiat Oncol Biol Phys. 2009, 73: 636-637. 10.1016/j.ijrobp.2008.09.004. author reply 637
Shapiro E, Kinsella TJ, Makuch RW, Fraass BA, Glatstein E, Rosenberg SA, Sherins RJ: Effects of fractionated irradiation of endocrine aspects of testicular function. J Clin Oncol. 1985, 3: 1232-1239.
Petersen PM, Giwercman A, Daugaard G, Rorth M, Petersen JH, Skakkeaek NE, Hansen SW, von der Maase H: Effect of graded testicular doses of radiotherapy in patients treated for carcinoma-in-situ in the testis. J Clin Oncol. 2002, 20: 1537-1543. 10.1200/JCO.20.6.1537.
Dixon SC, Knopf KB, Figg WD: The control of prostate-specific antigen expression and gene regulation by pharmacological agents. Pharmacol Rev. 2001, 53: 73-91.
Tinkler SD, Howard GC, Kerr GR: Sexual morbidity following radiotherapy for germ cell tumours of the testis. Radiother Oncol. 1992, 25: 207-212. 10.1016/0167-8140(92)90270-5.
Crawford ED, Barqawi AB, O'Donnell C, Morgentaler A: The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007, 100: 509-513. 10.1111/j.1464-410X.2007.07022.x.
Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A: Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008, 159: 507-514. 10.1530/EJE-08-0601.
Oermann E, Hanscom H, Lei S, Suy S, Collins B, Batipps G, McGeagh K, Jha R, Dawson N, Dritschilo A: A Pilot Study of Intensity Modulated Radiation Therapy Plus a Hypofractionated Stereotactic Body Radiation Therapy Boost for the Treatment of Intermediate to High Risk Prostate Cancer. Technol Cancer Res Treat. 2010, 9: 453-462.
Barry MJ, Fowler FJ, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT: The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992, 148: 1549-1557. discussion 1564
Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG: Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000, 56: 899-905. 10.1016/S0090-4295(00)00858-X.
Rosen RC, Cappelleri JC, Gendrano N: The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002, 14: 226-244. 10.1038/sj.ijir.3900857.
Zagars GK, Pollack A, von Eschenbach AC: Serum testosterone--a significant determinant of metastatic relapse for irradiated localized prostate cancer. Urology. 1997, 49: 327-334. 10.1016/S0090-4295(96)00619-X.
D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A: Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. Jama. 1998, 280: 969-974. 10.1001/jama.280.11.969.
Alcantara P, Hanlon A, Buyyounouski MK, Horwitz EM, Pollack A: Prostate-specific antigen nadir within 12 months of prostate cancer radiotherapy predicts metastasis and death. Cancer. 2007, 109: 41-47. 10.1002/cncr.22341.
Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR: Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001, 86: 724-731. 10.1210/jc.86.2.724.
Wheeler MJ, Barnes SC: Measurement of testosterone in the diagnosis of hypogonadism in the ageing male. Clin Endocrinol (Oxf). 2008, 69: 515-525. 10.1111/j.1365-2265.2008.03325.x.
Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, Lin X, Greenfield TK, Litwin MS, Saigal CS: Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008, 358: 1250-1261. 10.1056/NEJMoa074311.
Joos H, Sedlmayer F, Gomahr A, Rahim HB, Frick J, Kogelnik HD, Rettenbacher L: Endocrine profiles after radiotherapy in stage I seminoma: impact of two different radiation treatment modalities. Radiother Oncol. 1997, 43: 159-162. 10.1016/S0167-8140(97)00052-2.
Bruheim K, Svartberg J, Carlsen E, Dueland S, Haug E, Skovlund E, Tveit KM, Guren MG: Radiotherapy for rectal cancer is associated with reduced serum testosterone and increased FSH and LH. Int J Radiat Oncol Biol Phys. 2008, 70: 722-727. 10.1016/j.ijrobp.2007.10.043.
Pickles T, Graham P: What happens to testosterone after prostate radiation monotherapy and does it matter?. J Urol. 2002, 167: 2448-2452. 10.1016/S0022-5347(05)65002-1.
Tomic R, Bergman B, Damber JE, Littbrand B, Lofroth PO: Effects of external radiation therapy for cancer of the prostate on the serum concentrations of testosterone, follicle-stimulating hormone, luteinizing hormone and prolactin. J Urol. 1983, 130: 287-289.
Yau I, Vuong T, Garant A, Ducruet T, Doran P, Faria S, Liberman S, Richard C, Letellier F, Charlebois P: Risk of hypogonadism from scatter radiation during pelvic radiation in male patients with rectal cancer. Int J Radiat Oncol Biol Phys. 2009, 74: 1481-1486. 10.1016/j.ijrobp.2008.10.011.
Yoon FH, Perera F, Fisher B, Stitt L: Alterations in hormone levels after adjuvant chemoradiation in male rectal cancer patients. Int J Radiat Oncol Biol Phys. 2009, 74: 1186-1190. 10.1016/j.ijrobp.2008.09.027.
Hall EJ: Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006, 65: 1-7. 10.1016/j.ijrobp.2006.01.027.
King CR, Maxim PG, Hsu A, Kapp DS: Incidental testicular irradiation from prostate IMRT: it all adds up. Int J Radiat Oncol Biol Phys. 2010, 77: 484-489. 10.1016/j.ijrobp.2009.04.083.
Fuller DB: Testicular dose from prostate cyberknife: a cautionary note in regard to King et al. Int J Radiat Oncol Biol Phys. 2009, 73: 637-
Cooper CS, MacIndoe JH, Perry PJ, Yates WR, Williams RD: The effect of exogenous testosterone on total and free prostate specific antigen levels in healthy young men. J Urol. 1996, 156: 438-441. 10.1016/S0022-5347(01)65871-3. discussion 441-432
Monath JR, McCullough DL, Hart LJ, Jarow JP: Physiologic variations of serum testosterone within the normal range do not affect serum prostate-specific antigen. Urology. 1995, 46: 58-61. 10.1016/S0090-4295(99)80159-9.
Guay A, Seftel AD, Traish A: Hypogonadism in men with erectile dysfunction may be related to a host of chronic illnesses. Int J Impot Res. 2010, 22: 9-19. 10.1038/ijir.2009.46.
Lips IM, Dehnad H, van Gils CH, Boeken Kruger AE, van der Heide UA, van Vulpen M: High-dose intensity-modulated radiotherapy for prostate cancer using daily fiducial marker-based position verification: acute and late toxicity in 331 patients. Radiat Oncol. 2008, 3: 15-10.1186/1748-717X-3-15.
Ghadjar P, Vock J, Vetterli D, Manser P, Bigler R, Tille J, Madlung A, Behrensmeier F, Mini R, Aebersold DM: Acute and late toxicity in prostate cancer patients treated by dose escalated intensity modulated radiation therapy and organ tracking. Radiat Oncol. 2008, 3: 35-10.1186/1748-717X-3-35.
Pickles T, Duncan G, Graham P: Re: Hermann et al., low testosterone levels and quality of life. Radiother Oncol. 2006, 78: 107-108. 10.1016/j.radonc.2005.10.004.
Acknowledgements
We acknowledge Robert Meier, M.D., Debra Freeman, M.D., Alan Katz, M.D. and Donald Fuller, M.D. for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Declaration of Competing interests
BT Collins serves as a clinical consultant to Accuray Inc.
The other authors declare that they have no competing interests.
Authors' contributions
EO and SS participated in data collection, data analysis, manuscript drafting, table/figure creation and manuscript revision. HH, JK, BE, JR, NP, BS, DB, and VC participated in data collection, data analysis and manuscript revision. SL, XY and GZ participated in treatment planning, data collection, data analysis, and manuscript revision. GB, NC, SD, GB, JP, KM and JL participated in treatment planning, data analysis and manuscript revision. LA, RJ, ND, BC, and AD participated in the design and coordination of the study. SC drafted the manuscript, designed the study, and led the research effort. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Oermann, E.K., Suy, S., Hanscom, H.N. et al. Low incidence of new biochemical and clinical hypogonadism following hypofractionated stereotactic body radiation therapy (SBRT) monotherapy for low- to intermediate-risk prostate cancer. J Hematol Oncol 4, 12 (2011). https://doi.org/10.1186/1756-8722-4-12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-8722-4-12