Abstract
Synaptic transmission and long-term potentiation (LTP) in the CA1 region of hippocampal slices have been studied during ageing of a double transgenic mouse strain relevant to early-onset familial Alzheimer's disease (AD). This strain, which over-expresses both the 695 amino acid isoform of human amyloid precursor protein (APP) with K670N and M671L mutations and presenilin 1 with the A246E mutation, has accelerated amyloidosis and plaque formation. There was a decrease in synaptic transmission in both wildtype and transgenic mice between 2 and 9 months of age. However, preparing slices from 14 month old animals in kynurenic acid (1 mM) counteracted this age-related deficit. Basal transmission and paired-pulse facilitation was similar between the two groups at all ages (2, 6, 9 and 14 months) tested. Similarly, at all ages LTP, induced either by theta burst stimulation or by multiple tetani, was normal. These data show that a prolonged, substantially elevated level of Aβ are not sufficient to cause deficits in the induction or expression of LTP in the CA1 hippocampal region.
Similar content being viewed by others
Background
Three loci have been identified that account for nearly all the familial Alzheimer's disease (AD) cases. Mutations in the amyloid precursor protein (APP) gene account for around 2-3% percent of familial AD cases and mutations in presenilins 1 and 2 (PS1 and PS2) have been linked to 70-80% of early onset AD [1–3]. The mutations associated with early onset familial AD in a Swedish family, where the 695 amino acid APP protein contains the two mutations K670N and M671L (APP695SWE mutation), affect cleavage of APP at the β-secretase site [4]. Subsequent cleavage of APP at the intramembranous γ-secretase site results in the formation of Aβ. PS1 is intimately associated with the cleavage of APP at the γ-secretase site and familial AD mutations in PS1, such as the A246E mutation occurring in transmembrane domain 6 of the presenilin protein [5, 6], alter the efficiency of cleavage of APP and accelerate the production of Aβ [3, 6–8].
Mice expressing the human form of the APP695SWE mutation develop certain AD-like symptoms, such as increased Aβ deposits and plaques, increased glial cell number, and deficits in spatial memory learning tasks [9–12]. Physiological studies have focused on the hippocampus, a region affected by AD, and have studied, in particular, long-term potentiation (LTP) as a candidate synaptic mechanism involved in learning and memory [13]. However, the findings have been controversial. Thus, one study reported normal synaptic transmission but impaired LTP in the APP695SWE mutant [12] whilst in other studies synaptic transmission was impaired but LTP was normal in the mutant [14, 15]. In contrast, mice expressing the PS1A246E mutation showed enhanced LTP [16], along with increased production of Aβ(42), the longer form of Aβ associated with plaque formation [17].
Mice expressing mutations in both APP and PS1 exhibit accelerated Aβ production compared to those carrying mutations in only APP or PS1 [18]. Studies using double transgenic (DbTg) mice expressing both the APP695SWE mutations in APP and either the PS1M146L [19] or PS1P264L [20] mutation showed reduced hippocampal LTP at a younger age than basal synaptic transmission was compromised. However, a recent study found no alteration in hippocampal synaptic transmission or plasticity in mice overexpressing the APP695SWE and PS1ΔE9 mutations, even though the mice showed deficits in spatial learning [21]. Another DbTg strain of mouse which over-expresses both the 695 amino acid isoform of human amyloid precursor protein (APP) with K670N and M671L mutations (APP695SWE mice) and the A246E mutation in PS1 (PS1A246E) has much more aggressive amyloidosis and plaque deposition than the APP695SWE mice [17]. Given the relevance of these mouse models to human disease but the conflicting findings of the effect of double mutations on synaptic transmission and plasticity in the hippocampus, we report here an analysis of these DbTg animals.
Methods
Transgenic mice
The generation of the APP695SWE transgenic mice used in this study has been described previously [9, 22]. APP695SWE transgenic mice were originally in a hybrid 87.5% C57BL6 x 12.5% SJL genetic background and were subsequently backcrossed to C57BL6 x SJL F1 mice over several generations. APP695SWE transgenic mice in a background closer to 50% C57BL6-50% SJL were then crossed with the PS1A246E transgenic line overexpressing the familial AD PS1A246E mutation of human PS1 [23] to generate the DbTg line (APP695SWE x PS1A246E; Lewis et al, 2004). All animals used in this study were from the N4 generation. All procedures were carried out in accordance with The UK Animals (Scientific Procedures) Act 1986.
Four age groups were studied: 2 months (i.e., between 2 and 3 months of age), 6 (6-7), 9 (9-10) and 14 (14 -15) months of age. Mice older than 15 months were not studied because of their high mortality and ethical considerations. All mice in this study were either heterozygous for both the APP695SWE and PS1A246E transgene (DbTg) or their wildtype (Wt) littermates. Animals were genotyped using PCR based methods for detection of the APP695SWE transgene [9, 14, 22], the PS1A246E transgene [23] and for the rd (retinal degeneration) mutation [24]. Rd homozygous mice were excluded from this study as a precaution, since it has been suggested that this mutation may indirectly affect neuronal number within the hippocampus [25]. All experiments and analyses were performed with the experimenters blind as to the genotype of the animal.
Quantification of Aβ levels by HTRF
Levels of Aβ were determined by homogenous time-resolved fluorescence (HTRF) immunoassay as described previously [26]. Amyloid was extracted from the contralateral hemispheres by homogenisation in 10 volumes of 5 M GnHCl, 50 mM HEPES (pH 7.3), 5 mM EDTA plus 1x protease inhibitor cocktail (Complete™, obtained from Roche Diagnostics). Following 3 h rotation at room temperature, the homogenate was diluted ten-fold into ice-cold 25 mM HEPES (pH 7.3), 1 mM EDTA, 0.1% BSA plus 1x protease inhibitor cocktail and centrifuged (16,000g, 20 min, 4°C). Aliquots of the supernatant were stored at -20°C. The levels of amyloid peptides Aβ(40) and Aβ(42) were then detected by HTRF. All peptides (of > 95% purity; California Peptide Research Inc., California, U.S.A.) were frozen at 100 μM in 100% DMSO and serially diluted in buffer whose composition reflects that of the extracted samples (1 part GnHCl extraction buffer: 9 parts dilution buffer, as above). The HTRF signal was generated as a result of non-radiative transfer from europium cryptate-labelled Aβ (40)- or Aβ (42)-specific antibodies (G2-10 and G2-11 respectively; licensed from the University of Heidelberg; labelled at CIS bio international, Marcoule, France) to streptavidin-conjugated APC (Prozyme). The latter was brought into the complex by interaction with biotinylated antibody 4G8 (Senetek plc, Missouri, U.S.A.), which is specific for residues 17-24 of Aβ. Final reagent concentrations in a typical 96-well plate assay were: G2-10K (0.75 nM) or G2-11K (0.6 nM), 4G8 +/- biotin (1.0 nM), SA-XL665 (2.0 nM), KF (0.1-0.2 M). 50 μl of sample or synthetic peptide standard were assayed and a total volume of 200 μl/well was made up with dilution buffer. Blank values were determined by the use of non-biotinylated 4G8 antibody. The reaction mixture was left at 4°C for 20 h, and then read on the Discovery™ HTRF microplate analyser, providing simultaneous measurement at 665 nm (XL665 fluorescence) and 620 nm (EuK fluorescence). The ΔR ratio [= Ratio (sample) - Ratio (blank)] was used to extrapolate the amyloid concentrations of the brain extracts from the synthetic peptide standard curves.
Histology
Histology to demonstrate plaque deposition was performed as described previously (Lewis et al, 2004). Briefly, 6 μm sagittal hippocampal slices were prepared and monoclonal anti-human β-amyloid (clone 6F3D) (DAKO, UK) was used to demonstrate amyloid plaques whilst polyclonal anti-GFAP (glial fibrillary acidic protein: DAKO) used to label reactive astrocytes. Endogenous peroxidase activity was blocked in 0.3% H2O2 in 0.1 M pH 7.4 phosphate buffered saline (PBS) for 30 min; non-specific binding was blocked by incubation with 5% normal horse serum (5% NHS) (Vector Labs, UK) in PBS for 1 h. Mouse anti-β-amyloid was applied (1:100 in 5% NHS) overnight at 4°C. Biotinylated anti-mouse IgG (Vector Labs) was applied for 30 min, followed by ABC reagent (Vector Elite, Vector Labs) for 30 min. DAB (Menarini, UK) was used as the chromogenic substrate. Peroxidase activity was quenched in 0.3% H2O2/PBS and sections further incubated in polyclonal anti-GFAP (1:1000 in 5% normal goat serum) overnight at 4°C. Visualisation was achieved by incubation in biotinylated anti-rabbit IgG followed by ABC reagent (Vector Elite, Vector Labs).
Electrophysiology
Recordings were made form 350 μm thick hippocampal slices prepared from 2, 6, 9 and 14 month old DbTg and Wt mice. Animals were killed by decapitation, as licensed under the UK Animals (Scientific Procedures) Act 1986, and the brains rapidly removed in ice-cold artificial cerebrospinal fluid (aCSF). The composition of this aCSF was, in mM: NaCl, 126; NaH2PO4, 1.2; MgCl2, 1.3; CaCl2, 2.4; KCl, 2.5; NaHCO3, 26; glucose, 10. Brains were cut along the midline and parasagittal whole brain slices prepared from one hemisphere (randomly chosen) using a Vibratome. The hippocampus was then dissected out of these slices. The contralateral hemisphere was used for histology or determination of Aβ levels. Slices were allowed to recover for at least 1 h at room temperature before being transferred to a submerged recording chamber perfused with aCSF at 2 ml.min.-1 and maintained at 33°C. Kynurenic acid (1 mM) was included in the aCSF used for preparing slices from the 14 month old animals. Slices were then transferred to non-kynurenic acid containing aCSF approximately 30 min after dissection and allowed to recover for a further 30 min.
Field excitatory postsynaptic potentials (fEPSPs) were recorded from stratum radiatum of area CA1 and Schaffer collateral-commissural fibres were stimulated using a bipolar nickel-chromium electrode. The initial slope of the negative-going phase of the fEPSP was used as a measure of synaptic efficacy. Recordings were made using a SPIKE 2 script running on a CED1401plus interface (Cambridge Electronic Design). Stimulus-response curves were constructed by using stimulus intensities from 0 to 45 V in increments of 5 V. Responses were subsequently set to a level that gave a slope value of approximately 20% of the maximum obtained. Baseline responses were obtained every 30 s. Paired-pulse facilitation (PPF) was assessed using a succession of paired-pulses using inter-pulse intervals of 25, 50, 100, 200 and 300 ms. A further 30 min baseline period was obtained before attempting to induce LTP.
LTP was induced by delivery of a theta burst stimulation paradigm (TBS), which comprised 10 bursts, at an interburst frequency of 5 Hz and an intraburst frequency of 100 Hz, each burst consisting of 4 stimuli delivered at test stimulus intensity. In some experiments, LTP was induced using a stronger stimulation whereby a repetitive tetanic stimulus was applied (100 stimuli at 100 Hz at test intensity repeated 4 times at 5 min intervals). All data are presented as mean ± s.e.mean. n values are given as [x (y)] where x = number of slices and y = number of animals. Data were log transformed and analysed using a one-way ANOVA with repeated measures (BMDP statistical package, release 7). Linear regression of fEPSP slope vs fibre volley amplitude was done with Microsoft® Excel 97 Analysis Toolpack.
Results
Aβ levels and amyloid plaque deposition
Expression of the APP695SWE x PS1A246E transgenes produced a dramatic increase in both short (Aβ(40)) and long (Aβ(42)) forms of the Aβ peptide (Fig. 1). This elevation in Aβ levels occurred several months earlier than in the single APP695SWE transgenic [14, 26]. At 9 months, the Aβ load was significantly greater than in Wt brains; levels of Aβ(40) were 3.0 ± 0.5 compared with 0.03 ± 0.02 nmol/g wet weight tissue and levels of Aβ(42) were 1.6 ± 0.4 compared to 0.01 ± 0.009 nmol/g wet weight tissue, in DbTg and Wt mice, respectively. At 14 months the levels of Aβ(40) and Aβ(42) increased to 10.8 ± 2.2 and 5.6 ± 1.4 nmol/g wet weight tissue in DbTg mice, compared to 0.01 ± 0.01 and 0.004 ± 0.003 nmol/g wet weight tissue in Wt mice (Fig. 1B). In addition, compared to the single transgenic APP695SWE mouse, the Aβ(42)/Aβ(40) ratio is enhanced throughout the life of the animal [14, 26]. Plaques were also evident in aged DbTg mice throughout the hippocampus, but were not evident in Wt animals (Fig. 1A; [26]).
Basal synaptic transmission
Synaptic transmission was quantified over a wide range of stimulus intensities and was analysed in terms of fEPSP slope versus stimulus intensity (Fig. 2) and fEPSP slope versus fibre volley (FV) amplitude (Fig. 3). Using both methods of analysis there was a pronounced, age-dependent decline in synaptic transmission in slices obtained from both Wt and DbTg mice of between 2 and 9 months of age. Indeed, it was difficult to obtain viable synaptic responses from slices prepared form both Wt and DbTg animals in the 9 month age group and many failed to produce a fEPSP. For this reason we performed the dissections for the 14 month age group in the presence of kynurenic acid, since this treatment has been shown to greatly improve slice viability of some transgenic mouse strains [12, 14]. This treatment resulted in considerably improved synaptic viability (Figs 2 & 3).
Age-dependent reduction in synaptic transmission in CA1 region of Wt and DbTg mice. (A) Individual examples of fEPSPs recorded with a stimulus intensity of 10, 20 and 30 V from slices prepared from 14 month old Wt and DbTg mice. Scale bar 0.5 mV, 10 ms. Stimuli were delivered at the times indicated by the arrows and stimulus artefacts have been removed for clarity. (B - D) Pooled data showing relationship between fEPSP slope and stimulus intensity at 2, 6, 9 and 14 months. In this and following figures symbols are [black circle] for Wt and [white circle] for DbTg results.
The input-output curves were generally similar for both genotypes, at all ages studied (Figs 2 & 3). For example, in 14 month old mice the slope of the relationship between fEPSP slope and FV amplitude was 2.34 ± 0.25 V.s-1.mV-1 [47 (11)] in Wt animals and 2.42 ± 0.31 V.s-1.mV-1 [43 (13)] in DbTg mice (p > 0.05). The maximum obtained fEPSPs were also similar in the two groups. For example, in 14 month old mice the fEPSP evoked by 45 V stimulation was 2.32 ± 0.21 V.s-1 [47 (11)] and 2.21 ± 0.22 V.s-1 [43 (13)] (p > 0.05) in Wt and DbTg mice, respectively.
Paired-pulse facilitation
In all age groups, there was no difference in the level of PPF (slope of second response/slope of first response) between DbTg and Wt mice. For example, at an inter-stimulus interval (ISI) of 50 ms the PPF ratios for Wt and DbTg mice were, respectively, at 2 months: 1.86 ± 0.05 [30 (13)] and 1.99 ± 0.13 [20 (10)]; 6 months: 1.93 ± 0.07 [24 (8)] and 1.80 ± 0.15 [15 (7)]; 9 months: 1.81 ± 0.10 [28 (10)] and 1.75 ± 0.09 [19 (8)] and 14 months: 1.72 ± 0.03 [28 (8)] and 1.77 ± 0.04 [24 (6)] (in all cases, p > 0.05). There was also no age-related change in the level of PPF in either Wt or DbTg animals (p > 0.05 at all stimulus intervals used). PPF data over a range of inter-pulse intervals for the 2 and 14 month age group are presented in Fig. 4.
Long-term potentiation
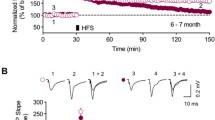
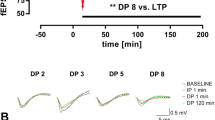
A theta burst stimulus (total of 40 stimuli) induced robust LTP in the CA1 region in all age groups. The level of LTP was similar in both genotypes across all ages, quantified for up to 60 min following theta burst stimulation (Fig. 5A-D; p > 0.05 at all age groups). For example, the level of LTP observed 60 minutes after theta burst stimulation in Wt and DbTg animals was, at 2 months: 156 ± 9% [9 (5)] and 171 ± 12% [8 (6)]; 6 months: 205 ± 17% [18 (9)] and 216 ± 32% [13 (7)]; 9 months: 171 ± 9 [15 (8)] and 211 ± 22% [8 (4)]; 14 months: 186 ± 10% [23 (13)] and 178 ± 15% [16 (9)]. There was no age-related change in the level of LTP seen following theta burst stimulation for either Wt of DbTg mice. In the 14 month age group, a subset of slices was followed for over 3 h following theta burst stimulation. There was no difference between genotypes, the level of LTP seen 3.5 hours post-theta burst stimulation in Wt and DbTg animals was 196 ± 28% [9 (5)] and 183 ± 20% [9 (5)] (p > 0.05; Fig. 5E).
LTP induced by a theta burst stimulus is normal in DbTg mice. (A) Example experiment taken from 14 month old Wt slice. LTP was induced at the time indicated by the arrowhead. Example traces are taken from the time points immediately prior to and 60 min after the induction of LTP. Scale bar 0.5 mV, 20 ms. (B) Example experiment taken from 14 month old DbTg slice. (C) Pooled data for 2 month-old animals ([black circle] Wt, [white circle] DbTg). In this and subsequent figures showing pooled data, data points are shown at two minute intervals. (D) Summary of LTP as a percentage of baseline at 60 min post-tetanus for the different age groups. (E) Pooled data from a subset of slices from 14 month old animals where LTP was followed for 3.5 h after the theta burst. Scale bar 0.5 mV, 10 ms.
In a second series of experiments, a stronger induction protocol was delivered (total of 400 stimuli) and LTP again followed for over 3 h (Fig. 6). The levels of LTP were similar for each genotype at all ages (p > 0.05 at 3.5 h), thus at 3.5 hours post-LTP induction the level of LTP in Wt and DbTg animals was, at 2 months: 189 ± 18% [10 (5)] and 188 ± 28 [7 (3)]; 6 months 151 ± 17% [11 (9)] and 139 ± 23% [8 (5)]; 9 months: 177 ± 26% [11 (6)] and 159 ± 23% [9 (5)]; 14 months: 203 ± 12% [20 (9)] and 168 ± 14% [16 (8)]. Again, there was no age-related change in the level of LTP induced by this strong induction protocol for either Wt or DbTg animals.
LTP induced by repeated tetani is normal in DbTg mice. (A-C) Pooled data for slices from 2, 6 and 9 month old animals ([black circle] Wt, [white circle] DbTg). (D) Summary of the LTP as a percentage of baseline at 3.5 hours after the last tetanus for the different age groups. (E) Pooled data for slices from 14 month old animals. Traces are taken from the time points immediately prior to induction of LTP and 3.5 h after the last tetanus. Scale bar 0.5 mV, 5 ms. LTP was induced by the delivery of four tetanic stimuli separated by 5 min at the times indicated by the arrowheads.
Discussion
In DbTg mice over-expressing the human familial AD transgenes APP695SWE and PS1A246E, Aβ peptides accumulate in an accelerated age-dependent manner with an early enhancement of the Aβ(42)/Aβ(40) ratio (see also [26]). Accelerated production of Aβ has also been reported in different mouse strains expressing double mutations of APP and PS1 (reviewed in [18]). However, there has been little work carried out into studying synaptic function and plasticity in these multiple transgenic mice lines. We find that normal LTP in the CA1 region can be recorded over a wide age range and that there is little, if any, difference between the DbTg and the Wt mice.
Effects on basal synaptic transmission
Consistent with our earlier report on the APP695SWE single transgenic mouse [14] we found that there was an age-dependent reduction in fEPSPs evoked by a fixed stimulus intensity over a wide range. The deficit was clearly present when synaptic transmission was compared with the amplitude of the presynaptic fibre volley and therefore reflects a reduction in the input-output relationship. This age-related decrease in basal synaptic transmission was similar in both Wt and DbTg mice, with little if any difference between the two groups at each age point. Similarly, Volianskis et al. [21] recently reported a similar age-related decrease in basal transmission in mice overexpressing APP695SWE and PS1ΔE9 transgenes, which was similar in both groups of animals. Consistent with previous studies [12, 14], the reduction in synaptic transmission was prevented by blockade of ionotropic glutamate receptors with kynurenic acid during slice preparation, suggesting that it was due to increased susceptibility to excitotoxicity, which may be a characteristic of the background mouse strain on which the transgenes are expressed. Interestingly, hippocampal cultures prepared from double transgenic animals (APPSWE and PS1L166P) show a reduction in excitatory synapses compared to cultures from wildtype or APPSWE mice, suggesting that the inclusion of the PS1 transgene may have an additional effect on glutamatergic synapse formation under some conditions [27].
Here we have shown that, even at 14 month of age, there is no deficit in basal transmission in DbTg compared to Wt animals. This is despite the fact that Aβ levels are greatly enhanced in these animals and plaques are also evident in the hippocampus and other brain regions (see [26]). Thus it appears that the increased production of Aβ in these mice does not impair basal synaptic function.
Effects on LTP
Previous studies using transgenic mouse modes of Alzheimer's disease have resulted in little consensus on the effects of mutations in APP and PS1 with respect to LTP. For example, a reduction of LTP has been reported in mice that over-express either the London (V642I; [28] or Swedish (APP695SWE; [12, 29] mutations in APP. In contrast, no impairment was observed in single transgenic mice that over-express the V717F mutant form of APP (APPInd; [30–32]) and in studies using the APP695SWE mutation [14, 15]. Both the ΔE9 [33] and A246E [16] mutations in PS1 have previously been shown to lead to enhanced LTP expression. Mice under-expressing PS1 have displayed reduced levels of LTP in one study [34] but normal LTP in another [35].
Few studies to date have been conducted using mice expressing double mutations in APP and PS1. One previous study reported an increased rate of decay in dentate gyrus LTP in vivo in 17-18 month old mice expressing the APP695SWE and PS1A246E, mutations [36], but normal CA1 LTP in vitro at this age. Two studies have found impairments in hippocampal LTP [37] and hippocampal-dependent learning [37, 38] in both aged wildtype and transgenic mice overexpressing mutated APP and PS1, suggesting that deficits were independent of Aβ or plaque load. Although impairments in CA1 LTP have been observed in mice expressing mutations in both APP and PS1 in some studies [19], [39], a recent study using mice expressing both APP695SWE and PS1ΔE9 found no deficit in hippocampal LTP in vitro at all ages studied (up to 12 months of age;[21]). In our study we were unable to detect any changes in LTP at CA1 synapses using two induction protocols (that employed respectively 40 and 400 stimuli). Of course, we cannot exclude the possibility that alterations in LTP may be observed with other patterns of activation or under different experimental conditions or in other pathways. Interestingly, a recent study has shown that deficits in LTP in vitro in mice overexpressing the Swedish mutant of APP are only seen if the animals are previously exposed to spatial training, not in naïve animals [40], suggesting that deficits in plasticity are subtle and subject to alterations based on prior experience. The primary conclusion, however, is that LTP is readily induced despite the pronounced and long-lasting increase in Aβ(42) and Aβ(40) levels in the hippocampus.
Alzheimer's disease is characterised by synaptic degeneration and changes in dendritic and axonal morphology [41–43]. Whilst these processes are not important for the earliest phase of LTP, they may become more important in the protein synthesis dependent phase of LTP. For this reason we extended our analysis of LTP beyond that which has been studied previously in slices obtained from transgenic models of Alzheimer's disease. However, we noticed little difference in LTP between the genotypes, even when followed for over 3 h post-induction. Theta patterns of activity are more physiological than tetanic stimulation and involve the activation of presynaptic GABAB receptors to transiently suppress GABA inhibition and thereby facilitate the activation of NMDA receptors [44]. Thus, LTP induced by theta patterns of activity may be more susceptible to regulatory influences, in particular those that affect GABA-mediated inhibition. In this context, we have previously observed that a deficit in LTP observed in APP null nice is normalised when GABAA receptor-mediated inhibition is blocked [45].
The finding that both basal synaptic transmission and LTP is normal in region CA1 of the hippocampus even when Aβ levels are greatly enhanced and plaques are present may be considered somewhat surprising, particularly as the presence of plaques would be expected to disrupt the neuronal organisation of this region. However, these findings are consistent with some of the previous studies utilising transgenic mice relevant to AD. This would suggest that synaptic physiology remains normal in situations where Aβ levels are elevated, at least in the CA1 region of the hippocampus. However, our experiments were performed without visualisation of plaques in the slices and thus we cannot rule out the possibility that the majority of our recording sites were in areas not containing plaques and that recording from regions neighbouring plaques may reveal a deficit in basal synaptic transmission and/or plasticity. This may also account for discrepancies between studies of transgenic mice expressing AD related proteins. Thus further experiments could target recordings from plaque containing regions. Ours and other studies investigating synaptic physiology from acutely prepared brain slices give an indication of transmission at individual or sets of synapses but do not provide information on the function of the hippocampus as a whole. If transmission and/or plasticity are impaired at only a fraction of the neurones in this structure then function (e.g. spatial learning) may be impaired.
Increasing evidence suggests that the form in which Aβ is present in the brain is crucial in terms of whether neuronal function and memory is impaired. Thus oligomers rather than monomers or fibrils of Aβ induce synapse loss and impair memory [46–48]. Aβ exists in the AD brain as a polydisperse mixture of high order oligomers and only these high molecular weight oligomers bind to hippocampal neurons [49]. Furthermore, cerebral injection of cell medium containing oligomers and monomers, but not fibrils, inhibits LTP in vivo [46]. As such the existence of Aβ oligomers rather then plaques may cause neuronal damage, and there is little correlation between the presence of plaques and severity of dementia in humans [50]. It may be, therefore, that although the mice used in the current study display Aβ plaques, they do not produce an oligomeric form of Aβ that is detrimental to neuronal health and function. Alternatively, it may be that a loss of normal APP function rather than an increase in Aβ may cause an impairment in LTP, as APP knockout mice show deficits in LTP [45], and so differences in secreted APP between transgenic strains, which can have trophic and neuroprotective effects [51], may lead to differences in synaptic transmission and plasticity.
Another possibility for our finding, a lack of effect on LTP in the DbTg mice, is that other factors may be required for generating AD-related impairments in hippocampal function, for example altered tau [52]. Consistent with this Roberson and colleagues have shown that reducing tau expression can affect amyloid related toxicity in transgenic mice without altering plaque deposition [53]. Human patients have additional pathology relative to transgenic mouse models utilising mutations in APP and PS1. In particular, although the mice show Aβ plaques, they do not display intracellular tangles of hyperphosphorylated tau protein [54]. Oddo and colleagues [55] generated a triple transgenic mouse expressing mutated APP (APPSWE), PS1 (M146V mutation) and tau (P301L mutation), which showed progressive development of both plaques and tangles as well as a reduction in hippocampal LTP. Such mice may represent a more robust model of AD.
Conclusions
In conclusion, it is clear from our present experiments that the cellular machinery for induction and expression of LTP at CA1 synapses is intact in these DbTg mice. This is apparent even in aged mice, which have been exposed to greatly elevated levels of Aβ peptides for over 6 months.
References
Hardy J: Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 1997, 20 (4): 154-159. 10.1016/S0166-2236(96)01030-2.
Price DL, Sisodia SS: Mutant genes in familial Alzheimer's disease and transgenic models. Annu Rev Neurosci. 1998, 21: 479-505. 10.1146/annurev.neuro.21.1.479.
Newman M, Musgrave IF, Lardelli M: Alzheimer disease: amyloidogenesis, the presenilins and animal models. Biochimica et biophysica acta. 2007, 1772 (3): 285-297.
Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ: Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992, 360 (6405): 672-674. 10.1038/360672a0.
Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al: Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995, 375 (6534): 754-760. 10.1038/375754a0.
Fraser PE, Yang DS, Yu G, Levesque L, Nishimura M, Arawaka S, Serpell LC, Rogaeva E, St George-Hyslop P: Presenilin structure, function and role in Alzheimer disease. Biochimica et biophysica acta. 2000, 1502 (1): 1-15.
Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, et al: Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nature medicine. 1997, 3 (1): 67-72. 10.1038/nm0197-67.
Zhang Z, Nadeau P, Song W, Donoviel D, Yuan M, Bernstein A, Yankner BA: Presenilins are required for gamma-secretase cleavage of beta-APP and transmembrane cleavage of Notch-1. Nat Cell Biol. 2000, 2 (7): 463-465. 10.1038/35017108.
Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G: Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science (New York, NY). 1996, 274 (5284): 99-102.
Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT: APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. JNeuropatholExpNeurol. 1997, 56 (9): 965-973.
Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM: Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998, 152 (1): 307-317.
Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, et al: Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nature neuroscience. 1999, 2 (3): 271-276. 10.1038/6374.
Fitzjohn SM, Doherty AJ, Collingridge GL: The use of the hippocampal slice preparation in the study of Alzheimer's disease. European journal of pharmacology. 2008, 585: 50-59. 10.1016/j.ejphar.2008.02.077.
Fitzjohn SM, Morton RA, Kuenzi F, Rosahl TW, Shearman M, Lewis H, Smith D, Reynolds DS, Davies CH, Collingridge GL, et al: Age-related impairment of synaptic transmission but normal long-term potentiation in transgenic mice that overexpress the human APP695SWE mutant form of amyloid precursor protein. Journal of Neuroscience. 2001, 21 (13): 4691-4698.
Brown JT, Richardson JC, Collingridge GL, Randall AD, Davies CH: Synaptic transmission and synchronous activity is disrupted in hippocampal slices taken from aged TAS10 mice. Hippocampus. 2005, 15 (1): 110-117. 10.1002/hipo.20036.
Parent A, Linden DJ, Sisodia SS, Borchelt DR: Synaptic transmission and hippocampal long-term potentiation in transgenic mice expressing FAD-linked presenilin 1. NeurobiolDis. 1999, 6 (1): 56-62. 10.1006/nbdi.1998.0207.
Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, et al: Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996, 17 (5): 1005-1013. 10.1016/S0896-6273(00)80230-5.
Duyckaerts C, Potier MC, Delatour B: Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008, 115 (1): 5-38. 10.1007/s00401-007-0312-8.
Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O: Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Annals of neurology. 2004, 55 (6): 801-814. 10.1002/ana.20101.
Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, Shirao T, Aoki C, Huerta PT: AMPA receptor downscaling at the onset of Alzheimer's disease pathology in double knockin mice. Proceedings of the National Academy of Sciences of the United States of America. 2006, 103 (9): 3410-3415. 10.1073/pnas.0507313103.
Volianskis A, Kostner R, Molgaard M, Hass S, Jensen MS: Episodic memory deficits are not related to altered glutamatergic synaptic transmission and plasticity in the CA1 hippocampus of the APPswe/PS1DeltaE9-deleted transgenic mice model of beta-amyloidosis. Neurobiology of aging. 2010, 31: 1173-1187. 10.1016/j.neurobiolaging.2008.08.005.
Hsiao KK, Borchelt DR, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younkin S, Price D, et al: Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron. 1995, 15 (5): 1203-1218. 10.1016/0896-6273(95)90107-8.
Qian S, Jiang P, Guan XM, Singh G, Trumbauer ME, Yu H, Chen HY, Van de Ploeg LH, Zheng H: Mutant human presenilin 1 protects presenilin 1 null mouse against embryonic lethality and elevates Abeta1-42/43 expression. Neuron. 1998, 20 (3): 611-617. 10.1016/S0896-6273(00)80999-X.
Kuenzi F, Rosahl TW, Morton RA, Fitzjohn SM, Collingridge GL, Seabrook GR: Hippocampal synaptic plasticity in mice carrying the rd mutation in the gene encoding cGMP phosphodiesterase type 6 (PDE6). Brain Res. 2003, 967 (1-2): 144-151. 10.1016/S0006-8993(02)04241-5.
Wimer RE, Wimer CC, Alameddine L, Cohen AJ: The mouse gene retinal degeneration (rd) may reduce the number of neurons present in the adult hippocampal dentate gyrus. Brain research. 1991, 547 (2): 275-278. 10.1016/0006-8993(91)90971-W.
Lewis HD, Beher D, Smith D, Hewson L, Cookson N, Reynolds DS, Dawson GR, Jiang M, Van der Ploeg LH, Qian S, et al: Novel aspects of accumulation dynamics and A beta composition in transgenic models of AD. Neurobiology of aging. 2004, 25 (9): 1175-1185. 10.1016/j.neurobiolaging.2003.12.009.
Priller C, Mitteregger G, Paluch S, Vassallo N, Staufenbiel M, Kretzschmar HA, Jucker M, Herms J: Excitatory synaptic transmission is depressed in cultured hippocampal neurons of APP/PS1 mice. Neurobiology of aging. 2009, 30 (8): 1227-1237. 10.1016/j.neurobiolaging.2007.10.016.
Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, et al: Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. JBiolChem. 1999, 274 (10): 6483-6492.
Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, et al: Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2006, 103 (13): 5161-5166. 10.1073/pnas.0600948103.
Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F: Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995, 373 (6514): 523-527. 10.1038/373523a0.
Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L: Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. ProcNatlAcadSciUSA. 1999, 96 (6): 3228-3233.
Larson J, Lynch G, Games D, Seubert P: Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res. 1999, 840 (1-2): 23-35. 10.1016/S0006-8993(99)01698-4.
Zaman SH, Parent A, Laskey A, Lee MK, Borchelt DR, Sisodia SS, Malinow R: Enhanced synaptic potentiation in transgenic mice expressing presenilin 1 familial Alzheimer's disease mutation is normalized with a benzodiazepine. NeurobiolDis. 2000, 7 (1): 54-63. 10.1006/nbdi.1999.0271.
Morton RA, Kuenzi FM, Fitzjohn SM, Rosahl TW, Smith D, Zheng H, Shearman M, Collingridge GL, Seabrook GR: Impairment in hippocampal long-term potentiation in mice under-expressing the Alzheimer's disease related gene presenilin-1. NeurosciLett. 2002, 319 (1): 37-40.
Yu H, Saura CA, Choi SY, Sun LD, Yang X, Handler M, Kawarabayashi T, Younkin L, Fedeles B, Wilson MA, et al: APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron. 2001, 31 (5): 713-726. 10.1016/S0896-6273(01)00417-2.
Gureviciene I, Ikonen S, Gurevicius K, Sarkaki A, van Groen T, Pussinen R, Ylinen A, Tanila H: Normal induction but accelerated decay of LTP in APP + PS1 transgenic mice. Neurobiology of disease. 2004, 15 (2): 188-195. 10.1016/j.nbd.2003.11.011.
Gruart A, Lopez-Ramos JC, Munoz MD, Delgado-Garcia JM: Aged wild-type and APP, PS1, and APP + PS1 mice present similar deficits in associative learning and synaptic plasticity independent of amyloid load. Neurobiology of disease. 2008, 30 (3): 439-450. 10.1016/j.nbd.2008.03.001.
Park SW, Ko HG, Lee N, Lee HR, Rim YS, Kim H, Lee K, Kaang BK: Aged wild-type littermates and APPswe+PS1/ΔE9 mice present similar deficits in associative learning and spatial memory independent of amyloid load. Genes & Genomics. 2010, 32: 63-70.
Gengler S, Hamilton A, Holscher C: Synaptic plasticity in the hippocampus of a APP/PS1 mouse model of Alzheimer's disease is impaired in old but not young mice. PLoS ONE. 5 (3): e9764-10.1371/journal.pone.0009764.
Middei S, Roberto A, Berretta N, Panico MB, Lista S, Bernardi G, Mercuri NB, Ammassari-Teule M, Nistico R: Learning discloses abnormal structural and functional plasticity at hippocampal synapses in the APP23 mouse model of Alzheimer's disease. Learning & memory (Cold Spring Harbor, NY. 2010, 17 (5): 236-240.
Knowles RB, Wyart C, Buldyrev SV, Cruz L, Urbanc B, Hasselmo ME, Stanley HE, Hyman BT: Plaque-induced neurite abnormalities: implications for disruption of neural networks in Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 1999, 96 (9): 5274-5279. 10.1073/pnas.96.9.5274.
Phinney AL, Deller T, Stalder M, Calhoun ME, Frotscher M, Sommer B, Staufenbiel M, Jucker M: Cerebral amyloid induces aberrant axonal sprouting and ectopic terminal formation in amyloid precursor protein transgenic mice. J Neurosci. 1999, 19 (19): 8552-8559.
Arendt T: Alzheimer's disease as a disorder of mechanisms underlying structural brain self-organization. Neuroscience. 2001, 102 (4): 723-765. 10.1016/S0306-4522(00)00516-9.
Davies CH, Collingridge GL: The physiological regulation of synaptic inhibition by GABAB autoreceptors in rat hippocampus. Journal of Physiology. 1993, 472: 245-265.
Fitzjohn SM, Morton RA, Kuenzi F, Davies CH, Seabrook GR, Collingridge GL: Similar levels of long-term potentiation in amyloid precursor protein -null and wild-type mice in the CA1 region of picrotoxin treated slices. Neuroscience letters. 2000, 288 (1): 9-12. 10.1016/S0304-3940(00)01204-0.
Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ: Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002, 416 (6880): 535-539. 10.1038/416535a.
Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH: A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006, 440 (7082): 352-357. 10.1038/nature04533.
Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL: Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007, 27 (11): 2866-2875. 10.1523/JNEUROSCI.4970-06.2007.
Hepler RW, Grimm KM, Nahas DD, Breese R, Dodson EC, Acton P, Keller PM, Yeager M, Wang H, Shughrue P, et al: Solution state characterization of amyloid beta-derived diffusible ligands. Biochemistry. 2006, 45 (51): 15157-15167. 10.1021/bi061850f.
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R: Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Annals of neurology. 1991, 30 (4): 572-580. 10.1002/ana.410300410.
Goodman Y, Mattson MP: Secreted forms of beta-amyloid precursor protein protect hippocampal neurons against amyloid beta-peptide-induced oxidative injury. Experimental neurology. 1994, 128 (1): 1-12. 10.1006/exnr.1994.1107.
Seabrook GR, Ray WJ, Shearman M, Hutton M: Beyond amyloid: the next generation of Alzheimer's disease therapeutics. Mol Interv. 2007, 7 (5): 261-270. 10.1124/mi.7.5.8.
Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L: Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science (New York, NY). 2007, 316 (5825): 750-754.
Ashe KH: Mechanisms of memory loss in Abeta and tau mouse models. Biochem Soc Trans. 2005, 33 (Pt 4): 591-594.
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM: Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003, 39 (3): 409-421. 10.1016/S0896-6273(03)00434-3.
Acknowledgements
We wish to thank David S. Reynolds for assistance in this project. The work was supported by a Medical Research Council (MRC) LINK award G9710681. The MRC was not involved in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Frederick Kuenzi, Thomas Rosahl, David Smith, Huw Lewis and Guy Seabrook were employees of Merck Research Laboratories when this work was carried out. There are no other conflicts of interest.
Authors' contributions
Conceived and designed the experiments: SMF, FK, RAM, TWR, GRS, GLC
Performed the experiments: FK, RAM, HL, DS, GRS
Analyzed the data: SMF, FK, RAM, GRS
Wrote the paper: SMF, FK, RAM, GRS, GLC.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Fitzjohn, S.M., Kuenzi, F., Morton, R.A. et al. A study of long-term potentiation in transgenic mice over-expressing mutant forms of both amyloid precursor protein and presenilin-1. Mol Brain 3, 21 (2010). https://doi.org/10.1186/1756-6606-3-21
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-6606-3-21