Abstract
Background
The red fox (Vulpes vulpes) is host to a community of zoonotic and other helminth species. Tracking their community structure and dynamics over decades is one way to monitor the long term risk of parasitic infectious diseases relevant to public and veterinary health.
Methods
We identified 17 helminth species from 136 foxes by mucosal scraping, centrifugal sedimentation/flotation and the washing and sieving technique. We applied rarefaction analysis to our samples and compared the resulting curve to the helminth community reported in literature 35 years ago.
Results
Fox helminth species significantly increased in number in the last 35 years (p-value <0.025). Toxascaris leonina, Mesocestoides litteratus, Trichuris vulpis and Angiostrongylus vasorum are four new veterinary-relevant species. The zoonotic fox tapeworm (E. multilocularis) was found outside the previously described endemic regions in the Netherlands.
Conclusions
Helminth fauna in Dutch red foxes increased in biodiversity over the last three decades.
Similar content being viewed by others
Background
Long-term studies on parasite communities of marine and terrestrial wildlife hosts were instrumental to evaluating the influence of natural and anthropogenic factors on environmental changes, especially when sampling series span more than ten years [1–3].
For larger mammals, like the red fox, many cross-sectional studies report on the parasitic helminth fauna [4–13] or focus on limited parasite species [10, 12, 14–19], but long-term studies are rare [9].
In the 1980's, Borgsteede [4] studied the helminth fauna in foxes from the border region in the eastern part of The Netherlands, collected between February 1978 and May 1979. For ensuing decades, this study has been the sole large scale surveillance of helminth fauna in red foxes in the Netherlands.
A series of additional large scale surveillance in red foxes became reality since the initial detection of Echinococcus multilocularis in the Netherlands in 1996 [20]. E. multilocularis tends to increase in the fox population over the last decades in Europe [21] and therefore, the European Food Safety Authority (EFSA) recommends monitoring this parasite in foxes, especially at the borders of its distribution area in Europe [22]. Following the initial detection in the Netherlands, E. multilocularis in foxes was found to disperse in southern Limburg, but not in the central and western part of the Netherlands [20]. Since the Netherlands are a densely populated country with an average human population density of 497/km2[23] and a pet population of around 1.5 million dogs [24], a high density of red foxes (0.5 to 4.0 per square kilometre) might potentially lead to exposure of humans and dogs to zoonotic parasites, like E. multilocularis[16].
Here, we compared our recent large-scale surveillance of helminth fauna in the population of red foxes from the border region in the eastern part of The Netherlands with the historic studies more than 35 years ago. We evaluated trends in parasite richness by applying the rarefaction analysis [25, 26]. In addition, we discuss the relevance of our findings for public health.
Methods
Animals
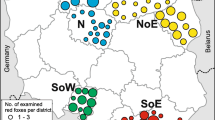
From October 2010 until April 2012, routinely shot foxes were collected by hunters and sent to the National Institute for Public Health and Environment (RIVM, Bilthoven, The Netherlands). The chosen fox sample size (288) originated from a strip with a width of 15 km and a length of 266 km at the border with Germany, between Groningen and Limburg (4000 km2), excluding the formerly found positive districts (Figure 1). Upon arrival, fox carcasses were stored at −80˚C to inactivate the eggs of E. multilocularis[27], according to WHO guidelines [28]. After a minimum period at −80˚C of one week, carcasses were thawed and dissected. Data on weight, measurements, age and gender were collected after thawing. From weight and body size, condition was estimated as the ratio of body weight in grams over body length (nose-anus) in millimetres (body weight/length index, BWL).
The age of the foxes was evaluated by examining tooth wear, especially the wear of the lower incisors and the upper and lower molars and by cutting the root of one or two canines into several 0.15 mm thin slices which were examined microscopically (magnification 20–40 times) under horizontal cross light [29]. Foxes without signs of wear were classified as first year animals [30].
During dissection, the jejunum and faecal material (if present) from the distal colon/rectum of each fox were sampled. The whole small intestines of 262 foxes were evaluated by microscopic examination of mucosal scrapings and macroscopic examination of the opened small intestine. Moreover, distal colon content was used for PCR (see E. multilocularis-specific PCR identification); 158 foxes had sufficient faecal content in the colon to be used for additional microscopic analysis after centrifugal sedimentation/flotation.
Microscopical examination of parasites
Small intestine mucosal scraping
The small intestine of each fox was separated and opened. Macroscopically visible helminths were scored and noted. Subsequently, mucosal scrapings were made to screen the mucosal content for small helminths microscopically [31, 32]. The presence of intestinal helminths was scored semi-quantitatively: ‘+’ 1–2 individuals, ‘++’ 3–10, ‘+++’ 11–50, ‘++++’ 51–100 and ‘+++++’ >100. Parasites were identified morphometrically and in cases where difficult to identify young adult stages were found, or the freezing/thawing process had damaged the morphology of cestode species, morphological identification was confirmed by PCR (see Molecular identification of parasites). For this purpose, parasite specimens were collected and stored in 70% ethanol until further use.
Sedimentation/flotation of gut content
When available, about 3 grams of distal colon content were suspended in 50 ml tap water, an 11 ml centrifuge tube was filled with this suspension and the product of centrifugal sedimentation/flotation was examined microscopically. A sucrose solution of 1.28-1.3 g/cm3 was used as flotation medium for the faecal examination of eggs and larvae. The centrifugal step for flotation was performed with the cover slip on top of the tube and one slide was examined per sample. The results were scored semi quantitatively using ‘+’ for 1–10 eggs per slide; higher numbers were scored as ’++’ for one to five per microscopic field at 100x (10x10) magnification and ‘+++’ for more than five per microscopic field at the same magnification.
Since fox carcasses were frozen to inactivate zoonotic parasites, the Baermann method could not be used to isolate first stage larvae of Crenosoma vulpis and Angiostrongylus vasorum. Larvae that were found by CSF, which were not too damaged by the freezing and thawing process were identified morphologically according to McGarry and Morgan [33].
Screening for cardio-pulmonary helminths
The lungs and hearts of 97 foxes were examined for helminths by opening the right heart and pulmonary arteries up to the level of small branches in the lungs [34]. The bronchi were opened, examined and washed with water, which was sieved through a 150 μm mesh size sieve. The same procedure was used for heart and vessels. Adult and juvenile worms were removed from the sieve and identified morphologically up to species level [35, 36].
Screening for helminths in the urinary bladder
In addition, four urinary bladders were opened to look for adult worms of Pearsonema plica.
Helminth species number
To evaluate a possible change in helminth species richness, we applied rarefaction analysis [25, 26] to the number of distinct helminth species that we identified in 136 foxes. We calculated the rarefaction curve with the software package EstimateS 9.0 [25, 26, 37] with default settings. Based on the rarefaction curve, we compared our findings with those of historical studies [4–6, 8, 9].
Foxes, for which biological parameters or geographical data were missing, were excluded from analysis. This limited the available dataset for multifactorial analysis to 136 foxes. For each parasite species, prevalence was calculated and significance of prevalence difference was analyzed with Fisher’s Exact test. Correlations between body condition, age, gender and parasite prevalence were determined by ANOVA (analysis of variance). Fisher’s exact test and ANOVA were performed and the resulting P-values were calculated using Quickcalc (GraphPad Software, Inc. La Jolla, California, USA) and the data analysis module of Microsoft Excel 2007.
E. multilocularis-specific PCR identification
To analyse the presence of E. multilocularis at sub-microscopical level, three grams of colon contents were tested in a single tube nested 12S ribosomal DNA PCR as described previously [20]. PCR products were specified by southern blot hybridization, using E. multilocularis- specific probes as described previously [38].
Molecular identification of parasites
DNA isolation and PCR
Parasites were transferred from 70% ethanol and soaked in demineralized water. DNA was isolated using the Qiagen Blood and Tissue Kit (Qiagen NV, Venlo, The Netherlands), according to the manufacturer’s instructions. To confirm the identification of cestode species, a fragment of the mitochondrial cytochrome c oxidase 1 (CO1) gene was amplified as described by Bowles et al. [39]. All PCRs were carried out in 50 μl final volume containing 3 μl genomic DNA, 0.5 μl of each forward and reverse primer (50 μM stock) and 25 μl of Qiagen HotstarTaq polymerase master mix (Qiagen NV, Venlo, The Netherlands ). The final reaction volume was adjusted to 50 μl with sterile demineralized water. PCR amplification of the partial CO1 gene was performed using the following conditions: denaturation at 95°C for 15 min, followed by 35 cycles of 1 min denaturation at 95°C, 1 min annealing at 45°C, 1:15 min elongation at 72°C, followed by a final extension step of 7 min at 72°C.
DNA sequencing of amplicons
PCR amplicons were purified using standard procedures (ExoSAP-IT®, Affymetrix, Cleveland, Ohio, USA). All DNA sequence PCR reactions were carried out on both DNA strands in 20 μl final volume containing 3 μl of amplicate, 7 μl sequence buffer, 1 μl of Big Dye Terminator and 1 μl of each PCR primer. Sequence PCR was performed under the following conditions: 95°C for 1 min, followed by 25 cycles of 96°C for 10 min, 50°C for 5 min and finally 60°C for 4 min. Trace files of the obtained sequences were generated on an automated ABI sequencer at the Institute’s DNA sequence facility.
DNA and phylogenetic analysis
DNA sequences were assembled, edited, and analysed with BioNumerics version 6.6 (Applied Maths NV, Sint-Martens-Latem, Belgium). Obtained CO1 gene sequences were compared to reference sequences present in Genbank after subtraction of the primer sequences. Cluster analysis of the sequences was conducted using the unweighted neighbour-joining algorithm of the BioNumerics program. Bootstrap proportions were calculated by the analysis of 2500 replicates for neighbour-joining trees. Available CO1 sequences of cestodes and trematodes from Genbank were included in the alignment. Sequence homology ≥99% and homology of morphological criteria were considered as proof of identity between isolated and Genbank species.
Unequivocally identified Alaria alata isolates from foxes from this study served as out-group in phylogenetic analysis.
Results
Animal age, gender and body weight
In total, 262 foxes were collected. Seventy per cent of the foxes were 7–12 months old at the time of sampling and seven foxes were older than 5 years. This age distribution of shot foxes indicates high hunting pressure as found in previous studies [30, 40].
Overall, 55% of the sampled foxes were males and 45% were females, which were evenly distributed over the study area (Figure 1). Males were heavier than females; average body weight / length (BWL) index of males and females differed significantly (ANOVA, P-value < 0.0001). Correlation between BWL index and infection classes was absent for both male (P-value = 0.626) and female foxes (P-value = 0.232).
Analysis of helminth species number
Seventeen helminth species were identified from our reference data set of 136 foxes. The 95% confidence interval was 14.39 – 19.61 parasite species. The number of parasite species in 137 foxes that were sampled 35 years ago [4] was twelve species, which is a significantly lower species richness (P-value < 0.025) (Figure 2).
Analysis of fox parasite species by rarefaction method. Open circle: the number of distinct parasite species identified from 136 Dutch foxes in this study. Solid circle: the number of distinct parasite species identified from the foxes described in a cited study. Solid line: expected number of distinct parasite species estimated by the rarefaction method based on our data set (i.e. open circle). Dotted line: 95% confidence interval. Nickel et al. [9] reported two independent fox populations from different regions, sampled in 1966 (green solid circle) and in 1980 (light green solid circle) respectively.
Multiple infections per fox
On average 97.1% of the foxes were infected with one or more out of 17 helminth species, with maximum co-infection levels of eight different species.
Foxes younger than 10 months were more frequently infected (35-37%) with 2–3 parasite species than foxes older than 10 months (10-27%) (Figure 3).
Number of co-infections per age group and per gender. Male foxes peak at three to four co-infections, females nine months of age and younger peak at two to three co-infections. Male foxes exhibit the highest numbers of co-infection (8). Zero co-infections mean no infection at all. Total number of foxes is 136.
Prevalence per helminth species and comparison with other studies
Parasite prevalence was higher in male foxes for the majority of the parasite species (Table 1), although this was only significant for Toxocara canis (Fisher’s Exact test, P = 0.013). T. canis and U. stenocephala were the most prevalent intestinal fox parasites in our study, like in other Western European countries [5–7, 14–16]. The prevalences of T. canis and Taenia spp. were significantly lower in this study compared to the earlier study of Borgsteede [4] (Table 2).
The combined prevalence of Toxocara canis and Toxascaris leonina reported in Belgian foxes in 2005 [16] was not different (Fisher’s Exact test, P = 0.315) from the prevalence in our study. The prevalence of T. canis in Danish foxes in 2006 [6] was 59.4%, which is almost identical to the level found in this present study, as was the case for Taenia species. In contrast, the prevalence of Uncinaria stenocephala was significantly higher in Denmark [6], compared to either our data (Fisher’s Exact test, P = 0.0018), historical data from northern Germany [5] (Fisher’s Exact test, P = 0.002), or historical data from the Netherlands [4] (Fisher’s Exact test, P = 0.054).
The prevalences of Strongyloides sp., Eucoleus aerophilus and Crenosoma vulpis was significantly higher than reported in 1984 [4] (Table 2). Trichuris vulpis, Angiostrongylus vasorum, Mesocestoides litteratus and Echinococcus multilocularis were new species in the studied area. The trematode Apophallus donicus, of which one individual was found by Borgsteede [4] was not identified in the present study. This was also the case for Hymenolepis spp., for which rodents are definitive hosts. Adult Hymenolepids are regarded as passing species from prey, as is Molineus patens, and these were thus excluded from analysis of helminth species parasitic to red fox.
E. Multilocularis-specific PCR identification
All foxes were negative for this species by microscopical examination of mucosal scrapings, but one fox out of 262 investigated foxes was positive for E. multilocularis (prevalence 0.7%; 95% CI 0.02-2.1%), using the 12S single tube nested PCR and subsequent southern blot analysis on faecal content. This positive result was confirmed after repeated testing of the faecal content. Up to this study, no positive foxes were identified in the presently studied area.
Molecular characterisation of intestinal parasites
PCR products of Taenia polyacantha, Taenia crassiceps and Alaria alata were all 403 bp in length. These DNA sequences were submitted to Genbank [accession numbers KF751222-KF751223 (T. crassiceps, isolates V1382 and V1336), KF751225-KF751226 (T. polyacantha, V1361 and V1269) and KF751233-KF751234 (A. alata, V1338 and V1359)].
Microscopic identification of cestodes was confirmed by cluster analysis of the partial CO1 gene sequences. The inferred Neighbour Joining tree shows very high homology between obtained CO1 sequences and Genbank entries for T. crassiceps from Russia and Norway (EU544548, EU544547), T. polyacantha from Denmark and Finland (EU544583, EU544584) and for the trematode A. alata from Lithuania and Germany (HM022221, HM022222 and HM022224), the latter of which served as outgroup (Figure 4).
CO1 Neighbour Joining Tree of European fox cestode isolates. Taenia species found in red fox (* this study) show high homology with other European isolates found in Genbank (bootstrap values of 2500 simulations). Alaria alata is used as outgroup and here too, the Dutch isolates show high homology with other European isolates from Genbank. Bar indicates base substitutions per site.
Discussion
This study shows an increased diversity in the helminth parasite community of Dutch red foxes compared to a study conducted in the same region 35 years ago [4]. We report four new records of veterinary importance: Toxascaris leonina, Mesocestoides litteratus, Trichuris vulpis and Angiostrongylus vasorum. The finding of a fifth (zoonotic) species –Echinococcus multilocularis– has been described earlier for the Netherlands [20], but not in this same geographical area.
We used a combination of microscopic and molecular techniques to evaluate the helminth fauna of red fox as described above, whereas Borgsteede [4] and Lucius et al. [5] used microscopy following the washing and sieving technique. Use of the more sensitive PCR technique in this present study might have biased the observed biodiversity to some extent, since it was not available in the period of the study of Borgsteede [4], but this does not explain the observed biodiversity increase compared to older studies. Confirmation of the identity of cestode species that had been found microscopically by PCR in this present study, did not lead to more cestode species compared to historic data. Moreover, even without E. multilocularis, which was demonstrated only by PCR, significantly more helminth species were found in this present study, compared to historical data (result not shown). The introduction of E. multilocularis and A. vasorum into the Netherlands is documented [20, 38, 41]; these independent studies support the increased biodiversity of helminth fauna in the population of red foxes in the Netherlands. The study of van der Giessen et al. [20], for which a combination of mucosal scraping and PCR was used, demonstrated presence of E. multilocularis in the eastern border region, both north and south to the present study area, but not in the latter, which was included in that study as well. This finding confirmed the observation of Borgsteede [4] at that time.
Parasites indicated as Capillaria spp. might include more fox specific species, like Eucoleus boehmi, which is endemic to the Netherlands (H. Cremers, unpublished data), and other species passing through the gut after predation; however these were not further identified to species level.
Rarefaction and extrapolation of parasite richness and abundance data (this study) revealed a significant increase of species richness compared to 12 different fox parasite species determined by Borgsteede [4], 11 species found by Lucius et al. [5] and 9–12 species found in two regions of the former German Democratic Republic respectively in 1966 and in 1980 [9]. Recent studies in the Northern European hemisphere [6, 8] show species richness that fits the asymptotic maximum of the estimated species richness calculated from our data. This increase might be driven by a combination of natural developments and or anthropogenic causes (global warming, climatic fluctuations). It is however, beyond the scope of this paper to identify the drivers for the observed increase in the parasite biodiversity.
Parasites of veterinary importance may be introduced into the environment through pet travel or translocation of wildlife hosts. Angiostrongylus vasorum only recently became endemic to the Netherlands [41] and is known for its endemic foci in Dutch dogs [41]. In the present study, we found A. vasorum-positive foxes in the southern half of the study area, outside and distant from the published endemic foci, which demonstrates a wider endemic area sustained by the red fox.
In this study, E. multilocularis parasite DNA was identified by PCR in the intestinal content of one red fox in the northern part of the Dutch-German border area. The identification based solely on molecular techniques suggests a very low intestinal abundance in the infected fox, well below the detection level of microscopy. Previous studies showed PCR to be more sensitive, compared to the mucosal scraping method, especially at low endemicity [20, 42].
The observed T. canis prevalence decline in foxes (−17%) is also recognised in the human population, since data from a Dutch cohort study show a moderate but significant decrease of T. canis exposure between 1998 and 2004 [43]. However, this is not recognised in prevalence of patent infections in dogs [44–47].
The prevalence of Taenia spp. showed the sharpest decline (−59%), followed by T. canis (−17%), compared to the study by Borgsteede [4]. Among fox prey are rodents, which are obligate intermediate hosts in the lifecycle of cestode parasites like E. multilocularis and Taenia spp., and facultative intermediate hosts of nematodes like T. canis. Small mammals, especially voles (Microtus arvalis and Arvicola terrestris), comprise almost 50% of the fox’s prey during autumn and winter [30, 48, 49]. The decreasing prevalence of Taenia spp. and T. canis in foxes might be correlated with the decreasing abundance of rodents [50, 51], which is also indicated by decline of raptor species exclusively preying on rodents [52, 53].
We were able to identify Taenia crassiceps and T. polyacantha from frozen material, using morphological data in combination with molecular techniques. A combination of detection techniques as presented in this study might be useful to increase sensitivity and specificity and to differentiate host-specific parasites from parasite eggs and/or larvae passing after ingestion of prey. CO1 gene sequences of A. alata, T. crassiceps and T. polyacantha from Dutch fox (this study) were homologous with isolates from European countries at the North or East of the Netherlands (Germany, Denmark, Lithuania, Finland and Russia). Previously, spatial prevalence analysis across borders demonstrated radiation of E. multilocularis, from the adjacent Belgian fox population to the southern Dutch fox population [20, 54].
In conclusion, we infer a significant increase in parasitic helminths diversity in the fox population at the eastern border of the Netherlands over a period of 35 years. In the same period, the prevalence of two zoonotic helminths species belonging to different genera declined. In addition, four veterinary-important species were identified for the first time in this present study, and three additional species showed higher prevalence over that period. We identified the fox tapeworm E. multilocularis for the first time outside the previously described endemic spots in the Netherlands. Due to the very low prevalence and abundance, the infection risk for humans in the studied area is considered limited. It remains important, however, to follow the spread of E. multilocularis in this area in the future.
References
Spratt DM: Helminth communities in small mammals in southeastern New South Wales. Int J Parasitol. 1987, 17 (1): 197-202. 10.1016/0020-7519(87)90041-5.
Haukisalmi V, Henttonen H: The impact of climatic factors and host density on the long-term population dynamics of vole helminths. Oecologia. 1990, 83: 309-315.
Dzikowski R, Paperna I, Diamant A: Use of fish parasite species richness indices in analyzing anthropogenically impacted coastal marine ecosystems. Helgol Mar Res. 2003, 57: 220-227. 10.1007/s10152-003-0138-2.
Borgsteede FH: Helminth parasites of wild foxes (Vulpes vulpes L.) in The Netherlands. Z Parasitenkd. 1984, 70 (3): 281-285. 10.1007/BF00927813.
Lucius R, Böckeler W, Pfeiffer AS: Parasieten der Haus-, Nutz-, und Wildtiere Schleswich-Holsteins: Parasiten der innere Organen des Rotfuchses (Vulpes vulpes). Z Jagdwiss. 1988, 34: 242-255.
Saeed I, Maddox-Hyttel C, Monrad J, Kapel CM: Helminths of red foxes (Vulpes vulpes) in Denmark. Vet Parasitol. 2006, 139 (1–3): 168-179.
Eira C, Vingada J, Torres J, Miquel J: The helminth community of the red fox, Vulpes vulpes, in Dunas de Mira (Portugal) and its effect on host condition. Wildl Biol Pract. 2006, 2 (1): 26-36.
Bruzinskaite-Schmidhalter R, Sarkunas M, Malakauskas A, Mathis A, Torgerson PR, Deplazes P: Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology. 2012, 139 (1): 120-127. 10.1017/S0031182011001715.
Nickel S, Hiepe T, Hansel U, Jurke E: Beitraege zur Parasitenfauna der DDR. 5. Mitteilung. Untersuchung zum Helminthenvorkommen beim Rotfuchs (Vulpes vulpes L.). AngewParasitol. 1980, 21: 94-100.
Richards DT, Harris S, Lewis JW: Epidemiological studies on intestinal helminth parasites of rural and urban red foxes (Vulpes vulpes) in the United Kingdom. Vet Parasitol. 1995, 59 (1): 39-51. 10.1016/0304-4017(94)00736-V.
Gortazar C, Villafuerte R, Lucientes J, Fernandez-de-Luco D: Habitat related differences in helminth parasites of red foxes in the Ebro valley. Vet Parasitol. 1998, 80 (1): 75-81. 10.1016/S0304-4017(98)00192-7.
Rajkovic-Janje R, Marinculic A, Bosnic S, Benic M: Prevalence and seasonal distribution of helminth parasites in red fox (Vulpes vulpes) from the Zagreb County (Croatia). Z Jagdwiss. 2002, 48: 151-160.
Magi M, Macchioni F, Dell'omodarme M, Prati MC, Calderini P, Gabrielli S, Iori A, Cancrini G: Endoparasites of red fox (Vulpes vulpes) in central Italy. J Wildl Dis. 2009, 45 (3): 881-885. 10.7589/0090-3558-45.3.881.
Criado-Fornelio A, Gutierrez-Garcia L, Rodriguez-Caabeiro F, Reus-Garcia E, Roldan-Soriano MA, Diaz-Sanchez MA: A parasitological survey of wild red foxes (Vulpes vulpes) from the province of Guadalajara. Spain. Vet Parasitol. 2000, 92 (4): 245-251. 10.1016/S0304-4017(00)00329-0.
Wolfe A, Hogan S, Maguire D, Fitzpatrick C, Vaughan L, Wall D, Hayden TJ, Mulcahy G: Red fox (Vulpes vulpes) in Ireland as hosts of parasites of potential zoonotic and veterinary significance. Vet Rec. 2001, 149: 759-763.
Vervaeke M, Dorny P, De Bruyn L, Vercammen F, Jordaens K, van den Berge K, Verhagen R: A survey of intestinal helminths of red fox (Vulpes vulpes) in northern Belgium. Acta Parasitol. 2005, 50 (3): 221-227.
Letkova V, Lazar P, Curlik J, Goldova M, Kocisova A, Kosuthova L, Mojzisova J: The red fox (Vulpes vulpes) as a source of zoonoses. Vet Archiv. 2006, 76 (suppl): S73-S81.
Barabási SS, Fok E, Gubányi A, Mészáros F, Cozma V: Helminth fauna of the small intestine in the European red fox, Vulpes vulpes with notes on the morphologicalidentification of Echinococcus multilocularis. Sci Parasitol. 2010, 11 (3): 141-151.
Suchentrunk F, Sattmann H: Prevalence of intestinal helminths in Austrian Red Foxes. Ann Naturhist Mus Wien. 1994, 96B: 29-38.
van der Giessen JW, Rombout YB, Franchimont JH, Limper LP, Homan WL: Detection of Echinococcus multilocularis in foxes in The Netherlands. Vet Parasitol. 1999, 82 (1): 49-57. 10.1016/S0304-4017(98)00263-5.
Hegglin D, Deplazes P: Control of Echinococcus multilocularis: strategies, feasibility and cost-benefit analyses. Int J Parasitol. 2013, 43 (5): 327-337. 10.1016/j.ijpara.2012.11.013.
EFSA: Development of harmonized schemes for the monitoring and reporting of Echinococcus in animals and foodstuffs in the European Union. European Food Safety Authority. 2010,http://www.efsa.europa.eu/en/supporting/doc/36e.pdf,
CBS P, Wageningen UR: Compendium voor de leefomgeving. 2013,www.compendiumvoordeleefomgeving.nl,
Borst N, Megens TPA, Overgaauw PAM, Teurlings MMEM: Feiten & Cijfers Gezelschapsdierensector. 2011,http://issuu.com/hasdenboschinternational/docs/feiten___cijfers_van_de_gezelschapsdierensector_20,
Gotelli NJ, Colwell RK: Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett. 2001, 4: 379-391. 10.1046/j.1461-0248.2001.00230.x.
Colwell RK, Chao A, Gotelli NJ, Lin S, Mao CX, Chazdon RL, Longino JT: Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol. 2012, 5 (1): 3-21. 10.1093/jpe/rtr044.
Veit P, Bilger B, Schad V, Schafer J, Frank W, Lucius R: Influence of environmental factors on the infectivity of Echinococcus multilocularis eggs. Parasitology. 1995, 110 (Pt 1): 79-86.
WHO: Guidelines for surveillance, prevention and control of Echinococcosis/hydatidosis. Edited by: Eckert J, Gemmell MA, Matyas Z, Soulsby EJL. 1984, Geneva: WHO, 2
Grue H, Jensen B: Review of the formation of incremental lines in tooth cementum of terrestrial mammals. Dan Rev Game Biol. 1979, 11 (3): 1-48.
Mulder J, Jansman HAH, Van Der Giessen JWB: Ecologisch onderzoek aan geschoten vossen in Zuid-Limburg, 2002–2003 met aanbevelingen voor het beheer van de vossenpopulatie in relatie tot hamsterpredatie [Ecological research on foxes shot in South Limburg, 2002–2003 with recommendations for managing the fox population in relation to hamster predation] Dutch. De Bilt: Bureau Mulder-natuurlijk. 2004, Available from: www.limburg.nl/dsresource?objectid=4791&type=org
Deplazes P, Eckert J: Diagnosis of the Echinococcus multilocularis infection in final hosts. Applied Parasitology. 1996, 37: 245-252.
Eckert J, Deplazes P, Craig PS, Gemmell MA, Gottstein B, Heath D, Jenkins DJ, Kamiya M, Lightowlers M: Echinococcosis in animals: clinical aspects, diagnosis and treatment. WHO/OIE Manual on echinococcosis in humans and animals: a public health problem of global concern. Edited by: Eckert J, Gemmell MA, Meslin F-X, Pawlowski ZS. 2001, World Organisation for Animal Health and World Health Organisation, 73-100. Available from: http://whqlibdoc.who.int/publications/2001/929044522X.pdf
McGarry JW, Morgan ER: Identification of first-stage larvae of metastrongyles from dogs. Vet Rec. 2009, 165 (9): 258-261. 10.1136/vr.165.9.258.
Bourque ACCG, Miller LM, Whitney H: Pathological findings in dogs naturally infected with Angiostrongylus vasorum in Newfoundland and Labrador. Canada. J Vet Diagn Invest. 2008, 20 (1): 11-20. 10.1177/104063870802000103.
Morgan ER, Tomlinson A, Hunter S, Nichols T, Roberts E, Fox MT, Taylor MA: Angiostrongylus vasorum and Eucoleus aerophilus in foxes (Vulpes vulpes) in Great Britain. Vet Parasitol. 2008, 154 (1–2): 48-57.
Rosen L, Ash LR, Wallace GD: Life history of the canine lungworm Angiostrongylus vasorum (Baillet). Am J Vet Res. 1970, 31 (1): 131-143.
Colwell RK: EstimateS 9.0 User's Guide Last. 2013, http://purl.oclc.org/estimates. 2013
van der Giessen JW, Rombout Y, Teunis P: Base line prevalence and spatial distribution of Echinococcus multilocularis in a newly recognized endemic area in the Netherlands. Vet Parasitol. 2004, 119 (1): 27-35. 10.1016/j.vetpar.2003.11.001.
Bowles J, Blair D, McManus DP: Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol. 1992, 54 (2): 165-173. 10.1016/0166-6851(92)90109-W.
Mulder J: De vos in Nederland [Red fox in The Netherlands]. Naar een effectief en geaccepteerd vossenbeheer [To an effective and generally accepted fox population control] Report of the symposium on the red fox at 12 May 2004 in Utrecht. Edited by: Mulder JL, Van Apeldoorn RC, Klok C. 2005, Utrecht: Faunafonds, 15-21.
Van Doorn DC, van de Sande AH, Nijsse ER, Eysker M, Ploeger HW: Autochthonous Angiostrongylus vasorum infection in dogs in The Netherlands. Vet Parasitol. 2009, 162 (1–2): 163-166.
Takumi K, De Vries A, Chu ML, Mulder J, Teunis P, van der Giessen J: Evidence for an increasing presence of Echinococcus multilocularis in foxes in The Netherlands. Int J Parasitol. 2008, 38 (5): 571-578. 10.1016/j.ijpara.2007.09.014.
Pinelli E, Herremans T, Harms MG, Hoek D, Kortbeek LM: Toxocara and Ascaris seropositivity among patients suspected of visceral and ocular larva migrans in the Netherlands: trends from 1998 to 2009. Eur J Clin Microbiol Infect Dis. 2011, 30 (7): 873-879. 10.1007/s10096-011-1170-9.
Rep BH: Roundworm infection (Toxocara and Toxascaris) in dogs in the Netherlands. Tijdschr Diergeneesk. 1980, 105 (7): 282-289.
Overgaauw PA: Prevalence of intestinal nematodes of dogs and cats in the Netherlands. Vet Quart. 1997, 19 (1): 14-17. 10.1080/01652176.1997.9694730.
Le Nobel WE, Robben SRM, Döpfer D, Hendrikx WML, Boersema JH, Franssen F, Eysker M: Infections with endoparasites in dogs in Dutch animal shelters [in Dutch]. Tijdschr Diergeneesk. 2004, 129 (2): 40-44.
Overgaauw PA, Van Zutphen L, Hoek D, Yaya FO, Roelfsema J, Pinelli E, Van Knapen F, Kortbeek LM: Zoonotic parasites in faecal samples and fur from dogs and cats in The Netherlands. Vet Parasitol. 2009, 163 (1–2): 115-122.
Klanszki J, Körmendi S, Hancz C, Zalewski A: Feeding habits and trophic niche overlap in a carnivora community in Hungary. Acta Theriol. 1999, 44 (4): 429-442.
Voigt DR: Red Fox in: Wild Furbearer Management and conservation in North America, Section IV: species biology, management, and conservation. Chapter. 1999, 30: 379-392. Available from: http://publicdocs.mnr.gov.on.ca/view.asp?document_id=10160
Van Apeldoorn AC: Alterra-rapport 1234. Muizenplagen in Nederland: oorzaken en bestrijding [in Dutch]. 2005, Wageningen: Alterra
Broekhuizen S, Hoekstra B, Van Laar V, Smeenk C, Thissen JBM: Atlas van de Nederlandse zoogdieren. Stichting Uitgeverij van de Koninklijke Nederlandse Natuurhistorische Vereniging III ISBN: 90-5011-051-7 [in Dutch]. 1992
Herremans M: Wordt het bidden voor de Torenvalk (Is the Kestrel hovering on the brink?) [in Dutch, with abstract in English and French]. Natuur.oriolus. 2011, 77 (2): 60-67.
Bijlsma RG: Trends en broedresultaten van roofvogels in Nederland in 2010 (Raptor breeding succes in The Netherlands in 2010) [in Dutch with summary in English]. Takkeling. 2011, 19 (1): 6-51.
Vervaeke M, van der Giessen J, Brochier B, Losson B, Jordaens K, Verhagen R, Coulander Cde L, Teunis P: Spatial spreading of Echinococcus multilocularis in Red foxes (Vulpes vulpes) across nation borders in Western Europe. Prev Vet Med. 2006, 76 (3–4): 137-150.
Dillard KJ, Saari SA, Anttila M: Strongyloides stercoralis infection in a Finnish kennel. Acta Vet Scand. 2007, 49: 37-10.1186/1751-0147-49-37.
Acknowledgements
This study was partially financed by the Dutch Food Safety Organisation (NVWA). The authors thank Merel Langelaar, Marieke Opsteegh and Manoj Fonville for their valuable contribution to the study coordination, dissection of foxes and the parasitological examinations. We are also thankful to Margriet Montizaan of the Dutch Hunters Association (KNJV) and hunters for providing foxes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they do not have competing interests.
Authors’ contributions
FF generated and analysed parasitological data, performed molecular lab work and sequence analysis, and wrote the manuscript, RN generated parasitological data and wrote the manuscript, JM generated biological data concerning the collected foxes, HC generated parasitological data concerning non-intestinal helminths, CD did the molecular lab work concerning E. multilocularis, KT wrote the study design and manuscript, and helped with statistical analysis, JvdG wrote the study design, conceived and wrote the project proposal, coordinated the study, generated parasitological data and contributed to the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Franssen, F., Nijsse, R., Mulder, J. et al. Increase in number of helminth species from Dutch red foxes over a 35-year period. Parasites Vectors 7, 166 (2014). https://doi.org/10.1186/1756-3305-7-166
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-3305-7-166