Abstract
Co-infection of tuberculosis and parasitic diseases in humans is an important public problem in co-endemic areas in developing countries. However, there is a paucity of studies on co-infection and even fewer reviews. This review examines 44 appropriate papers by PRISMA from 289 papers searched in PubMed via the NCBI Entrez system (no grey literature) up to December 2012 in order to analyze the factors that influence epidemic and host’s immunity of co-infection. The limited evidence in this review indicates that most common parasite species are concurrent with Mycobacterium tuberculosis in multiple organs; socio-demographics such as gender and age, special populations with susceptibility such as renal transplant recipients, patients on maintenance haemodialysis, HIV positive patients and migrants, and living in or coming from co-endemic areas are all likely to have an impact on co-infection. Pulmonary tuberculosis and parasitic diseases were shown to be risk factors for each other. Co-infection may significantly inhibit the host’s immune system, increase antibacterial therapy intolerance and be detrimental to the prognosis of the disease; in addition, infection with parasitic diseases can alter the protective immune response to Bacillus Calmette-Guerin vaccination against Mycobacterium tuberculosis.
Similar content being viewed by others
Introduction
Rationale
Both tuberculosis (TB) and parasitic diseases in humans are infectious diseases that exhibit an extensive distribution, causing serious harm to humans. The World Health Organization Special Programme for Research and Training in Tropical Diseases (WHO TDR) provided the TDR disease portfolio in 1999 to deal with the deterioration in the health situation, including leprosy, TB and eight kinds of parasitic diseases, such as malaria, schistosomiasis, etc. [1]. WHO estimated that there was about one third of the global population infected by TB, and in 2010, there were an estimated 8.8 million incident cases of TB globally, mostly occurring in Asia (59%) and Africa (26%) [2]. Meanwhile, in 2009 WHO also reported that there were an estimated 225 million malaria cases, mainly distributed in Africa (78%), South-East Asia (15%) and the Eastern Mediterranean (5%) [3]. In 2012, there were an estimated 436 million people at risk of Schistosomiasis haematobium infection in Sub-Saharan Africa, of which 112 million were infected, with an estimated 393 million people at risk of Schistosomiasis mansoni infection, of which 54 million were infected [4]; an estimated 120 million people in tropical and subtropical areas of the world were infected with lymphatic filariasis in 2009 [5]. These figures suggest that there is an overlap of endemic regions between TB and parasitic disease, which may lead to co-infection of these diseases in the population.
The earliest report we found was from 1945 and interpreted how to treat a pulmonary TB (PTB) case running concurrently with malaria [6]. A report from 1946 described co-existence of TB with hookworm [7]. The co-infection of TB and parasitic diseases have been reported in many studies for almost the past 70 years, although great achievements have been gained in the fields of TB and parasitic disease control and prevention respectively [2–5]. Up to 2012, some cases of co-infection between TB and parasitic diseases were reported around the world [8–29], and some epidemiological surveys of co-infection in hospitals or communities were carried out [30–34]. Some of these studies showed that the immune response was modified in the co-infection situation [35–51].
Inevitably, co-infection would increase the complexity of control and prevention on TB and parasitic diseases. The current systematic reviews on the co-infection of TB and parasitic diseases help to clarify the complexity of co-infections; however, there are only a few systematic reviews on co-infection. We only found that Enwere et al.[35] reviewed the host response of co-infection between TB and malaria and 4 reviews focused on the influence of chronic helminth infections on immunity against TB [44, 45, 47, 48].
Objectives
This paper reviewed studies globally over the past 70 years on the co-infection of TB and parasitic diseases using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [52], in order to learn more about which parasites are concurrent with TB, the epidemiological situation regarding co-infection, and the human immune function affected by co-infection.
Methods
Protocol and registration
We did not register the protocol for this review.
Eligibility criteria
Published articles were included if they involved case reports of co-infection with TB and any parasitic diseases in human participants, or epidemiological surveys of co-infection in populations, or clinical or laboratory research on the immune responses during co-infection, were eligible for inclusion in the systematic review. Journal articles published with full text or abstracts in English before 2013 were eligible for inclusion.
Information sources
We mainly searched PubMed via the NCBI Entrez system (any date to December, 2012) (http://www.ncbi.nlm.nih.gov) for studies on the association between TB and parasitic diseases. We also searched bibliographies of identified reports, including previous reviews, for additional references.
Search
The search was limited to studies of human beings or animal models and no language limits were applied. The terms that were used as MeSH terms or Direct keywords for the search and our search strategy are described in Table 1. All titles, abstracts and full texts from each of the searches were examined and reviewed.
Study selection
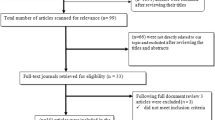
The study selection process is illustrated in Figure 1.
Papers that were not co-infection studies, or were not for humans, or were published in non-English language without an abstract in English, were excluded. Eligible papers were tabulated.
Data collection process
Two reviewers extracted data from each eligible study independently, and differences were resolved by discussion with a third. Extracted data was tabulated on the basis of data items. No formal meta-analysis was carried out and analysis to investigate statistical heterogeneity or publication bias was not performed because most of the studies were case reports and immunological research, and there were very few epidemiological surveys.
Data items
Data extracted included country, year, co-infection of diseases, sex, age, human immunodeficiency virus (HIV) test and medical history for case reports. For epidemiological surveys, the detailed information included was country, year, survey site, number of population screened, number of people with PTB, number of people with parasitic disease, number of people co-infected and prevalence. For clinical or laboratory research on immunity during co-infection, we collected detailed information on country, year, co-infection of disease, subject and conclusions.
Summary measures
Risk ratios (RR) and their 95% confidence intervals (CI) were calculated using the Statistical Analysis System (SAS 9.2; SAS Institute Inc., Cary, NC, USA) through Chi square test. All P-values of <0.05 were taken as statistically significant.
Results
Two hundred and eighty nine papers were retrieved from the search of published work, of which, 10 were excluded because of duplication and 235 were irrelevant to co-infection with TB and parasitic diseases. Finally, 44 studies of co-infection were included in the analysis, of which, 22 were case reports, 5 were epidemiological surveys and 17 were immunological research/reviews (Figure). There was no grey literature included.
Table 2 summarizes the case reports of co-infection. Twenty-four cases were reported in 22 studies from 13 countries during 1984 to 2012, in which 7 cases were from India. Fourteen studies investigated co-infection with PTB and parasites diseases and 8 studies involved the extrapulmonary TB, such as renal TB, lymph node TB, abdominal TB, TB in liver, tuberculous lymphadenitis and TB verrucosa cutis. The youngest case reported was 34 days old and the eldest case was 82 years old, and the median age of all cases reported was 36 years old. Fifteen of a total 24 cases were male. Ten patients with different clinical conditions co-infected with TB and leishmaniasis were reported, of which, visceral leishmaniasis was the highest. There were also 6 cases co-infected with TB and hydatid disease. In addition, parasitic diseases accompanied by TB included trichomoniasis, malaria, toxoplasmosis, toxocariasis, schistosomiasis, strongyloidiasis and filarial elephantiasis in different organs. Fourteen of a total of 24 cases reported had been tested for HIV test and 4 were positive.
It can be seen in Table 3 that 5 studies from 3 East African countries and 1 East Asian country were conducted for epidemiology of co-infection between TB and parasitic diseases during 1984 to 2012. Two studies screened across the general population with 382 and 782 participants in a community and a hospital respectively, one study showed 329 and 215 PTB patients in two hospitals respectively, one study showed 309 PTB patients who were HIV positive and 346 who were HIV negative in a hospital, in the other study, there were 112 smear positive TB patients in a town, whose parasite species involved Entamoeba histolytica, Leishmania donovani, Giardia lamblia, malaria, Clonorchis sinensis, Schistosoma mansoni, taenia species, Ascaris lumbricoides, Trichocephalus trichiurus, hookworm and Strongyloides stercoralis. The prevalence of co-infection between TB and parasitic diseases varied widely among different participants, s species or survey sites.
The interrelationship of infection with TB and parasitic diseases have been evaluated in Table 4 through two studies screened in the general population, with 382 and 782 participants in a community and a hospital respectively. Those who were Leishmania donovani positive, Giardia lamblia positive or Strongyloides stercoralis positive more easily suffered from PTB than those who were negative and RR values (95% CI) were 1.73 (1.14-2.62), 1.80 (1.10-2.93) and 3.11 (1.53-6.35), respectively. Likewise, persons with PTB were more easily infected by Leishmania donovani, Giardia lamblia or Strongyloides stercoralis than persons without PTB and RR values (95% CI) were 1.24 (1.08-1.43), 1.86 (1.09-3.16) and 4.26 (1.42-12.77), respectively.
Immunological research of co-infection between TB and parasitic diseases during 1989 to 2012 are shown in Table 5. In four studies that reported a change of activity of the host’s immune system when the course of TB was aggravated by opisthorchiasis invasion, the activity increased at the acute stage of invasion and decreased at the subacute stage or in the chronization. A study from France showed that co-infecton of American cutaneous leishmaniasis, lepromatous leprosy and PTB downregulated the T-helper (Th) 1 cell response. Four studies on co-infection with Mycobacterium tuberculosis (M. TB) and malaria showed that malarial parasites decreased the host’s effective humoral and cellular immune responses to M. TB, and co-infection exacerbated chronic TB, suggesting a competitive antagonist effect between heat shock protein 70 (HSP70) from M. TB and adenosine triphosphate-binding protein (ATPBP) of malaria may exist. Two studies suggested that the impact of co-infection between filarial infection and M. TB infection on the immune response was uncertain. Four studies reported immunomodulation characteristics of co-infection beween TB and intestinal helminths. A study from China indicated that as the echinococcosis chronicity increased, the immune profile in TB patients changed from a Th1 to Th2 response. In three other studies in which the effect of infection of intestinal parasites and Schistosoma mansoni on the protective immune response to Bacillus Calmette-Guerin (BCG) vaccination against M. TB and the effect of malaria infection on the effectiveness of novel TB vaccines in protecting against TB were evaluated, the protective efficacy of BCG vaccination was reduced but the effectiveness of novel TB vaccines was unaffected.
Discussion
This review only found 24 cases of co-infection with TB and parasitic diseases but they distributed widely in 13 countries and covered PTB, extrapulmonary TB, and diseases caused by protozoa and helminths [8–29]. We also found 5 epidemiological studies in which prevalence of co-infection varied widely [30–34], and found that it was evident in 18 studies that the activity of the host’s immune system was altered during co-infection happened [27, 34–42, 44–51]. Therefore, assessing co-infection characteristics, influencing factors and impact on immunity is important to control and prevention whether for TB or parasitic diseases.
About 17 parasite genera concurrent with M. TB were reviewed as case reports, epidemiological surveys and immunological research, which were divided into protozoa (Entamoeba[32, 49], Leishmania[8–16, 31], Giardia lamblia[28, 32], Trichomonas[17], Plasmodium[18, 33, 35, 37, 38], Toxoplasma[19–21]) and helminths (Clonorchis sinensis[30], Opisthorchis[39, 40], Schistosoma[22, 33, 34], Taenia[32], Echinococcus[23–27], Ascaris lumbricoides[32, 34, 49], Toxocara[20], Trichuris trichiura[30, 32, 34], Ancylostoma[32–34], Strongyloides stercoralis[28, 32, 34, 49], Filaria[29, 43, 44]) and covered most of the common parasites species. Of those, leishmaniasis, hydatid disease and malaria were reported to coexist more freuequently with TB in the human body. In addition to co-infection of TB and a single parasitic disease, there was also co-infection of TB and multiple parasitic diseases, such as co-infection of pneumocystis carinii pneumonia, visceral leishmaniasis and PTB, co-infection of toxoplasmosis, toxocariasis and TB, and co-infection of strongyloidiasis, giardiasis and TB [9, 20, 28].
Possibly gender differences exist in co-infection of TB and different parasitic diseases. Among ten cases reported to be co-infected with TB and leishmaniasis, only two cases were female [8–16], however, only one of six cases co-infected with TB and hydatid disease was male [23–27]. In spite of these limited data without epidemiological evidence, we can infer that different social roles associated by gender possibly result in different probabilities of contacting different parasites resulting in different incidence and prevalence. An epidemiological survey in subtropical Ecuador suggested that male gender was one of risk factors associated with cutaneous leishmaniasis [53]. Contrarily, a population-based study in the Hamar of Ethiopia indicated that hydatid disease was a public health problem for women [54]. Irrefutably, gender differentials also exist in the prevalence of TB [55].
Although no significant age clustering was found in 23 cases of co-infecion who had a huge age span ranging from 34 days old to 82 years old [8–29], however, there were fewer reported cases of less than 20 years old and more than 60 years old. Likewise, this limited data were not epidemiological results. Nevertheless, age differentials of co-infection also exist and should not be a neglected factor. A cross-sectional household survey in Sudan showed that percentages of leishmania skin tests and tuberculin positivity by age group increased [31], which possibly reflects that with the increasing age, not only does the immune response increase following exposure to infection but also opportunities of exposure to infection increase gradually.
Among TB patients, prevalence of parasitic disease varies widely in different areas and different survey sites. TB and parasitic disease co-infection is common in clinical practice in East Africa. In Sudan, up to 77% of TB patients were positive for the leishmania skin test in the community [31]; 32% of TB patients from hospitals had intestinal parasites and 29% of TB patients from the community had intestinal helminths in Ethiopia [32, 34]; and 32.4-38.2% of TB patients from hospitals were also infected with Schistosomes in Tanzania [33]. We also noticed the prevalence differentials in Korea, where the infection rates of Trichocephalus trichiurus and Clonorchis sinensis were 20.7% and 17.6% respectively among TB patients in one hospital, but the infection rates were 6.5% and 6.0% respectively in another hospital [30]. Among the PTB patients from a hospital in Tanzania, the HIV-positive patients had a significantly lower prevalence of hookworm and Schistosome infection and a higher prevalence of malaria than the HIV-negative patients [33], which indicates HIV also has an impact on prevalence of co-infection.
Therefore, it is no surprise to find many factors that possibly affect co-infection of TB and parasitic diseases. First, socio-demographics, such as gender and age as previously mentioned above, maybe relate to prevalence of co-infection. Second, some special patients, such as renal transplant recipients, patients on maintenance haemodialysis, HIV positive patients and migrants [8, 10–12, 14, 16, 21, 22, 28, 33], are likely to be susceptible populations of co-infection. Last but not least, there are higher probabilities of co-infection in some areas with a higher prevalence of TB and parasitic diseases, such as India and East Africa [11, 12, 16, 18, 23, 29, 31–34]. These might provide useful information to control and prevent co-infection of TB and parasites under the background of very few epidemiological surveys for co-infection to date.
It was observed that PTB and parasitic diseases were risk factors for each other. We analyzed the data of an epidemiological survey in Sudan and found that Leishmaniasis patients had a 1.73 times higher risk for PTB than individuals without Leishmania and PTB patients had a 1.24 times higher risk for Leishmaniasis than individuals without PTB [31]. We also found data from an epidemiological survey in Ethiopia that Giardiasis and Strongyloidiasis patients all had higher risks for PTB than persons without Giardia lamblia and Strongyloides stercoralis, respectively, and PTB patients had a higher risk for Giardiasis and Strongyloidiasis than persons without PTB [32].
In comparison with mono-infection, co-infection makes the host’s immune system have to deal with a more complex internal environment. The usual manifestation is that clinical signs might become more frequent and serious and therapy may be affected when TB patients are also infected by parasites. Both clinical and laboratory based studies showed that when the course of TB was aggravated by opisthorchiasis invasion, clinical signs of TB became more pronounced, disorders in the functions of the liver and pancreas became more frequent, antibacterial therapy intolerance increased and prognosis of the disease deteriorated [39, 40]. In order to clarify these situations, some studies were conducted to evaluate the immuno-pathogenesis of co-infection. Limited data from two studies showed that the immune response of TB patients increased at the acute stage of opisthorchiasis invasion and decreased at the subacute stage or in the chronization [41, 42]. Two studies indicated that malarial parasites decreased the hosts effective humoral and cellular immune responses to M. TB [35, 36], and another study using an experimantal animal model showed co-infection between TB and malaria exacerbated chronic TB while rendering mice less refractory to Plasmodium[38]. Another study found that with the increase of echinococcosis chronicity, the immune profile in TB patients displayed an elevated Th2 immune reponse with subsequent suppression of the Th1 immune response [27]. Although one study suggested chronic filarial infections do not exacerbate M. TB infection [43], a review pointed out that filarial infections very clearly alter the magnitude and quality of the mycobacteria-specific cytokine response [44]. Generally, it is observed that the host’s immune system is inhibitited to a great extent during co-infection.
Some investigations observed the change of levels of immune indexes during co-infection. A study on co-infection of PTB and opisthorchiasis found that among co-infected patients, activity of the α1-proteinase inhibitor was more frequently higher and carriers of two markers i.e. Hp 2–2 and Gc 1–1 were more frequent [39]. A study on triple infection of leishmaniasis, TB and leprosy showed that patients were unable to mount a Th cell response to upregulate the interleukin-12 receptor expression after stimulation of the triple infection [14]. The investigation of co-infection between TB and intestinal helminths suggested that compared to either TB patients or healthy controls, the absolute frequencies of CD3+, CD4+, CD8+, Natural killer T cell and CD4+CD25high T cell increased in co-infected patients [46]. Indeed, the change of immune index levels reflects a ‘weakened’ immune response.
It is worthwhile evaluating the impact of parasitic disease infection on the efficacy of BCG vaccination against M. TB. Ferreira et al.[49] and Elias et al.[50] found that intestinal parasitic infections might significantly alter the protective immune response to BCG vaccination and/or polarize the general immune response to the Th2 profile since Th2-like interleukin-10 responses induced by intestinal parasites might interfere in the BCG-induced Th1-like interferon-γ response. Therefore, in areas of high prevalence of co-infection, anti-parasitic chemotherapy prior to immunization may greatly enhance the efficacy of BCG vaccination.
Conclusion
This review has found limited evidence of factors that influence epidemic and host’s immunity of co-infection between TB and parasitic disease in humans. Most of the common parasites species are concurrent with M. TB in multiple organs, which increase antibacterial therapy intolerance and deteriorate prognosis of disease. Socio-demographics such as gender and age, special populations with susceptibility, such as renal transplant recipients, patient on maintenance haemodialysis, HIV positive patients and migrants, and living in or coming from co-endemic areas likely have impacts on co-infection. PTB and parasitic diseases were shown to be risk factors for each other. Co-infection may inhibit the hosts immune system to a great extent. In addition, infection with parasites can alter the protective immune response to BCG vaccination against M. TB.
References
UNDP, World Bank: WHO Special Programme for Research and Training in Tropical Diseases (TDR): Tropical Disease Research: Progress 1999–2000. 2001, Geneva, Switzerland: World Health Organization Press, WHO/TDR/GEN/01.5,
World Health Organization: Global tuberculosis control: WHO report 2011. 2011, Geneva, Switzerland: World Health Organization Press, WHO/HTM/TB/2011.16
World Health Organization: World malaria report: 2010. 2010, Geneva, Switzerland: World Health Organization Press
World Health Organization: Schistosomiasis: progress report 2001–2011, strategic plan 2012–2020. 2013, Geneva, Switzerland: World Health Organization Press
World Health Organization: Progress report 2000–2009 and strategic plan 2010–2020 of the global programme to eliminate lymphatic filariasis: halfway towards eliminating lymphatic filariasis. 2010, Geneva, Switzerland: World Health Organization Press, WHO/HTM/NTD/PCT/2010.6
Chatterjee Bahadur GC: How to treat pulmonary tuberculosis case running concurrently with malaria. Indian Med Rec. 1945, 65: 260-263.
Black TC: Coexistent hookworm and tuberculosis. South Med J. 1946, 39: 881-884. 10.1097/00007611-194611000-00007.
Peces R, de la Torre M, Alcazar R: Visceral leishmaniasis and renal tuberculosis in a patient on maintenance haemodialysis. Nephrol Dial Transplant. 1996, 11: 707-708. 10.1093/oxfordjournals.ndt.a027367.
Toledo AC, de Castro MR: Pneumocystis carinii pneumonia, pulmonary tuberculosis and visceral leishmaniasis in an adult HIV negative patient. Braz J Infect Dis. 2001, 5: 154-157.
Ersoy A, Gullulu M, Usta M, Ozcelik T, Ylmaz E, Uzaslan EK, Vuruskan H, Yavuz M, Oktay B, Dilek K, Yurtkuran M: A renal transplant recipient with pulmonary tuberculosis and visceral leishmaniasis: review of superimposed infections and therapy approaches. Clin Nephrol. 2003, 60: 289-294.
Pandey K, Sinha PK, Ravidas VN, Kumar N, Verma N, Lal CS, Bimal S, Sur D, Bhattacharya SK: Nexus of infection with human immunodeficiency virus, pulmonary tuberculosis and visceral leishmaniasis: a case report from Bihar, India. Am J Trop Med Hyg. 2005, 72: 30-32.
Das VN, Pandey K, Kumar N, Hassan SM, Bimal S, Lal CS, Siddiqui NA, Bhattacharya SK: Visceral leishmaniasis and tuberculosis in patients with HIV co-infection. Southeast Asian J Trop Med Public Health. 2006, 37: 18-21.
Escobar MA, Saravia NG, Weigle KA: Concurrent mucosal leishmaniasis and pulmonary tuberculosis. Clin Infect Dis. 1996, 23: 836-837. 10.1093/clinids/23.4.836.
Delobel P, Launois P, Djossou F, Sainte-Marie D, Pradinaud R: American cutaneous leishmaniasis, lepromatous leprosy, and pulmonary tuberculosis coinfection with downregulation of the T-helper 1 cell response. Clin Infect Dis. 2003, 37: 628-633. 10.1086/376632.
Rathnayake D, Ranawake RR, Sirimanna G, Siriwardhane Y, Karunaweera N, De Silva R: Co-infection of mucosal leishmaniasis and extra pulmonary tuberculosis in a patient with inherent immune deficiency. Int J Dermatol. 2010, 49: 549-551. 10.1111/j.1365-4632.2010.04376.x.
Das VN, Pandey K, Verma N, Bimal S, Lal CS, Singh D, Das P: Post-kala-azar dermal leishmaniasis (PKDL), HIV and pulmonary tuberculosis. Natl Med J India. 2010, 23: 88-89.
Duboucher C, Farto-Bensasson F, Cheron M, Peltier JY, Beaufils F, Perie G: Lymph node infection by Trichomonas tenax: report of a case with co-infection by Mycobacterium tuberculosis. Hum Pathol. 2000, 31: 1317-1321. 10.1053/hupa.2000.18502.
Thapa R, Mallick D, Biswas B: Perinatal malaria and tuberculosis co-infection: a case report. Int J Infect Dis. 2010, 14: e254-e256.
Schroeder HW, Yarrish RL, Perkins TF, Lee C: Sequential disseminated tuberculosis and toxoplasmosis in a Haitian refugee. South Med J. 1984, 77: 533-534. 10.1097/00007611-198404000-00037.
Guneratne R, Mendis D, Bandara T, Fernando SD: Toxoplasma, toxocara and tuberculosis co-infection in a four year old child. BMC Pediatr. 2011, 11: 44-10.1186/1471-2431-11-44.
Hwang EH, Ahn PG, Lee DM, Kim HS: Cerebral toxoplasmosis combined with disseminated tuberculosis. Journal of Korean Neurosurgical Society. 2012, 51: 316-319. 10.3340/jkns.2012.51.5.316.
Torresi J, Sievert W: Hepatosplenic schistosomiasis presenting as granulomatous hepatitis in an immigrant from the Philippines with pulmonary tuberculosis, tuberculous lymphadenitis, and a history of alcohol abuse. J Travel Med. 2001, 8: 216-218.
Karande SC, Sheth SS, Lahiri KR, Shah MD: Coexistent hydatid disease and pulmonary tuberculosis in a five year old girl. J Assoc Physicians India. 1991, 39: 353-354.
Zamani A, Aydemir Y, Gormus N, Odev K, Solak H: Cardiac hydatid cyst in a patient with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2002, 6: 1033-1034.
Chen SY, Du XL, Yan W: A case of liver multiple hydatidosis complicated by tuberculosis in liver and abdominal cavity. Zhonghua Gan Zang Bing Za Zhi. 2004, 12: 359-
Saeed MY, Ahmed AH, Elhassan NB, Elhassan AM: Concomitant tuberculosis and hydatid cyst in a solitary pulmonary nodule of left lower lobe. BMJ Case Rep. 2009, 2009: bcr04.2009.1738-
Yang YR, Gray DJ, Ellis MK, Yang SK, Craig PS, McManus DP: Human cases of simultaneous echinococcosis and tuberculosis - significance and extent in China. Parasit Vectors. 2009, 2: 53-10.1186/1756-3305-2-53.
Dwarakanath AD, Welton M, Ellis CJ, Allan RN: Interrelation of strongyloidiasis and tuberculosis. Gut. 1994, 35: 1001-1003. 10.1136/gut.35.7.1001.
Kumar MV, Kaviarasan PK, Thappa DM, Jaisankar JJ: Tuberculosis verrucosa cutis complicating tropical elephantiasis. Indian J Dermatol Venereol Leprol. 2001, 67: 49-51.
Choi WY, Yoo JE, Kim WG, Yun BH, Kim SG, Yoo WH: Prevalence of intestinal helminthic infections and skin tests for paragonimus and clonorchis in tuberculosis patients. Kisaengchunghak Chapchi. 1984, 22: 209-214.
El-Safi SH, Hamid N, Omer A, Abdel-Haleem A, Hammad A, Kareem HG, Boelaert M: Infection rates with Leishmania donovani and Mycobacterium tuberculosis in a village in eastern Sudan. Trop Med Int Health. 2004, 9: 1305-1311. 10.1111/j.1365-3156.2004.01337.x.
Manuel Ramos J, Reyes F, Tesfamariam A: Intestinal parasites in adults admitted to a rural Ethiopian hospital: Relationship to tuberculosis and malaria. Scand J Infect Dis. 2006, 38: 460-462. 10.1080/00365540500525187.
Range N, Magnussen P, Mugomela A, Malenganisho W, Changalucha J, Temu MM, Mngara J, Krarup H, Friis H, Andersen AB: HIV and parasitic co-infections in tuberculosis patients: a cross-sectional study in Mwanza, Tanzania. Ann Trop Med Parasitol. 2007, 101: 343-351. 10.1179/136485907X176373.
Abate E, Belayneh M, Gelaw A, Idh J, Getachew A, Alemu S, Diro E, Fikre N, Britton S, Elias D: The impact of asymptomatic helminth co-infection in patients with newly diagnosed tuberculosis in north-west Ethiopia. PLoS One. 2012, 7: e42901-10.1371/journal.pone.0042901.
Enwere GC, Ota MO, Obaro SK: The host response in malaria and depression of defence against tuberculosis. Ann Trop Med Parasitol. 1999, 93: 669-678. 10.1080/00034989957907.
Scott CP, Kumar N, Bishai WR, Manabe YC: Short report: modulation of Mycobacterium tuberculosis infection by Plasmodium in the murine model. Am J Trop Med Hyg. 2004, 70: 144-148.
Wiwanitkit V: Co-infection between tuberculosis and malaria: A consideration on interaction of molecules and pathogenesis. J Vector Borne Dis. 2006, 43: 195-197.
Mueller AK, Behrends J, Hagens K, Mahlo J, Schaible UE, Schneider BE: Natural transmission of Plasmodium berghei exacerbates chronic tuberculosis in an experimental co-infection model. PLoS One. 2012, 7: e48110-10.1371/journal.pone.0048110.
Vasil’ev AV, Shenderova RI, Ginzburg ZI, Vasil’ev VI: Tuberculosis of the lungs complicated by opisthorchiasis under conditions of the extreme north. Probl Tuberk. 1989, 6: 41-44.
Kashuba EA, Rusakova LI: Anthelmintic therapy of opisthorchiasis in patients with active tuberculosis. Probl Tuberk. 1992, 3-4: 33-36.
Kalenova LF, Zolotukhin VA, Kashuba EA, Kostina GI, Postnikova TF: The formation of host-parasite relationships in an experimental mixed pathology of opisthorchiasis-tuberculosis as dependent on the phase of the Opisthorchis infestation. Zh Mikrobiol Epidemiol Immunobiol. 1992, 4: 20-22.
Kashuba EA, Podkletnova LF, Stepanova TF, Chebysheva EV: Functioning of opisthorchiasis-tuberculosis parasitocenosis at different stages of invasion (experimental investigation). Zh Mikrobiol Epidemiol Immunobiol. 2003, 1: 17-23.
Hubner MP, Killoran KE, Rajnik M, Wilson S, Yim KC, Torrero MN, Morris CP, Nikonenko B, Blanco JC, Hemming VG, Mitre E: Chronic helminth infection does not exacerbate Mycobacterium tuberculosis infection. PLoS Negl Trop Dis. 2012, 6: e1970-10.1371/journal.pntd.0001970.
Metenou S, Babu S, Nutman TB: Impact of filarial infections on coincident intracellular pathogens: Mycobacterium tuberculosis and Plasmodium falciparum. Curr Opin HIV AIDS. 2012, 7: 231-238. 10.1097/COH.0b013e3283522c3d.
Elias D, Britton S, Kassu A, Akuffo H: Chronic helminth infections may negatively influence immunity against tuberculosis and other diseases of public health importance. Expert Rev Anti Infect Ther. 2007, 5: 475-484. 10.1586/14787210.5.3.475.
Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R: Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol. 2007, 147: 45-52.
Mendez-Samperio P: Immunological mechanisms by which concomitant helminth infections predispose to the development of human tuberculosis. Korean J Parasitol. 2012, 50: 281-286. 10.3347/kjp.2012.50.4.281.
Rafi W, Ribeiro-Rodrigues R, Ellner JJ, Salgame P: Coinfection-helminthes and tuberculosis. Curr Opin HIV AIDS. 2012, 7: 239-244. 10.1097/COH.0b013e3283524dc5.
Ferreira AP, Aguiar AS, Fava MW, Correa JO, Teixeira FM, Teixeira HC: Can the efficacy of bacille calmette-guerin tuberculosis vaccine be affected by intestinal parasitic infections?. J Infect D. 2002, 186: 441-442. 10.1086/341656. author reply 442–443
Elias D, Akuffo H, Pawlowski A, Haile M, Schon T, Britton S: Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine. 2005, 23: 1326-1334. 10.1016/j.vaccine.2004.09.038.
Parra M, Derrick SC, Yang A, Tian J, Kolibab K, Oakley M, Perera LP, Jacobs WR, Kumar S, Morris SL: Malaria infections do not compromise vaccine-induced immunity against tuberculosis in mice. PLoS One. 2011, 6: e28164-10.1371/journal.pone.0028164.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D: The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009, 6: e1000100-10.1371/journal.pmed.1000100.
Armijos RX, Weigel MM, Izurieta R, Racines J, Zurita C, Herrera W, Vega M: The epidemiology of cutaneous leishmaniasis in subtropical Ecuador. Trop Med Int Health. 1997, 2: 140-152. 10.1046/j.1365-3156.1997.d01-236.x.
Klungsoyr P, Courtright P, Hendrikson TH: Hydatid disease in the Hamar of Ethiopia: a public health problem for women. Trans R Soc Trop Med Hyg. 1993, 87: 254-255. 10.1016/0035-9203(93)90114-6.
Sharma PP, Kumar A, Singh P: A study of gender differentials in the prevalence of tuberculosis based on NFHS-2 and NFHS-3 data. Indian J Community Med. 2010, 35: 230-237. 10.4103/0970-0218.66869.
Acknowledgement
This work is supported in part by National S & T Major Program (grant no. 2012ZX10004-220).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declare that they have no conflicts of interest.
Authors’ contributions
XZ and XL conceived and designed the review; XL conducted the review of the literature, extracted the pertinent data, performed analysis of data, and wrote the first draft of the manuscript; XZ provided strategic advice and assisted with editing of the manuscript. All authors read and approved the final version of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Li, XX., Zhou, XN. Co-infection of tuberculosis and parasitic diseases in humans: a systematic review. Parasites Vectors 6, 79 (2013). https://doi.org/10.1186/1756-3305-6-79
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-3305-6-79