Abstract
Background
Anopheles albimanus is a key malaria vector in the northern neotropics. Current vector control measures in the region are based on mass distributions of long-lasting insecticidal nets (LLINs) and focal indoor residual spraying (IRS) with pyrethroids. Resistance to pyrethroid insecticides can be mediated by increased esterase and/or multi-function oxidase activity and/or mutations in the voltage-gated sodium channel gene. The aim of this work was to characterize the homologous kdr region of the voltage-gated sodium channel gene in An. albimanus and to conduct a preliminary retrospective analysis of field samples collected in the 1990’s, coinciding with a time of intense pyrethroid application related to agricultural and public health insect control in the region.
Methods
Degenerate primers were designed to amplify the homologous kdr region in a pyrethroid-susceptible laboratory strain (Sanarate) of An. albimanus. Subsequently, a more specific primer pair was used to amplify and sequence the region that contains the 1014 codon associated with pyrethroid resistance in other Anopheles spp. (L1014F, L1014S or L1014C).
Results
Direct sequencing of the PCR products confirmed the presence of the susceptible kdr allele in the Sanarate strain (L1014) and the presence of homozygous-resistant kdr alleles in field-collected individuals from Mexico (L1014F), Nicaragua (L1014C) and Costa Rica (L1014C).
Conclusions
For the first time, the kdr region in An. albimanus is described. Furthermore, molecular evidence suggests the presence of kdr-type resistance in field-collected An. albimanus in Mesoamerica in the 1990s. Further research is needed to conclusively determine an association between the genotypes and resistant phenotypes, and to what extent they may compromise current vector control efforts.
Similar content being viewed by others
Background
Anopheles albimanus is one of the key malaria vectors of Latin America and is widely distributed throughout the region [1, 2]. In recent years, insecticide resistance has emerged in malaria vectors worldwide as a result of increased intensity of insecticide use, principally via the widespread use of indoor residual spraying (IRS) and long-lasting insecticidal nets (LLINs) in malaria endemic areas [3–5]. Malaria control in the region currently relies heavily on the use of LLINs, which are treated with pyrethroid insecticides [6]. The widespread use of insecticide treated nets (ITNs) [7–11], LLINs [12–14] and both the historical and ongoing use of DDT and pyrethroid insecticides for IRS [13, 15–17] elicit selection pressures on local vector populations. As such, the routine surveillance of insecticide resistance must be implemented in the context of vector control programs to verify that control tools are maintaining their efficacy. The timely detection of insecticide resistance and the characterization of the mechanisms underlying insecticide resistance in a vector population can provide valuable data regarding which insecticides should be used to maintain maximum vector control impact.
Resistance to pyrethroid insecticides in malaria vectors can be primarily mediated by either metabolic mechanisms or target site insensitivity, such as mutations on the voltage-gated sodium channel (VGSC) gene [3, 18]. Despite reports of pyrethroid resistance throughout the region, none of these mechanisms have been well-described at the molecular level for malaria vectors in Latin America [19]. Previous studies using biochemical assays and bioassays with synergists on pyrethroid resistant An. albimanus from Guatemala and Mexico suggest that an increase in the activity levels of esterases and multi-function oxidases are at least partially responsible for the resistance detected in these populations [20–24]. Elevated oxidase activity has been associated with cross-resistance to pyrethroids and DDT in An. albimanus[23]. One previous study carried out on An. albimanus from Mexico suggested that a target-site mechanism may be involved in cross-resistance between pyrethroids and DDT [25]. Knock-down resistance (kdr) is a target-site mechanism reported in other anopheline species that results in cross-resistance to both pyrethroids and DDT [26, 27]. In anophelines, kdr is linked to single nucleotide polymorphisms on transmembrane segment 6 of domain II of the VGSC gene. The mutations previously reported for anophelines occur on codon 1014, resulting in an amino acid change of leucine to phenylalanine, serine or cysteine [28–34]. To date, similar mutations have not been described in An. albimanus.
The present study describes for the first time the homologous kdr region of the VGSC gene in An. albimanus where mutations in other anopheline species have been detected that are associated with kdr-type resistance. Further, we report molecular evidence of kdr resistant-type alleles in field mosquitoes collected in Mexico, Nicaragua and Costa Rica in the 1990s.
Methods
Primer design
DNA and cDNA sequences of the VGSC gene of different Anopheles spp. were retrieved from GenBank (Table 1). Conserved regions were identified from a multiple alignment (MEGA 5.0 [35]) and degenerate primers were designed based on conserved codons using An. punctipennis as a basis [GenBank: AY283039-AY283041]. The strategy used to design the primers to amplify the VGSC gene in An. albimanus is presented in Figure 1A.
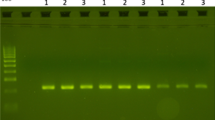
Strategy to amplify segment 6 of domain II of the VGSC gene in Anopheles albimanus . (A) Diagrammatic representation of the design of degenerate and specific primers for An. albimanus [GenBank: KF137581] based on An. gambiae [GenBank: Y13592] and An. punctipennis [GenBank: AY283041]. The identical positions are indicated by an asterisk and mutation site is enclosed by a box. Intron position is indicated by a black line below the sequence. AAKDRF (5′-AGATGGAAYTTYACNGAYTTC-′3); AAKDRF2 (5′-CATTCATTTATGATTGTGTTTCGTG-′3); AAKDRR (5′-GCAANGCTAAGAANAGRTTNAG-′3). (B) PCR products using degenerate and specific primers. The PCR products were separated on a 2% agarose gel containing ethidium bromide. Lane 1: 50 bp DNA ladder (Novagen); Lane 2: degenerate PCR products (using AAKDRF and AAKDRR primers); Lane 3: negative control of degenerate PCR (H2O); Lane 4: specific PCR product (using AAKDRF2 and AAKDRR primers); Lane 5: negative control of specific PCR (H2O).
Mosquito population
The An. albimanus Sanarate laboratory strain, maintained in the insectary of Center for Health Studies (CHS) of Universidad del Valle de Guatemala (Guatemala, Guatemala) was used to validate the designed primers. The Sanarate strain is susceptible to DDT, deltamethrin, permethrin, bendiocarb and malathion (unpublished observations) according to bottle bioassay susceptibility tests [36]. Genomic DNA from individual mosquitoes was isolated following the method described by Collins et al. [37].
Amplification, cloning and sequencing of the VGSC gene
The amplification of segment 6 of domain II of the VGSC gene with degenerate primers was carried out in a 50 μl reaction mixture containing 1X Colorless GoTaq® Flexi Buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 2.5 μM of each degenerate primer (AAKDRF and AAKDRR), 1 unit of GoTaq® HotStart Polymerase (Promega, Fitchburg, Wisconsin) and 10 to 30 ng of genomic DNA. The degenerate PCR conditions were 95°C for 3 min, followed by 35 cycles of 95°C for 45 sec, 40.5°C for 45 sec and 72°C for 1 min with a final extension step at 72°C for 5 min in a Px2 Thermal Cycler (Thermo Fisher Scientific, Waltham, Massachusetts).
Non-specific amplification was obtained in An. albimanus from the Sanarate strain using the degenerate primers (Figure 1B). Four different-sized PCR products were isolated for specific amplification using the band-stab PCR technique [38]. These purified PCR products were directly sequenced by Macrogen Inc. (Korea) using AAKDRF and AAKDRR as sequencing primers. BLAST analysis showed that a fragment of approximately 250 bp corresponded to the VGSC gene in An. albimanus. To confirm these findings and to obtain a high-quality DNA sequence of this fragment, PCR products were cloned using a TA Cloning® Kit (Invitrogen, Carlsbad, California) according to the manufacturer’s instructions. The plasmids of the positive clones that contained the fragment of VGSC gene were isolated with the PureLink™ HQ Mini Plasmid Purification Kit (Invitrogen, Carlsbad, California) according to the manufacturer’s instructions. Plasmids were sequenced with M13 universal primers using 3500XL Genetic Analyzer (Applied Biosystems, Foster City, California) with BigDye® Terminator v1.1.
PCR assay to detect kdr-type resistance
A second, non-degenerate forward primer (AAKDRF2) was designed based on the sequence of the VGSC gene of An. albimanus (GenBank: KF137581) obtained with the degenerate primers (Figure 1A). The amplification with the specific forward (AAKDRF2) and AAKDRR primer was performed using the same reaction specifications as in the degenerate PCR, except that 0.5 μM of each primer were used. The PCR conditions consisted of an initial denaturation at 95°C for 3 min, followed by 40 cycles at 95°C for 45 sec, 51.5°C for 45 sec and 72°C for 1 min, with a final extension step at 72°C for 5 min in an iCycler (BioRad, Hercules, California). The PCR assay with AAKDRF2 and AAKDRR primers amplified a single band of 225 bp in An. albimanus from the Sanarate strain (Figure 1B), which corresponds to the VGSC gene of An. albimanus. These primers were used to amplify the VGSC gene in DNA samples of An. albimanus from Guatemala (collected in 1995), Mexico (collected in 1991), Nicaragua (collected in 1995), Costa Rica (collected in 1995), Ecuador (collected in 1991) and Colombia (collected in 1992) previously used in population genetic studies [39, 40]. The PCR products were sequenced by Macrogen Inc. (Korea) using AAKDRF2 and AAKDRR primers.
Results and discussion
Sequence analysis showed that segment 6 of domain II of the VGSC gene (excluding the intron sequence) of An. albimanus has a sequence identity of 92% with An. gambiae and 83% with An. punctipennis at the nucleotide level. Variations in the nucleotide sequence of An. albimanus did not produce changes in the amino acid sequence (100% identity with An. gambiae and An. punctipennis, Figure 2). The position of intron II was established through comparison with the VGSC cDNA sequence from An. gambiae [GenBank: Y13592]. The size of intron II in An. albimanus (71 bp) was greater than in An. gambiae (57 bp) and An. punctipennis (68 bp). Variation in the size of intron II has been detected in An. vestitipennis and An. pseudopunctipennis (unpublished observations), and may potentially be used for taxonomic identification of malaria vectors from Latin America, as proposed for other anopheline species [41].
Amino acid sequence comparison of kdr region of Anopheles albimanus with other anopheline species. The sequence of the segment 6 of domain II of the VGSC gene of An. albimanus was compared to An. gambiae [GenBank: CAA73920] and An. punctipennis [GenBank: AAP60053]. Identical positions are indicated by an asterisk and mutation site (codon 1014) is enclosed by a red box. The amino acid at the mutation site corresponds to the pyrethroid and DDT susceptible (wild-type) genotypes.
Sequence results from the Sanarate strain of An. albimanus showed that the individuals contained the susceptible/wild type kdr allele, TTG (L1014), previously reported in An. sacharovi, An. sinensis and other anopheline species from the Mekong region [34, 42, 43]. In the field-collected mosquitoes from Latin America, polymorphisms at codon 1014 were detected in several of the samples (Figure 3A). The field samples from Guatemala, Ecuador and Colombia also contained the susceptible TTG (L1014) allele. A non-synonymous homozygous mutation, TGT (cysteine, L1014C), was detected in field samples from Mexico and Nicaragua. This mutation has previously been associated with permethrin, deltamethrin and beta-cypermethrin resistance in An. sinensis[34, 44, 45]. A field sample from Costa Rica contained a homozygous TTC polymorphism (phenylalanine, L1014F), previously reported in populations of An. gambiae resistant to permethrin and DDT, An. sinensis resistant to deltamethrin and An. peditaeniatus resistant to DDT, permethrin, alpha-cypermethrin, lambda-cyhalothrin and etofenprox [28, 43, 45]. With the exception of certain individuals from Nicaragua and Guatemala, all kdr alleles were found to be homozygous (Figure 3B). The heterozygote alleles from Nicaragua were TKY and from Guatemala were TKK. Interestingly, the kdr allele reported in An. gambiae from East Africa (L1014S) [29] was not detected.
Kdr alleles detected on the segment 6 of domain II of the VGSC gene of Anopheles albimanus. (A) DNA alignment of the VGSC gene of An. albimanus from different regions of Latin America. The identical positions are indicated by an asterisk and polymorphic site (codon 1014) is enclosed by a red box. (B) Electropherograms for kdr alleles detected on the VGSC gene of An. albimanus.
An. albimanus populations are panmictic over at least 600 km in Central America, West of Panama [46]. In this region, insecticide resistance in An. albimanus has been reported and the main source of its selection has been the extensive use of pesticides in large scale agricultural activities [47–50]. During the nineties, populations in the area in continued exposure to agricultural insecticides plus pressures from the use of insecticides for vector control could have maintained a constant selection pressure on Mesoamerican An. albimanus populations, possibly explaining the finding of three homozygous kdr variants in Mexico, Nicaragua and Costa Rica with mutations that have been associated with pyrethroid and DDT resistance in other anopheline species. Even though to date the role of kdr has not been directly implicated in the insecticide resistance documented in the region, it is highly likely that kdr is an important resistance mechanism in Latin American malaria vectors.
Conclusions
Our findings describe for the first time the kdr region in An. albimanus, including the presence of polymorphisms associated with insecticide resistance in other anopheline species. We have documented the presence of homozygous kdr alleles associated with resistance in other anopheline species in An. albimanus individuals collected across Mesoamerica at a time of intense agricultural and public health insecticide use. This suggests that pyrethroid and DDT resistance in the region could have been mediated in the past by a kdr mechanism. Future work will endeavor to link resistant phenotypes with the kdr polymorphisms described here, as well as lead to the development of allele-specific diagnostic assays for An. albimanus and other malaria vectors across the region.
References
Zimmerman RH: Ecology of malaria vectors in the Americas and future direction. Mem Inst Oswaldo Cruz. 1992, 87: 371-383.
Sinka ME, Rubio-Palis Y, Manguin S, Patil AP, Temperley WH, Gething PW, Van Boeckel T, Kabaria CW, Harbach RE, Hay SI: The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010, 3: 72-10.1186/1756-3305-3-72.
Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V: Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control?. Trends Parasitol. 2011, 27: 91-98. 10.1016/j.pt.2010.08.004.
Czeher C, Labbo R, Arzika I, Duchemin JB: Evidence of increasing Leu-Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long-lasting insecticide-treated nets implementation. Malar J. 2008, 7: 189-10.1186/1475-2875-7-189.
Balkew M, Ibrahim M, Koekemoer LL, Brooke BD, Engers H, Aseffa A, Gebre-Michael T, Elhassen I: Insecticide resistance in Anopheles arabiensis (Diptera: Culicidae) from villages in central, northern and south west Ethiopia and detection of kdr mutation. Parasit Vectors. 2010, 3: 40-10.1186/1756-3305-3-40.
WHO: World malaria report: 2010. 2010, Geneva: World Health Organization
Kroeger A, Gonzalez M, Ordonez-Gonzalez J: Insecticide-treated materials for malaria control in Latin America: to use or not to use?. Trans R Soc Trop Med Hyg. 1999, 93: 565-570. 10.1016/S0035-9203(99)90048-2.
Kroeger A, Mancheno M, Alarcon J, Pesse K: Insecticide-impregnated bed nets for malaria control: varying experiences from Ecuador, Colombia, and Peru concerning acceptability and effectiveness. Am J Trop Med Hyg. 1995, 53: 313-323.
Fanello C, Kolaczinski JH, Conway DJ, Carnevale P, Curtis CF: The kdr pyrethroid resistance gene in Anopheles gambiae: tests of non-pyrethroid insecticides and a new detection method for the gene. Parassitologia. 1999, 41: 323-326.
Kolaczinski JH, Fanello C, Herve JP, Conway DJ, Carnevale P, Curtis CF: Experimental and molecular genetic analysis of the impact of pyrethroid and non-pyrethroid insecticide impregnated bednets for mosquito control in an area of pyrethroid resistance. Bull Entomol Res. 2000, 90: 125-132.
Vulule JM, Beach RF, Atieli FK, Roberts JM, Mount DL, Mwangi RW: Reduced susceptibility of Anopheles gambiae to permethrin associated with the use of permethrin-impregnated bednets and curtains in Kenya. Med Vet Entomol. 1994, 8: 71-75. 10.1111/j.1365-2915.1994.tb00389.x.
Yewhalaw D, Bortel WV, Denis L, Coosemans M, Duchateau L, Speybroeck N: First evidence of high knockdown resistance frequency in Anopheles arabiensis (Diptera: Culicidae) from Ethiopia. Am J Trop Med Hyg. 2010, 83: 122-125. 10.4269/ajtmh.2010.09-0738.
Protopopoff N, Verhaeghen K, Van Bortel W, Roelants P, Marcotty T, Baza D, D’Alessandro U, Coosemans M: A significant increase in kdr in Anopheles gambiae is associated with an intensive vector control intervention in Burundi highlands. Trop Med Int Health. 2008, 13: 1479-1487. 10.1111/j.1365-3156.2008.02164.x.
Ndiath MO, Sougoufara S, Gaye A, Mazenot C, Konate L, Faye O, Sokhna C, Trape JF: Resistance to DDT and pyrethroids and increased kdr mutation frequency in An. gambiae after the implementation of permethrin-treated nets in Senegal. PLoS One. 2012, 7: e31943-10.1371/journal.pone.0031943.
Himeidan YE, Chen H, Chandre F, Donnelly MJ, Yan G: Short report: permethrin and DDT resistance in the malaria vector Anopheles arabiensis from eastern Sudan. Am J Trop Med Hyg. 2007, 77: 1066-1068.
Arevalo-Herrera M, Quinones ML, Guerra C, Cespedes N, Giron S, Ahumada M, Pineros JG, Padilla N, Terrientes Z, Rosas A: Malaria in selected non-Amazonian countries of Latin America. Acta Trop. 2012, 121: 303-314. 10.1016/j.actatropica.2011.06.008.
Brown AW: Insecticide resistance in mosquitoes: a pragmatic review. J Am Mosq Control Assoc. 1986, 2: 123-140.
Aizoun N, Aikpon R, Padonou GG, Oussou O, Oke-Agbo F, Gnanguenon V, Osse R, Akogbeto M: Mixed-function oxidases and esterases associated with permethrin, deltamethrin and bendiocarb resistance in Anopheles gambiae s.l. in the south–north transect Benin, West Africa. Parasit Vectors. 2013, 6: 223-10.1186/1756-3305-6-223.
Penilla RP, Rodriguez AD, Hemingway J, Torres JL, Arredondo-Jimenez JI, Rodriguez MH: Resistance management strategies in malaria vector mosquito control. Baseline data for a large-scale field trial against Anopheles albimanus in Mexico. Med Vet Entomol. 1998, 12: 217-233. 10.1046/j.1365-2915.1998.00123.x.
Dzul FA, Patricia Penilla R, Rodriguez AD: Susceptibility and insecticide resistance mechanisms in Anopheles albimanus from the southern Yucatan Peninsula, Mexico. Salud Publica Mex. 2007, 49: 302-311. 10.1590/S0036-36342007000400010.
Beach RF, Cordon-Rosales C, Brogdon WG: Detoxifying esterases may limit the use of pyrethroids for malaria vector control in the Americas. Parasitol Today. 1989, 5: 326-327.
Brogdon WG, McAllister JC, Corwin AM, Cordon-Rosales C: Independent selection of multiple mechanisms for pyrethroid resistance in Guatemalan Anopheles albimanus (Diptera: Culicidae). J Econ Entomol. 1999, 92: 298-302.
Brogdon WG, McAllister JC, Corwin AM, Cordon-Rosales C: Oxidase-based DDT-pyrethroid cross-resistance in guatemalan anopheles albimanus. Pestic Biochem Physiol. 1999, 64: 101-111. 10.1006/pest.1999.2415.
Scott JA, Collins FH, Feyereisen R: Diversity of cytochrome P450 genes in the mosquito, Anopheles albimanus. Biochem Biophys Res Commun. 1994, 205: 1452-1459. 10.1006/bbrc.1994.2828.
Penilla RP, Rodriguez AD, Hemingway J, Trejo A, Lopez AD, Rodriguez MH: Cytochrome P450-based resistance mechanism and pyrethroid resistance in the field Anopheles albimanus resistance management trial. Pestic Biochem Physiol. 2007, 89: 111-117. 10.1016/j.pestbp.2007.03.004.
Soderlund DM, Knipple DC: The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochem Mol Biol. 2003, 33: 563-577. 10.1016/S0965-1748(03)00023-7.
Dong K: Insect sodium channels and insecticide resistance. Invert Neurosci. 2007, 7: 17-30. 10.1007/s10158-006-0036-9.
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, Guillet P, Pasteur N, Pauron D: Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998, 7: 179-184. 10.1046/j.1365-2583.1998.72062.x.
Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH: Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000, 9: 491-497. 10.1046/j.1365-2583.2000.00209.x.
Syafruddin D, Hidayati AP, Asih PB, Hawley WA, Sukowati S, Lobo NF: Detection of 1014F kdr mutation in four major Anopheline malaria vectors in Indonesia. Malar J. 2010, 9: 315-10.1186/1475-2875-9-315.
Enayati AA, Vatandoost H, Ladonni H, Townson H, Hemingway J: Molecular evidence for a kdr-like pyrethroid resistance mechanism in the malaria vector mosquito Anopheles stephensi. Med Vet Entomol. 2003, 17: 138-144. 10.1046/j.1365-2915.2003.00418.x.
Singh OP, Dykes CL, Das MK, Pradhan S, Bhatt RM, Agrawal OP, Adak T: Presence of two alternative kdr-like mutations, L1014F and L1014S, and a novel mutation, V1010L, in the voltage gated Na+ channel of Anopheles culicifacies from Orissa. India. Malar J. 2010, 9: 146-10.1186/1475-2875-9-146.
Singh OP, Dykes CL, Lather M, Agrawal OP, Adak T: Knockdown resistance (kdr)-like mutations in the voltage-gated sodium channel of a malaria vector Anopheles stephensi and PCR assays for their detection. Malar J. 2011, 10: 59-10.1186/1475-2875-10-59.
Kim H, Baek JH, Lee W, Lee SH: Frequency detection of pyrethroid resistance allele in Anopheles sinensis populations by real-time PCR amplification of specific allele (rtPASA). Pestic Biochem Physiol. 2007, 87: 54-61. 10.1016/j.pestbp.2006.06.009.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S: MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011, 28: 2731-2739. 10.1093/molbev/msr121.
Brogdon WG, McAllister JC: Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. J Am Mosq Control Assoc. 1998, 14: 159-164.
Collins FH, Mendez MA, Rasmussen MO, Mehaffey PC, Besansky NJ, Finnerty V: A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am J Trop Med Hyg. 1987, 37: 37-41.
Bjourson AJ, Cooper JE: Band-stab PCR: a simple technique for the purification of individual PCR products. Nucleic Acids Res. 1992, 20: 4675-10.1093/nar/20.17.4675.
De Merida AM, De Mata MP, Molina E, Porter CH, Black WC: Variation in ribosomal DNA intergenic spacers among populations of Anopheles albimanus in South and Central America. Am J Trop Med Hyg. 1995, 53: 469-477.
De Merida AM, Palmieri M, Yurrita M, Molina A, Molina E, Black WC: Mitochondrial DNA variation among Anopheles albimanus populations. Am J Trop Med Hyg. 1999, 61: 230-239.
Henry-Halldin CN, Nadesakumaran K, Keven JB, Zimmerman AM, Siba P, Mueller I, Hetzel MW, Kazura JW, Thomsen E, Reimer LJ, Zimmerman PA: Multiplex assay for species identification and monitoring of insecticide resistance in Anopheles punctulatus group populations of Papua New Guinea. Am J Trop Med Hyg. 2012, 86: 140-151. 10.4269/ajtmh.2012.11-0503.
Luleyap HU, Alptekin D, Kasap H, Kasap M: Detection of knockdown resistance mutations in Anopheles sacharovi (Diptera: Culicidae) and genetic distance with Anopheles gambiae (Diptera: Culicidae) using cDNA sequencing of the voltage-gated sodium channel gene. J Med Entomol. 2002, 39: 870-874. 10.1603/0022-2585-39.6.870.
Verhaeghen K, Van Bortel W, Trung HD, Sochantha T, Keokenchanh K, Coosemans M: Knockdown resistance in Anopheles vagus, An. sinensis, An. paraliae and An. peditaeniatus populations of the Mekong region. Parasit Vectors. 2010, 3: 59-10.1186/1756-3305-3-59.
Tan WL, Wang ZM, Li CX, Chu HL, Xu Y, Dong YD, Wang ZC, Chen DY, Liu H, Liu DP: First report on co-occurrence knockdown resistance mutations and susceptibility to beta-cypermethrin in Anopheles sinensis from Jiangsu Province, China. PLoS One. 2012, 7: e29242-10.1371/journal.pone.0029242.
Zhong D, Chang X, Zhou G, He Z, Fu F, Yan Z, Zhu G, Xu T, Bonizzoni M, Wang MH: Relationship between Knockdown Resistance. Metabolic Detoxification and Organismal Resistance to Pyrethroids in Anopheles sinensis. PLoS One. 2013, 8: e55475-10.1371/journal.pone.0055475.
Molina-Cruz A, de Merida AM, Mills K, Rodriguez F, Schoua C, Yurrita MM, Molina E, Palmieri M, Black WC: Gene flow among Anopheles albimanus populations in Central America, South America, and the Caribbean assessed by microsatellites and mitochondrial DNA. Am J Trop Med Hyg. 2004, 71: 350-359.
WHO: Vector resistance to pesticides. Fifteenth Report of the WHO Expert Committee on Vector Biology and Control. World Health Organ Tech Rep Ser. 1992, 818: 1-62.
PAHO: Results of insecticide susceptibility tests carried out in four Central American Countries between 1994–1997. Epidemiol Bull. 1997, 18: 11-14.
Lines JD: Do agricultural insecticides select for insecticide resistance in mosquitoes? A look at the evidence. Parasitology today. 1988, 4: S17-S20. 10.1016/0169-4758(88)90083-X.
Mouchet J: Agriculture and vector resistance. Insect Science and its Application. 1988, 9: 297-302.
Acknowledgements
Partial funding for this work was provided by the US Agency for International Development (USAID) under Amazon Malaria Initiative, Cooperative Agreement Guatemala Public Health/No. 1U51GH000011 and the Center for Health Studies, Universidad del Valle de Guatemala. Ana María de Mérida and Álvaro Molina for providing historic mosquito samples. We also thank Ellen Dotson from CDC (MR4) for providing the kdr positive controls and primers, and William Brogdon for his helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declared that they have no competing interests.
Authors’ contributions
JCL carried out the molecular assays, data analysis and drafted the manuscript. MEC conducted molecular assays and contributed to the manuscript. KL conducted molecular assays and sequencing. AL assisted with the analysis and interpretation of results and contributed to the manuscript. PMP designed and guided the study, performed data analysis and contributed to the manuscript. NRP conceived the study and participated in analysis and interpretation of data and contributed to the drafting of the manuscript. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lol, J.C., Castellanos, M.E., Liebman, K.A. et al. Molecular evidence for historical presence of knock-down resistance in Anopheles albimanus, a key malaria vector in Latin America. Parasites Vectors 6, 268 (2013). https://doi.org/10.1186/1756-3305-6-268
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-3305-6-268