Abstract
Background
Hymenolepis microstoma (Dujardin, 1845) Blanchard, 1891, the mouse bile duct tapeworm, is a rodent/beetle-hosted laboratory model that has been used in research and teaching since its domestication in the 1950s. Recent characterization of its genome has prompted us to describe the specific strain that underpins these data, anchoring its identity and bringing the 150+ year-old original description up-to-date.
Results
Morphometric and ultrastructural analyses were carried out on laboratory-reared specimens of the 'Nottingham' strain of Hymenolepis microstoma used for genome characterization. A contemporary description of the species is provided including detailed illustration of adult anatomy and elucidation of its taxonomy and the history of the specific laboratory isolate.
Conclusions
Our work acts to anchor the specific strain from which the H. microstoma genome has been characterized and provides an anatomical reference for researchers needing to employ a model tapeworm system that enables easy access to all stages of the life cycle. We review its classification, life history and development, and briefly discuss the genome and other model systems being employed at the beginning of a genomic era in cestodology.
Similar content being viewed by others
Background
Species of Hymenolepis Weinland, 1858 (Platyhelminthes: Cestoda: Cyclophyllidea) have been used as tapeworm models in research and teaching since the 1950s when they were first domesticated in the laboratory of Clark P. Read [1]. Adult parasites of rodents with beetle intermediate hosts, they benefit from easy culture in vivo using natural hosts that are themselves model organisms (e.g. Mus musculus L., Tribolium confusum Jacquelin du Val). Research on Hymenolepis, and especially H. diminuta (Rudolphi, 1819), H. nana (von Siebold, 1852) and H. microstoma, is underpinned by an extensive literature that includes much of our classical knowledge of tapeworm biology [e.g. [2]]. A recently initiated effort sponsored by The Wellcome Trust Sanger Institute to characterize the genome and adult and larval transcriptomes of H. microstoma http://www.sanger.ac.uk/sequencing/Hymenolepis/microstoma/ has brought this classical model into the genomic era, greatly advancing its utility for researchers interested in employing a practical tapeworm system that allows access to all life cycle stages. In light of this development, and the fact that laboratory isolates can vary in features of their biology [3], it is desirable to have a description of the exact strain on which the genome is based, and to thus anchor the data to a well-defined entity.
Hymenolepis microstoma was first described from the bile ducts of mice in 1845 by Dujardin [4] who placed it in the genus Taenia L., 1758, which housed all tapeworms known at that time. In 1891, Blanchard [5] transferred the species to the genus Hymenolepis and provided an expanded description of the species. Although Bear and Tenora [6] suggested synonymy between H. microstoma and H. straminea (Goeze, 1782), species status of H. microstoma historically has been widely accepted, and molecular data have shown both species to represent independent, albeit closely related, lineages [7, 8]. In contrast, the genus Hymenolepis has itself been overhauled on several occasions and its membership and internal structure remain controversial. For example, whereas Hughes [9, 10] accepted the generic assignment H. microstoma by Blanchard, Spasskii [11] subdivided the genus and transferred H. microstoma to the genus Rodentolepis Spasskii, 1954, which he erected to house the rodent-hosted species of Hymenolepis with armed rostella. At the same time Spasskii erected the genus Vampirolepis Spasskii, 1954, which Schmidt subsequently considered a senior synonym of Rodentolepis, thus resulting in the new combination Vampirolepis microstoma (Dujardin, 1854) Schmidt, 1986 [12]. The genus Rodentolepis was retained by Czaplinski and Vaucher [13] in the most recent synoptic treatment of tapeworms [14], but this work did not consider species level taxa and therefore did not arbitrate on the generic assignment of H. microstoma. Thus although Vampirolepis microstoma [12] represents the most recent formal taxonomic assignment of the species, few investigators have adopted this name, and most reports refer to it as either a member of the genus Hymenolepis, or with less frequency, Rodentolepis. In our view, a natural circumscription of hymenolepid species will not be attained without the application of molecular data [15].
To this end, Haukisalmi et al. [8] recently used 28S rDNA to analyze phylogenetic relationships among 32 hymenolepidid species from rodents, shrews and bats, showing that both Hymenolepis and Rodentolepis represented paraphyletic assemblages. Although their work assigned H. microstoma to a 'Rodentolepis' clade, the lack of resolution and widespread paraphyly of the taxa in their analyses indicate that greater taxonomic representation and more robust data are needed before such nomenclatural circumscriptions can be made reliably. We therefore follow Blanchard [5] in recognizing the mouse bile duct tapeworm as a member of the genus Hymenolepis, employing the most common name in usage, whilst appreciating that a more comprehensive understanding of hymenlepidid interrelationships is likely to warrant generic reassignment.
Here we provide a description of a 'Nottingham' strain of H. microstoma based on light and scanning electron microscopy of laboratory-reared specimens from the same culture used to characterize the genome. History of the isolate, dating back to the laboratory of C. P. Read [1], suggests that it represents a model that has been widely employed and disseminated within the parasitological community for over 50 years, making the genome data directly relevant to a significant pre-existing literature on its biology.
Results
Description of Hymenolepis microstoma (Nottingham strain)
Hymenolepis microstoma (Dujardin, 1845) Blanchard, 1891
Recorded synonyms
Taenia microstoma Dujardin, 1845; Cercocystis tenebrionis Villot, 1882; Cysticercus tenebrionis (Villot, 1882) Leuckart, 1886; Cysticercus taenia-microstomae Dolly, 1894; Cysticercoides tenebrionis (Villot, 1882) Braun, 1898; Scolex (= Onchoscolex) decipiens (Diesing, 1853) Joyeux and Kobozieff, 1928; Rodentolepis microstoma (Dujardin, 1845) Spasskii, 1954; Vampirolepis microstoma (Dujardin, 1845) Schmidt, 1986.
Common name
mouse bile duct tapeworm
Laboratory strain designation
'Nottingham'
Laboratory strain history
2005-present, The Natural History Museum, London (PDO); 1977-2005, University of Nottingham, UK (Prof. Jerzy Behnke); 1964-1977, University of Glasgow, UK (Prof. Adrian Hopkins); before 1964, Texas Rice University, USA (Prof. Clark P. Read).
Laboratory hosts
flour beetles (Tribolium confusum) and BKW outbred conventional mice (Mus musculus).
Voucher specimens
20 whole-mounted specimens (BMNH 2010.12.8.1-20), 22 slides of histological sections of adult worms (scolex and neck: BMNH 2010.12.8.21-30; immature strobila: BMNH 2010.12.8.31-36; mature strobila: BMNH 2010.12.8.37-42), and 12 whole and partial specimens prepared for SEM, retained by the corresponding author.
No. chromosomes
12 diploid, all acrocentric [16, 17]
Genome size
~140 Mb (haploid)
Genome data
http://www.sanger.ac.uk/resources/downloads/helminths/hymenolepis-microstoma.html
Description
(based on 14-16 day old in vivo laboratory-reared specimens: 20 whole-mounted, 2 sectioned, and 12 specimens prepared for SEM; Figures. 1-2; all measurements are given as length × width in μm except where noted): worms anapolytic, weakly craspedote, 4.7 (2.5-8.1) cm long, with 659 (291-1,087) total segments (Figure 1A); scolex 138 (116-157) × 232 (204-284) with four muscular suckers 102 (79-129) × 96 (76-113) (Figure 1B). Rostellum 38 (26-52) × 71 (51-75) with an irregular surface lacking microtriches (Figures. 2A, B), armed with 25 (22-26) hooks, retractable into contractile rostellar pouch 104 (83-139) × 101 (79-140) (Figure 1B). Hooks cricetoid; α = 13.9, β = 12.3, γ = 6, γ' = 4.4 (Figure 1C). Width at level of neck 175 (94-225). Immature segments 62 (38-83) × 404 (437-463), mature segments 117 (70-167) × 729 (360-887), gravid terminal segments 164 (131-262) × 1,160 (454-1,426). Paired osmoregulatory vessels and longitudinal nerve cords lateral (Figure 1E, F). Entire worm covered by short (~2 um), uniform and densely packed filiform microtriches [18] (Figure 2C).
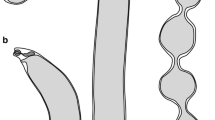
Illustrations of adult Hymenolepis microstoma (Nottingham strain). A. Whole worm. B. Hook showing measurement vectors. C. Egg. D. Scolex. E. Mature proglottide. F. Cross section of mature proglottide. Abbreviations: b, blade; c, cirrus; cs, cirrus sac; doc, dorsal osmoregulatory canal; eb, embryophore; eh, embryonic hooks; es, eggshell; esv, external seminal vesicle; g, guard; h, handle; isv, internal seminal vesicle; nc, nerve cord; o, ovary; oc, oncosphere; pf; polar filaments; r, rostellum; rp, rostellar bulb; s, shell; sr, seminal receptacle; t, testis; u, uterus; va, vagina; voc, ventral osmoregulatory canal. Scale bars: A = 1 mm; B = 10 μm; C = 50 μm; D-F = 100 μm.
Scanning electron micrographs of adult Hymenolepis microstoma (Nottingham strain). A. Scolex and rostellum. B. Rostellar hooks. C. Microtriches on the scolex. D. Internal view of gravid strobila. E. Seminal receptacle with spermatozoa surrounded by eggs. F. Three-day old transforming oncosphere showing larval hooks (arrows and insets). Scale bars: A = 50 μm; B = 5 μm; C = 2 μm; D = 100 μm; E-F = 20 μm (insets = 2 μm).
Male system consisting of three spherical to oval testes 72 (51-114) × 78 (61-115), arranged one poral and two aporal (occasionally reversed in individual proglottides). Vas deferens expands to form an external seminal vesicle 105 (73-195) × 56 (38-93) (Figure 1E). Cirrus pouch ovoid 58 (45-99) × 153 (97- 302), enclosing coiled cirrus and internal seminal vesicle, 52 (38-93) × 94 (72-195). Female system consisting of a central lobed ovary 63 (34-103) × 234 (130-360) partially overlapping a compact vitellarium 38 (41-94) × 56 (32-68). Seminal receptacle median. Vagina 299 (197-431) × 13 (9-18) situated ventral to male system (Figure 1F). Common genital pore dextral, unilateral, near mid-point of margin. Eggs thin-shelled (Figure 2E), enclosing embryophore with 3 polar filaments and oncosphere with embryonic hooks arranged in parallel (Figure 1D).
Remarks
Hymenolepis species exhibit the well-documented 'crowding effect' in which overall size and egg production are inversely related to the intensity of infection [19]. Consequently, size is dependent not only on the age of the worms, but on the number of worms present in the host, and cannot be used diagnostically [20]. Crowding in H. microstoma has been shown to decrease linear growth, egg production and the rate of proglottide formation [21]. Moreover, we chose to document gravid adult specimens at an age and size most useful for laboratory manipulations in which larger worms pose unnecessary practical problems (e.g. assays involving whole mount in situ hybridization or in vitro culture). de Rycke [22] showed that H. microstoma is in rapid state of growth starting around 12-14 days post-infection in Mus musculus, and whereas our length measurements correspond to those reported by de Rycke for the relevant age class (see Table 1), they are obviously less comparable to reports based on older specimens, such as those stemming from natural infections.
Discussion
Life history
Hymenolepis microstoma is most probably cosmopolitan in distribution [20] and is not known from human infections outside of a single report in which mixed infections of H. nana and H. microstoma were identified in four individuals from a remote region of Western Australia [23]. Reported natural definitive hosts include a large range of rodent genera that include mice (e.g. Apodemus Kaup, Dendromus Smith, Leggada Gray, Mastomys Thomas, Mus L.), gerbils (Meriones Illiger) and voles (Microtus Schrank) [9, 12, 24]. Infections in rats is controversial: whereas Joyeux and Kobozieff [25] reported successful infection of laboratory rats, Dvorak et al. [20] found rats to be refractory to H. microstoma, and Litchford [24] showed that rats became refractory with age. Similarly, although infections can be established in golden hamsters (Mesocricetus Nehring), they result in underdeveloped worms and cause severe pathology to the host [20, 24]. Dvorak et al. [20] demonstrated that mice could not be infected via eggs, as is the case with H. nana (ie. auto-infection) [26]. However, in congenitally athymic mice, Andreassen et al. [27] found that autoinfection was possible, showing that oncospheres penetrated the intestinal tissues and developed into cysticeroids that subsequently excysted and developed normally in the bile duct and duodenum, in a manner similar to the direct cycle of H. nana. Autoinfection of BALB/c mice was also implied by the detection of stage-specific antigens [28].
The life history of H. microstoma (Figure 3) has been described in detail previously [20, 25, 29] and is typical of other hymenolepid species, save its unusual location in the bile duct of the mammalian host. In brief, eggs containing patent oncospheres are expelled with faeces into the environment and may be ingested by either the adult or larval stage of an appropriate beetle host (e.g. Tribolium confusum, T. castaneum, Tenebrio molitor, and Oryzaephilus surinamensis). Oncospheral larvae (~20 μm; Figure 1D; Figure 2F) are released from their thin shells (Figure 2E; n.b. appearing as a 'hymen' via light microscopy and the eponym of the genus) through the action of the host mouthparts, and after ingestion use their three pairs of hooks and proteolytic secretions [30] to enter the haemocoel. There they undergo a complete metamorphosis, reconstituting their bodies into cycsticeroid larvae [31] in approximately seven days, the phases of which have been documented by both Voge [32] and Goodchild and Stullken [33]. Upon infection of the definitive host, the combination of pepsin and HCl in the stomach act to dissolve the larval membranes, and juvenile worms are then activated in the duodenum in response to trypsin and bile salts. de Rycke [22] described adult growth and organogenesis in Mus musculus (summarized in Table 1): in the first three days the juveniles move anteriorly in the upper 20% of the small intestine and duodenum before establishing permanently in the bile duct, where they commence strobilation. Within approximately 14 days terminal segments are gravid and most of their strobila extends outside of the bile duct and into the duodenum. Thus the entire life cycle, from egg to gravid adult, can be completed in the laboratory in only three weeks. Although the germinative ('neck') region of tapeworms has the potential for 'immortality' as demonstrated in H. diminuta by Read [34], infections of H. microstoma in mice persist for an average of six months, whereas those in the intermediate host can remain infective for the life of the beetle (> one year).
Life cycle of Hymenolepis microstoma. Infected adult or larval beetles (e.g. Tribolium confusum) are consumed by rodents (e.g. Mus musculus), releasing the cysticercoids which excyst and locate in the bile duct before commencing strobilation. Gravid adult worms develop in 12-14 days in vivo and release embryonated eggs in the duodenum that are expelled with the host faeces. Oncosphereal larvae are released when the eggs are consumed by beetles, allowing them penetrate the gut wall and metamorphose into patent cysticercoids in the haemocoel (apx. one week). Illustration adapted from Olsen [63].
The Hymenolepis genome
Through collaboration with The Wellcome Trust Sanger Institute, a draft genome of H. microstoma derived from the cultures described herein is now publically available: http://www.sanger.ac.uk/resources/downloads/helminths/hymenolepis-microstoma.html. The latest assembly (October 2010) includes more than 40× coverage of the estimated 140 Mb haploid genome and is based on data produced by a combination of Roche 454 and Illumina Solexa next-generation sequencing technologies. Gene annotation is presently being conducted using a combination of RNA-Seq [35] and automated gene prediction tools, revealing intron-exon structures and other aspects of their genomic organization, and additional tools are being used to characterize non-coding regions (M. Zarowiecki and M. Berriman, pers. comm.).
Hymenolepis microstoma is one of four tapeworm species to have complete genomes characterized: a reference genome of Echinococcus multilocularis Leukart, 1863 and draft genome of E. granulosus (Batsch, 1786) have been produced by the Sanger Institute (available from http://www.sanger.ac.uk/resources/downloads/helminths/) in collaboration with Profs. Klaus Brehm and Cecelia Fernandez, respectively, and a consortium in Mexico are currently working to characterize the genome of Taenia solium L., 1758 [36]. These data herald the beginning of the genomic era in cestodology and are already accelerating advances in our understanding of tapeworm biology and infection. At present the only published platyhelminth genome is that of the human blood fluke, Schistosoma mansoni Sambon, 1907 [37]. However, genome data for Schistosoma Weinland, 1858 and Echinococcus Rudolphi, 1801, as well as the free-living flatworm models Schmidtea mediterranea Benazzi, Baguna, Ballester, Puccinelli and Del Papa, 1975 [38] and Macrostomum lignano Ladurner, Scharer, Salvenmoser and Rieger, 2005 (http://www.macgenome.org/), have been available for some time and full reports on the characteristics of all of these genomes, including that of H. microstoma, are expected soon.
Model systems in the genomic era of cestodology
Of the three Hymenolepis species that have been employed in laboratory research, most literature concerns the rat tapeworm H. diminuta, followed by the medically important dwarf tapeworm, H. nana, and finally by the mouse bile duct tapeworm, H. microstoma. As a model for research in the genomic age, however, H. microstoma has advantages over both of these alternative systems. For example, compared to H. diminuta, it is both smaller and mouse-hosted, enabling smaller, and thus less expensive, assay sizes (e.g. for RNAi), as well as less expensive animal costs, whereas the mouse-hosted H. nana is both a human pathogen (albeit controversy persists regarding the conspecficity of human and mouse strains) and capable of infecting other laboratory animals through faecal contamination via its direct life cycle [26]. Moreover, whereas H. nana survives only weeks in the mouse host [39], H. microstoma persist for ~6 months and thus require less frequent passage. Although the smaller size of H. nana would be preferable for assays, on balance H. microstoma provides the best practical solution for contemporary research programmes that wish to employ a tapeworm model providing easy access to all stages of their life cycle at minimal expense and risk to human and animal health.
Completion of the H. microstoma life cycle in vitro from egg to gravid adult was demonstrated in the 1960s and 70s by De Rycke and Berntzen [40], Evans [41, 42] and Seidel [43, 44], but to our knowledge no report of research employing these techniques has been published subsequently. Our initial attempts to follow these protocols for the cultivation of adult worms resulted in only limited growth (3× increase in length) without the onset segmentation (unpub. data). However, as many of the reported media used by previous authors are no longer commercially available, more work is needed to develop contemporary protocols for in vitro culture. Among the most advanced in vitro systems available for tapeworm research today has been developed by Brehm and colleagues for Echinococcus [45–48], the genus on which most of our understanding of tapeworm molecular biology is based [49]. Development of an axenic culture system of the hydatid stage of E. multilocularis has allowed them to introduce transgenic and functional genomic techniques (e.g. RNAi) to cestodology, and their system is currently being used to pioneer research on stem-cells and developmental biology in parasitic flatworms [45, 50]. Although not yet supported by genome characterization, another currently employed in vitro system is that of Mesocestoides Vaillant, 1863 [e.g. [51]] which are readily maintained in the larval tetrathyridial stage [31] and can increase their numbers in culture via asexual fission [52]. Adult worms have also been grown in vitro and induced to strobilate through the addition of bile salts [53]. However, as with species of Echinoccocus and Taenia, in vivo development of strobilar stages of Mesocestoides is prohibited by the legalities and expense of maintaining large vertebrate hosts in the laboratory. Rodent hosted Hymenolepis species therefore remain the most convenient systems for research on the biology of adult tapeworms, and for this reason we have been developing H. microstoma as a model to study the development and evolution of tapeworm segmentation [54].
Although the basic framework of cestode evolution has been revealed by previous molecular studies [55–58] and the interrelationships of select groups are now well resolved [59–61], there has yet to be a comprehensive molecular phylogenetic study of the largest and most important group of tapeworms with regard to human and animal health, the Cyclophyllidea. All of the tapeworm species for which genomes have been characterized thus far belong to this order and thus it is especially important that we elucidate the relative phylogenetic positions of the 350+ described genera [14]. Such knowledge will provide an evolutionary underpinning for comparative genomic studies within the group and allow us to identify the sister lineages whose genomes share the closest evolutionary histories to the species for which full genome data are now available.
Methods
A seed culture of Hymenolepis microstoma infected beetles was obtained from Nottingham University in 2005 courtesy of Prof. Jerzy Behnke and subsequently maintained in vivo at the Natural History Museum (London) using flour beetles (Tribolium confusum) and BKW outbred conventional mice (full protocols can found at http://www.olsonlab.com; please contact the corresponding author to enquire about seed cultures). Gravid, 14-16 day old specimens were removed from the bile ducts and duodenum of mice and quickly swirled in near-boiling 0.85% saline for ~4 secs to fully extend the worms prior to fixation in cold 4% paraformaldehyde overnight at -4 C. Whole-mounted specimens were dehydrated in a graded ethanol series, stained using Gill's haematoxylon or left unstained, cleared in beachwood creosote and mounted in Canada balsam. Sections were prepared by paraffin embedding using standard histological techniques and stained with Mayer's Haemalum [62]. Measurements and illustrations were made under differential interference contrast on a Leica DM5000B compound microscope equipped with a camera lucida and digital documentation system. Specimens used for SEM were dehydrated as above, critically-point dried, sputter-coated with gold/palladium and viewed on a JEOL XL30 scanning electron microscope. Internal structures were imaged by SEM by cutting worms crudely using a razor blade.
References
Stewart GL, Lumsden RD, Fisher FM: The contributions of Clark P. Read on ecology of the vertebrate gut and its parasites. Bios. 1975, 46: 3-21.
Arai HP: Biology of the tapeworm Hymenolepis diminuta. 1980, New York: Academic Press
Pappas PW, Leiby DA: Variation in the sizes of eggs and oncospheres and the numbers and distributions of testes in the tapeworm, Hymenolepis diminuta. J Parasitol. 1986, 72: 383-391. 10.2307/3281677.
Dujardin MF: Histoire Naturelle des Helminthes ou vers intestinaux. 1845, 1-680.
Blanchard R: Histoire Zoologique et Médicale des Téniadés du genre Hymenolepis Weinland. 1891, Paris
Bear JG, Tenora F: Some species of Hymenolepis (Cestoidea) from rodents and from primates. Acta Scientarium Naturalium Academiae Scientarium Bohemoslovacae--Brno. 1970, 4: 1-32.
Casanova JC, Santalla F, Durand P, Vaucher C, Feliu C, Renaud F: Morphological and genetic differentiation of Rodentolepis straminea (Goeze, 1752) and Rodentolepis microstoma (Dujardin, 1845) (Hymenolepididae). Parasitol Res. 2001, 87: 439-444.
Haukisalmi V, Hardman LM, Foronda P, Feliu C, Laakkonen J, Niemimaa J, Lehtonen JT, Henttonen H: Systematic relationships of hymenolepidid cestodes of rodents and shrews inferred from squences of 28S ribosomal RNA. Zool Scr. 2010, 39: 631-641. 10.1111/j.1463-6409.2010.00444.x.
Hughes RC: The genus Hymenolepis Weinland 1858. Oklahoma Agricultural and Mechanical College Agricultural Experiment Station. 1940, 8: 1-42.
Hughes RC: A key to the species of tapeworms in Hymenolepis. Trans Am Microsc Soc. 1941, 60: 378-414. 10.2307/3222833.
Spasskii AA: Classification of Hymenolepididae from mammals. Tr Gel'mintol Lab. 1954, 7: 120-167.
Schmidt GD: Handbook of Tapeworm Identification. 1986, Boca Raton, Florida: CRC Press
Czaplinski B, Vaucher C: Family Hymenolepididae Ariola, 1899. Keys to the Cestode Parasites of Vertebrates. Edited by: Khalil LF, Jones A, Bray RA. 1994, Wallingford, U.K.: CAB International, 595-663.
Khalil LF, Jones A, Bray RA, eds: Keys to the Cestode Parasites of Vertebrates. 1994, Wallingford: CAB International
Olson PD, Tkach VV: Advances and trends in the molecular systematics of the parasitic Platyhelminthes. Adv Parasitol. 2005, 60: 165-243. 10.1016/S0065-308X(05)60003-6.
Hossain MM, Jones AW: The chromosomes of Hymenolepis microstoma (Dujardin 1845). J Parasitol. 1963, 49: 305-307. 10.2307/3276001.
Proffitt MR, Jones AW: Chromosome analysis of Hymenolepis microstoma. Exp Parasitol. 1969, 25: 72-84. 10.1016/0014-4894(69)90053-8.
Chervy L: Unified terminology for cestode microtriches: a proposal from the International Workshops on Cestode Systematics in 2002-2008. Folia Parasitol. 2009, 56: 199-230.
Read CP: The "Crowding Effect" in tapeworm infections. J Parasitol. 1951, 37: 174-178. 10.2307/3273449.
Dvorak JA, Jones AW, Kuhlman HH: Studies on the biology of Hymenolepis microstoma (Dujardin, 1845). J Parasitol. 1961, 47: 833-838. 10.2307/3275481.
Jones AW, Tan BD: Effect of crowding upon the growth and fecundity in the mouse bile duct tapeworm, Hymenolepis microstoma. J Parasitol. 1971, 57: 88-93. 10.2307/3277757.
de Rycke PH: Development of the cestode Hymenolepis microstoma in Mus musculus. Zeitschrift fur Parasitenkunde. 1966, 27: 350-354.
Macnish MG, Ryan UM, Behnke JM, Thompson RCA: Detection of the rodent tapeworm Hymenolepis (= Rodentolepis) microstoma in humans: a new zoonosis?. Int J Parasitol. 2003, 33: 1079-1085. 10.1016/S0020-7519(03)00137-1.
Litchford RG: Observations on Hymenolepis microstoma in three laboratory hosts: Mesocricetus auratus, Mus musculus, and Rattus novegicus. J Parasitol. 1963, 49: 403-410. 10.2307/3275808.
Joyeux C, Kobozieff NI: Recherches sur l'Hymenolepis microstoma (Dujardin, 1845). Annales de Parasitologie. 1928, 6: 59-79.
Heyneman D: Auto-reinfection in white mice resulting from infection by Hymenolepis nana. J Parasitol. 1953, 39: 28-10.2307/3274055.
Andreassen J, Ito A, Ito M, Nakao M, Nakaya K: Hymenolepis microstoma: direct life cycle in immunodeficient mice. J Helminthol. 2004, 78: 1-5. 10.1079/JOH2003207.
Ito A, Itoh M, Andreassen J, Onitake K: Stage-specific antigens of Hymenolepis microstoma recognized in BALB/c mice. Parasite Immunol. 1989, 11: 453-462. 10.1111/j.1365-3024.1989.tb00681.x.
Hickman JL: The biology of Hymenolepis microstoma (Dujardin). Pap Proc R Soc Tasman. 1964, 98: 73-77.
Fairweather I, Threadgold LT: Hymenolepis nana: the fine structure of the 'penetration gland' and nerve cells within the oncosphere. Parasitology. 1981, 82: 445-458. 10.1017/S003118200006697X.
Chervy L: The terminology of larval cestodes or metacestodes. Syst Parasitol. 2002, 52: 1-33. 10.1023/A:1015086301717.
Voge M: Development of Hymenolepis microstoma (Cestoda: Cyclophyllidea) in the intermediate host Tribolium confusum. J Parasitol. 1964, 50: 77-80. 10.2307/3276032.
Goodchild CG, Stullken RE: Hymenolepis microstoma: cysticercoid morphologenesis. Trans Am Microsc Soc. 1970, 89: 224-229. 10.2307/3224378.
Read CP: Longevity of the tapeworm, Hymenolepis diminuta. J Parasitol. 1967, 53: 1055-10.2307/3276836.
Wang Z, Gerstein M, Snyder M: RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009, 10: 57-63. 10.1038/nrg2484.
Santamaría RI, Soberón X, de la Torre P, Valdés V, Yánez J: The Taenia solium genome. Parasitol Int. 2005, 55: S127-S130.
Berriman M, Haas BJ, Loverde PT, Wilson RA, Dillon GP, Cerqueira GC, Mashiyama ST, Al-Lazikani B, Andrade LF, Ashton PD: The genome of the blood fluke Schistosoma mansoni. Nature. 2009, 460: 352-358. 10.1038/nature08160.
Sánchez Alvarado A, Newmark PA, Robb SMC, Juste R: The Schmidtea mediterranea database as a molecular resource for studying platyhelminthes, stem cells and regeneration. Development. 2002, 129: 5659-5665. 10.1242/dev.00167.
Ito A, Smyth JD: Adult cestodes. Immune responses in parasitic infections: immunology, immunopathology, and immunoprophilaxis. Edited by: Soulsby EJL. 1987, Boca Raton: CRC Press, 2: 115-163.
de Rycke PH, Berntzen AK: Maintenance and growth of Hymenolepis microstoma (Cestoda: Cyclophyllidea) in vitro. J Parasitol. 1967, 53: 352-354. 10.2307/3276588.
Evans WS: The in vitro cultivation of Hymenolepis microstoma from cysticercoid to egg-producing adult. Can J Zool. 1970, 48: 1135-1137. 10.1139/z70-198.
Evans WS: The cultivation of Hymenolepis in vitro. Biology of the tapeworm Hymenolepis diminuta. Edited by: Arai HP. 1980, New York: Academic Press, 425-448.
Seidel JS: Hemin as a requirement in the development in vitro of Hymenolepis microstoma (Cestoda: Cyclophyllidea). J Parasitol. 1971, 57: 566-570. 10.2307/3277917.
Seidel JS: The life cycle in vitro of Hymenolepis microstoma (Cestoda). J Parasitol. 1975, 61: 677-681. 10.2307/3279462.
Brehm K: Echinococcus multilocularis as a model in stem cell research and molecular host-parasite interaction. Parasitology. 2010, 137: 537-555. 10.1017/S0031182009991727.
Brehm K, Spiliotis M: Recent advances in the in vitro cultivation and genetic manipulation of Echinococcus multilocularis metacestodes and germinal cells. Exp Parasitol. 2008, 119: 506-515. 10.1016/j.exppara.2008.03.007.
Spiliotis M, Lechner S, Tappe D, Scheller C, Krohne G, Brehm K: Transient transfection of Echinococcus multilocularis primary cells and complete in vitro regeneration of metacestode vesicles. Int J Parasitol. 2008, 38: 1025-1039. 10.1016/j.ijpara.2007.11.002.
Spiliotis M, Mizukami C, Oku Y, Kiss F, Brehm K, Gottstein B: Echinococcus multilocularis primary cells: Improved isolation, small-scale cultivation and RNA interference. Mol Biochem Parasitol. 2010, 174: 83-87. 10.1016/j.molbiopara.2010.07.001.
Hemphill A, Kern P: Special issue: Experimental studies in Echinococcus. Exp Parasitol. 2008, 119: 437-438. 10.1016/j.exppara.2008.04.020.
Brehm K: The role of evolutionary conserved signalling systems in Echinococcus multilocularis development and host-parasite interaction. Med Microbiol Immunol. 2010, 199: 247-259. 10.1007/s00430-010-0154-1.
Koziol U, Dominguez MF, Marin M, Kun A, Castillo E: Stem cell proliferation during in vitro development of the model cestode Mesocestoides corti from larva to adult worm. Front Zool. 2010, 7: 1-12. 10.1186/1742-9994-7-22.
Sprecht D, Voge M: Asexual multiplication of Mesocestoides tetrathyrdidia in laboratory animals. J Parasitol. 1965, 51: 268-272. 10.2307/3276097.
Markoski MM, Bizarro CV, Farias S, Espinoza I, Galanti N, Zaha A, Ferreira HB: In vitro segmentation induction of Mesocestoides corti (Cestoda) tetrathyridia. J Parasitol. 2003, 89: 27-34. 10.1645/0022-3395(2003)089[0027:IVSIOM]2.0.CO;2.
Olson PD: Hox genes and the parasitic flatworms: New opportunities, challenges and lessons from the free-living. Parasitol Int. 2008, 57: 8-17. 10.1016/j.parint.2007.09.007.
Olson PD, Caira JN: Evolution of the major lineages of tapeworms (Platyhelminthes: Cestoidea) inferred from 18S ribosomal DNA and elongation factor-1α. J Parasitol. 1999, 85: 1134-1159. 10.2307/3285679.
Olson PD, Littlewood DTJ, Bray RA, Mariaux J: Interrelationships and evolution of the tapeworms (Platyhelminthes: Cestoda). Mol Phylogenet Evol. 2001, 19: 443-467. 10.1006/mpev.2001.0930.
Olson PD, Poddubnaya LG, Littlewood DTJ, Scholz T: On the position of Archigetes and its bearing on the early evolution of the tapeworms. J Parasitol. 2008, 94: 898-904. 10.1645/GE-1456.1.
Waeschenbach A, Webster BL, Bray RA, Littlewood DTJ: Added resolution among ordinal level relationships of tapeworms (Platyhelminthes: Cestoda) with complete small and large subunit nuclear ribosomal RNA genes. Mol Phylogenet Evol. 2007, 45: 311-325. 10.1016/j.ympev.2007.03.019.
Healy CJ, Caira JN, Jensen K, Webster B, Littlewood DTJ: Proposal for a new tapeworm order, Rhinebothriidea. Int J Parasitol. 2009, 39: 497-511. 10.1016/j.ijpara.2008.09.002.
Kuchta R, Scholz T, Brabec J, Bray RA: Suppression of the tapeworm order Pseudophyllidea (Platyhelminthes: Eucestoda) and the proposal of two new orders, Bothriocephalidea and Diphyllobothriidea. Int J Parasitol. 2008, 38: 49-55. 10.1016/j.ijpara.2007.08.005.
Olson PD, Caira JN, Jensen K, Overstreet RM, Palm HW, Beveridge I: Evolution of the trypanorhynch tapeworms: parasite phylogeny supports independent lineages of sharks and rays. Int J Parasitol. 2010, 40: 223-242. 10.1016/j.ijpara.2009.07.012.
Cooper D: The preparation of serial sections of platyhelminth parasites, with details of the materials and facilities required. Syst Parasitol. 1988, 12: 211-229. 10.1007/BF00007769.
Olsen OW: Animal parasites: their biology and life cycles. 1962, Minneapolis: Burgess Publishing Co
Acknowledgements
We thank especially Jerzy Behnke for providing a seed culture of H. microstoma and for assistance in tracking the history of the laboratory isolate. Special thanks also to Matt Berriman, Magdalena Zarowiecki and Alejandro Sanchez-Flores for leading the genome initiative at the Wellcome Trust Sanger Institute. Thanks to Jayne King and Natasha Pouchkina-Stantcheva for assistance with maintenance of the model, to Dave Cooper for histological sectioning, and to Lauren Howard and Alex Ball for assistance with SEM. Thanks also to Rod Bray for commenting on an earlier draft of the manuscript. This work was supported in part by a BBSRC grant to PDO (BBG0038151).
This work is dedicated to the memory of Clark P. Read: father of the Hymenolepis model and a scientist who was in his time "Parasitology's ambassador to the fields of physiology, biochemistry and molecular biology" [1].
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PDO designed the study and drafted the manuscript. LC carried out research. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cunningham, L.J., Olson, P.D. Description of Hymenolepis microstoma (Nottingham strain): a classical tapeworm model for research in the genomic era. Parasites Vectors 3, 123 (2010). https://doi.org/10.1186/1756-3305-3-123
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-3305-3-123