Abstract
Background
Interstitial deletions of chromosome 11 long arm are rarely observed and the associated phenotype ranges from normal to severe, depending on the position and size of the deletion and on the presence of unmasked recessive genes on the normal homologous. To our knowledge 32 cases are reported in literature with three family cases. Phenotype-genotype correlation is not very clear and the most common features are characteristic facial dysmorphisms, palate anomalies and developmental delay. Growth retardation is not typical and other major malformations are reported in some cases.
Case Presentation
We described a child with 11q interstitial deletion diagnosed at birth with hypotonia and minor dysmorphisms using standard cytogenetic techniques; array CGH was subsequently performed to define the deletion at a molecular level.
Conclusions
This case gave us the opportunity to attempt a genotype-phenotype correlation reviewing the literature and to describe a rehabilitative program that improved the development perspectives of this child.
Similar content being viewed by others
Background
Terminal 11q deletions are reported in literature to be associated with the well-described Jacobsen Syndrome. On the contrary interstitial deletions are very uncommon and heterogeneous in size and location of breakpoints, spanning from bands 11q13 to 11q23. From the first description by Taillemite et al. [1], 31 more cases were described [2–21], case ID 4366 in the European Cytogenetists Association Register of Unbalanced Chromosome Aberration, ECARUCA (http://www.ecaruca.net), case ID 3945 ECARUCA. Only in seven reports the deletion was characterized by cytogenetic molecular techniques [2–8]. The majority of deletions are de novo, but three family cases are described with normal or borderline phenotype [2, 4, 7]. Position and size of deletions are heterogeneous. The definition of the breakpoints of an interstitial 11q deletion by conventional techniques is difficult because the banding pattern could be confusing, but new molecular methods such as array CGH and SNPs allow an optimal definition of the breakpoint regions. Clinical phenotype includes several dysmorphic features, palate anomalies and developmental delay. Growth retardation, hypotonia, seizures, congenital heart malformation, kidney and skeletal anomalies are associated features in some cases.
Here we describe a case of de novo interstitial deletion of chromosome 11 long arm identified at birth while investigating mild dysmorphisms and hypotonia. The long term rehabilitative program is described and the good results obtained allow us to be positive in the prognosis of similar cases of 11q interstitial deletions. Finally we attempt a genotype/phenotype correlation reviewing the literature to search candidate genes with a role in the phenotype.
Case presentation
The proband is a boy and he is the only child born from a 36 year old father and a 27 year old mother. The parents were healthy and unrelated; paternal family history was negative for genetic diseases, while in the maternal family, eight members were affected by retinitis pigmentosa with an autosomal dominant pattern of transmission. Prenatal ultrasonographic evaluation gave normal results in fetal growth and morphology. No prenatal invasive genetic test was performed.
The child was born by natural delivery after a 40 week gestation plus 5 days. Birth weight was 3420 g (25°- 50° centile for gestational age), length at birth was 52 cm, (between 50°- 75° centile), occipito-frontal circumference (OCF) was 35.5 cm (50°- 75° centile), APGAR score was 9/10. In neonatal period, the child presented hyperpyrexia (blood, urine and cerebrospinal fluid microbiological analyses were all negative) and greater than 10% weight loss, which regressed with adequate nutrition. Episodes of bronchospasm and tirage, repeated episodes of cyanosis and desaturation resolved spontaneously shortly before discharge (at one month of age). During childhood his height growth was at the lower limit. He did not show any major malformation, besides submucous cleft palate. Brain MRI scan did not show any structural anomalies.
He had mild myopia and his hearing was normal. He did not show any significant medical complications.
At the age of 12 years and 1 month his weight was 41.6 kg (25°-50° percentile), his height was 134.2 cm (<3° percentile) and his OFC was 53 cm (50° percentile). His Body Mass Index (BMI) was 23 (75°-90° percentile). Facial dysmorphic features included long face, high nasal bridge, short and smooth philtrum, micrognathia. He had small hands and nasal speech.
Endocrinological analysis (IGF-1 dosage and growth hormone stimulation test with arginine) showed results consistent with isolated growth hormone (GH) deficiency. A further brain MRI scan, carried out when he was twelve, revealed a small pituitary gland. GH treatment has been advised and the patient has just started this therapy.
Written informed consent was obtained from the parents for publication of this case report and any accompanying images.
At birth, karyotype on peripheral blood lymphocytes was requested because of mild hypotonia and minor dysmorphic features.
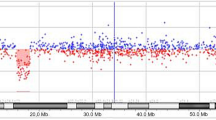
Conventional cytogenetic analysis revealed an interstitial deletion on chromosome 11 from bands 11q13.5 to 11q21 (Figure 1A). The parents’ karyotypes were both normal and the analysis was extended to 100 metaphases to rule out 11q deletion mosaicism. At the age of twelve, a comparative genomic hybridization array (Array-CGH) was performed using a CGH + SNP 4 × 180 K microarray kit (Agilent Technologies). The microarray contained 110,172 probes with a median probe spacing of 25.3 Kb (5 KB in International Standards for Cytogenomic Arrays Consortium, ISCA) and 59,647 SNP (UCSC hg19, http://genome.ucsc.edu/). Array-CGH was performed according to the manufacturer’s instructions (Agilent Technologies) and allowed the identification of a more distal deletion of 17,2 Mb in size: from 11q14.3 to 11q22.3 (from nt 92,434,272 to nt 109,584,301)(555 probes with median Log2ratio value of −1.04). In the same region, 337 SNP probes showed a single allele (Figure 1B,C). No additional pathogenetic Copy Number Variations (CNVs) were detected. The molecular karyotype, defined following the International System of Chromosome Nomenclature 2013, was: arr 11q14.3q22.3(92,434,272-109,584,301)x1.
Cytogenetic, arrayCGH and WISC-III results. (A) QFQ and GTG banded chromosome 11 homologues. The deleted chromosome 11 is on the right. (B) Array-CGH analysis showing 17.2 Mb deletion (Log2ratio: −1.04) at 11q14.3q22.3 (nt 92434272–109584301; hg19, NCBI build 37) (light blue bar) and loss of heterozygosity with 337 single alleles SNP. (C) enlargement of the deleted region (CytoGenomics software Agilent). (D): WISC-III results: Verbal(VIQ), Performance (PIQ) and Total IQ (TIQ) at 9.11 ys and after 2 years of rehabilitation and counselling to parents and teachers as regard as learning abilities.
At 1 month of age, the proband’s neurological examination showed no major dysfunctions; clinical observation according to Prechtl´s Method on the Qualitative Assessment of General Movements [22] showed a pattern characterized by Poor Repertoire. His motor milestones were not significantly delayed: control of the head at 4 months, sitting without support at 8 months, hands and knees crawling at 11 months, standing with assistance at 13 months, walking alone at 24 months.
Parents reported vocalization, babbling and the first word “mamma” being uttered at one year of age. “Mamma” remained the only word used to name everything word until 19 months of age. After a period of hospitalization following an accidental fall which caused clavicle fracture and a second spell in hospital due to gastroenteritis, the child presented an arrest in speech and psychomotor competencies. These aspects were inscribed in a global regression of communicational and relational capacities.
Speech development between 20 and 36 months was characterized by: aphonia, amimia, pseudo- articulatory movements, reduced opening of the labial rima. Moreover, the child showed a poor communicative intent even at a non verbal level.
At 3 years he was a clumsy, passive and withdrawn child, not interested in playing. Therefore, at 3 years of age a psychomotor treatment with attention to relational aspects was introduced twice a week.
During the first year of rehabilitation, the proband reduced his more passive traits becoming more and more purposeful, actively responsive and assertive. Communicative intention emerged together with the increasing awareness of his speech difficulties.
There was a large discrepancy between the verbal comprehension (discrete) and expressive skills: he used gestures associated with vocalization to indicate, to formulate questions, to express disappointment or excitement. Then, speech therapy sessions began twice a week at the age of 4.
The proband showed a deficit of phonetic and non-phonetic phonation. Later, rhinolalia and rhinophonia, and a deficit in coordination and in phonological memory were observed. Consequently, a double diagnosis of specific expressive language disorder and of childhood apraxia of speech was formulated.
At 5 years, after one year of speech therapy, he achieved a quite complete phonetic repertoire, vocabulary increase with risk threshold for semantic errors, and emerging narrative skills. While he showed an adequate lexical comprehension, morphosyntactic comprehension was delayed of one year and communication was supported by mimics and gestures. At the same time, he developed social skills, showing more relational and playing capacities with peers and adults.
At age 6, verbal speech was sufficiently intelligible despite the persistence of hypotonic and uncoordinated glosso-velo-pharyngeal muscles.
His cognitive development, as measured by WIPPSI Scale, was in the lower limits of the normal range, with a discrepancy between verbal (VIQ) and performance (PIQ) scores (VIQ =85; PIQ =96; Full Scale IQ = 89) [23].
At the age of 8, the proband presented an impairment in the ability of naming objects and in lexical retrieval with the help of phonemic cues. On using the Italian version of the Peabody Picture Vocabulary Test [24], comprehension was almost adequate (Language quotient = 82).
Morphosyntactic comprehension using the Rustioni Metz test [25] showed a delay of about 2 years. The verbal short term memory span was 3 (inadequate).
Moreover, learning difficulties in the presence of a cognitive development at the lower limit of the normal range emerged. In particular, reading comprehension, measured using the MT battery of tests [26], was inadequate (correct answers: 6/10), in the absence of dyslexia and dysorthography, as measured with the Dyslexia and Orthographic coding test [27]. Therefore, the logopedic rehabilitation continued until the child was 9 years old, with the aim to improve oral and written language comprehension. In the meantime, his writing and reading abilities supported him in planning the phonological string in speech.
Afterwards, counselling to parents and teachers, together with neuropsychiatric follow up continued until today with longitudinal controls (twelve years). We considered highly important to support parents and teachers in finding a harmonious global development which could take into account not only the educational aspects, but also the emotional and relational sides.
We were able to observe a global improvement in cognitive abilities (as illustrated in Figure 1D, where WISC III at 9 and 11 years of age are compared) [28].
Currently the child shows good skills at school in the application of the learned mechanisms, but he has more difficulties when logic skills and problem solving abilities are required. However, he is able to evaluate his work and adopt compensatory strategies in order to overcome his difficulties. He is competitive with his schoolmates, comparing their tests results and scores. He shows a good personal and social independence and participates in curricular and extracurricular activities with peers.
Conclusions
Interstitial 11q deletions are rare with only 32 patients described in literature to our knowledge [1–21] (Additional file 1: Table S1) they span from bands 11q13 to 11q23, but the majority of them has not been characterized with a molecular approach. A map was drawn up, in which deletions were positioned on the 11q chromosome ideogram (550 band resolution level) on the basis of the deletion sizes (from the largest to the smallest). When breakpoints were defined with a low resolution level (i.e. 11q13), we assumed the breakpoint to be in the middle (11q13.2). We disregarded two cases because of uncertain breakpoints [9, 15]. We report some frequent clinical signs selected from Table 1. Heterogeneity in position and size is evident and a minimal common deleted region could not be identified (Figure 2). Moreover, cases with a molecularly-defined deletion (Figure 2, light blue bar) are only five [3, 5–8]. Since they do not overlap and they are different in size a genotype/phenotype correl cannot be assessed. The first observation is that moderate/severe developmental delay correlates with the deletion size: in fact it was only reported in patients with larger loss, whereas growth retardation is distributed in the entire region. Growth is likely to be influenced by many factors, both genetic and environmental; it is therefore difficult to identify a close relationship with the deletion itself. Palate anomalies and seizures seem to correlate with more distal deletions (11q22-q23), while kidney/genital anomalies and cardiac malformations are reported in both proximal (11q13q21) and in distal ones (11q21q23.3) and don’t seem to correlate with the deletion size. The two families reported by Goumy et al. [2] and Li et al. [4] (Additional file 1: Table S1) with phenotypically normal deletion carriers are very interesting. Goumy et al. [2] describe a morphologically normal female fetus that inherited a 11q14.3q21.1 deletion from the mother whose clinical features were toe camptodactyly and ophtalmologic disorders. Her phenotypically normal maternal grandfather carried the same deletion. Prenatal diagnosis was carried out because of a positive Down Syndrome maternal serum screening. Li et al. [4] reported a 6 year old boy with mild intellectual impairment, short stature and a 11q14.3q21 deletion. Family study showed four males through three generations with the same deletion and apparently normal phenotype. The authors hypothesized a casual association between chromosomal anomaly and proband phenotype. In both reports, FISH with BAC clones was performed in order to partially define deletion breakpoints: Li identified a 3.6 Mb deletion (from rp11-792 M23 to rp11-573 M3) while Goumy defined the lowest deleted size of 8.5 Mb [from rp11-372E19 (91,733 Mb) to rp11-775E2 (100,424 Mb)]. These regions range approximately from nucleotide 89,255,000 (11q14.3) to 100,424,000 (11q22.1). Sparkes et al. [7] (Additional file 1: Table S1) described the third familial case: a male fetus tested for ultrasound multiple anomalies (choroid plexus cysts, echogenic intracardiac foci, a suspected structural cardiac malformation, left club foot and small cerebellum) with a maternally inherited 11q14.3q22.3 deletion. The mother was of normal intellect and healthy, but she had a surgically repaired bilateral club foot and high myopia. Moreover a cerebral MRI in the mother showed multifocal white matter changes and mild cerebral atrophy. Array CGH evidenced a 17,3 Mb deletion (from nt 89,492,818 to nt 106,832,040) and a 0,9 Mb duplication (from nt 88,258,744 to nt 89,103,489) in 11q21q23. From a molecular point of view, this case partially overlaps with our case (17,2 Mb deletion from nt 92,434,372 to nt 109,584,30), but we could not find any common clinical features: the case we are presenting showed mild developmental delay and submucous cleft palate; a cerebral MRI scan did not evidence any atrophy and he did not suffer from club foot.
Deletions map with major clinical signs reported as coloured dots. Chromosome 11q arm ideogram with on the left gene density (from Ensemble http://www.ensembl.org/index.html) and, on the right, vertical bars are deletions reported in literature from the largest to the smallest. Red bar PC: present case. Green bars: family cases. Light blue bars: cases with molecularly defined deletions (13a and 13 b are family cases). The numbers above the bars correspond to those reported in Additional file 1: Table S1. Specific clinical signs are reported as coloured dots.
In the deleted region of our patient, about 94 genes are mapped [http://genome.ucsc.edu/]: 53 of them have known functions, some of them being associated with autosomal recessive disease, others have no OMIM phenotype. A biological role is still unkwnown for the other 41 genes.
Eight OMIM genes are expressed in the brain (FAT 3, MED17, PANX1, GPR83, CNTN5, KIAA1377,GRIA4 and GUCY1A2). The CNTN5 gene, coding for contactins which mediate cell surface interactions in the development of the nervous system, is described as been involved in autism spectrum disorders [29]. The GRIA4 gene belongs to the glutammate receptor family that plays an important role in excitatory synaptic transmission. Patients with mental retardation and multiple congenital abnormalities showed a significant copy number change in the glutammate receptor family [30]. Even if it is very difficult to correlate particular clinical features to a specific gene, we may hypothesize the role of haploinsufficiency of these two genes in the patient phenotype.
In literature [8, 10, 12, 16, 20] a psychomotor delay and a language disorder were described, in a few cases, as an expressive disorder. Our patient also showed the particular trait of a childhood apraxia of speech.
The phenotype we observed in our case has only been described in one other report [11] in which the proband shows poor eye contact and low interest in his surrounding. The rehabilitation process proposed and the neuropsychiatric follow up and counselling have allowed the recovery of adequate relational competences, improved speech and language abilities and a positive evolution of his global cognitive and adaptive capacities and social and school skills.
After considering all the collected clinical information, we drew the conclusion that it is not possible to define a distinctive phenotype of the 11q partial monosomy due to the heterogeneity in size and position of the deletions and to the absence of a minimal common deleted region. About family cases showing normal phenotype [2, 4] we could hypothesize the effect of modifier genes or a compensatory gene expression of the allele on the normal chromosome 11. More literature data and a better molecular characterization are needed to confirm this hypothesis. Moreover, the phenotype is not only due to haploinsufficient genes but it is the result of complex gene – gene and gene-environment interactions.
We found this case particularly interesting because it gave us the opportunity to describe a rehabilitation program that we believe has improved the development perspectives of this child and therefore his quality of life.
Consent
Written informed consent was obtained from the patient’s parents for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
Taillemite JL, Morlier BG, Roux C: Interstitial deletion of the long arm of one 11 chromosome. Ann Genet. 1975, 18 (1): 61-63.
Goumy C, Gouas L, Tchirkov A, Roucaute T, Giollant M, Veronèse L, Francannet C, Vago P: Familial deletion 11q14.3-q22.1 without apparent phenotypic consequences: a haplosufficient 8.5 Mb region. Am J Med Genet A. 2008, 146A (20): 2668-2672. 10.1002/ajmg.a.32511.
Krgovic D, Marcun Varda N, Zagorac A, Kokalj-Vokac N: Submicroscopic interstitial deletion of chromosome 11q22.3 in a girl with mild mental retardation and facial dysmorphism: Case report. Mol Cytogenet. 2011, 4: 17-10.1186/1755-8166-4-17.
Li L, Moore P, Ngo C, Petrovic V, White SM, Northrop E, Ioannou PA, McKinlay Gardner RJ, Slater HR: Identification of a haplosufficient 3.6-Mb region in human chromosome 11q14.3->q21. Cytogenet Genome Res. 2002, 97 (3–4): 158-162.
Li P, Zhang HZ, Huff S, Nimmakayalu M, Qumsiyeh M, Yu J, Szekely A, Xu T, Pober BR: Karyotype-phenotype insights from 11q14.1-q23.2 interstitial deletions: FZD4 haploinsufficiency and exudative vitreoretinopathy in a patient with a complex chromosome rearrangement. Am J Med Genet A. 2006, 140 (24): 2721-2729.
Melis D, Genesio R, Cozzolino M, Del Giudice E, Mormile A, Imperati F, Ronga V, Della Casa R, Nitsch L, Andria G: An emerging phenotype of proximal 11q deletions. Eur J Med Genet. 2010, 53 (5): 340-343. 10.1016/j.ejmg.2010.07.010.
Sparkes RL, Shetty S, Chernos JE, Mefford HC, Micheil IA: Interstitial deletion of 11q in a mother and fetus: implications of directly transmitted chromosomal imbalances for prenatal genetic counseling. Prenat Diagn. 2009, 29 (3): 283-286. 10.1002/pd.2209.
Wincent J, Schoumans J, Anderlid BM: De novo deletion of chromosome 11q13.4-q14.3 in a boy with microcephaly, ptosis and developmental delay. Eur J Med Genet. 2010, 53: 50-53. 10.1016/j.ejmg.2009.10.003.
Carnevale A, Blanco B, Grether P, Castillejos AR: Interstitial deletion of the long arm of chromosome 11. Ann Genet. 1987, 30 (1): 56-8.
De Pater JM, Ippel PF, Bijlsma JB, Van Nieuwenhuizen O: Interstitial deletion 11q. Case report and review of the literature. Genet Couns. 1997, 8 (4): 335-339.
Guć-Sćekić M, Pilić-Radivojević G, Mrdjenović G, Djurić M: Interstitial deletion of 11q. J Med Genet. 1989, 26 (3): 205-206. 10.1136/jmg.26.3.205.
Horelli-Kuitunen N, Gahmberg N, Eeva M, Palotie A, Järvelä I: Interstitial deletion of bands 11q21->22.3 in a three-year-old girl defined using fluorescence in situ hybridization on metaphase chromosomes. Am J Med Genet. 1999, 86 (5): 416-9. 10.1002/(SICI)1096-8628(19991029)86:5<416::AID-AJMG4>3.0.CO;2-S.
Ikegawa S, Ohashi H, Hosoda F, Fukushima Y, Ohki M, Nakamura Y: Pseudoachondroplasia with de novo deletion [del(11)(q21q22.2)]. Am J Med Genet. 1998, 77 (5): 356-359. 10.1002/(SICI)1096-8628(19980605)77:5<356::AID-AJMG3>3.0.CO;2-L.
Joyce CA, Zorich B, Pike JS, Barber JCK, Dennis NR: Williams-Beuren syndrome: phenotypic variability and deletions of chromosome 7, 11, and 22 in a series of 52 patients. J Med Genet. 1996, 33: 986-992. 10.1136/jmg.33.12.986.
Klep-de Pater JM, de France HF, Bijlsma JB: Interstitial deletion of the long arm of chromosome 11. J Med Genet. 1985, 22 (3): 224-226. 10.1136/jmg.22.3.224.
Meyer MF, Gerresheim F, Pfeiffer A, Epplen JT, Schatz H: Association of polycystic ovary syndrome with an interstitial deletion of the long arm of chromosome 11. Exp Clin Endocrinol Diabetes. 2000, 108 (8): 519-523. 10.1055/s-2000-11023.
Ono J, Hasegawa T, Sugama S, Sagehashi N, Hase Y, Oku K, Endo Y, Ohdo S, Ishikiriyama S, Tsukamoto H, Okada S: Partial deletion of the long arm of chromosome 11: ten Japanese children. Clin Genet. 1996, 50 (6): 474-478.
Sachdeva R, Sears JE, Rychwalski PJ: A novel case of bilateral high myopia, cataract, and total retinal detachment associated with interstitial 11q deletion. Ophthalmic Genet. 2010, 31 (2): 84-8. 10.3109/13816811003628833.
Stratton RF, Lazarus KH, Ritchie EJ, Bell AM: Deletion (11)(q14.1q21). Am J Med Genet. 1994, 49 (3): 294-298. 10.1002/ajmg.1320490310.
Syrrou M, Fryns JP: Interstitial deletion of chromosome 11 (q22.3-q23.2) in a boy with mild developmental delay. J Med Genet. 2001, 38 (9): 621-624. 10.1136/jmg.38.9.621.
Wakazono A, Masuno M, Yamaguchi S, Tsubouchi K, Kondo N, Orii T: Interstitial deletion of the long arm of chromosome 11: report of a case and review of the literature. Jpn J Hum Genet. 1992, 37 (3): 229-234. 10.1007/BF01900717.
Einspieler C, Prechtl HFR, Bos AF, Ferrari F, Cioni G: Prechtl's Method on the Qualitative Assessment of General Movements in Preterm, Term and Young Infants. 2004, New York: Cambridge University Press
Wechsler D: Wechsler Preschool and Primary Scale of Intelligence – Revised. 1989, San Antonio, TX: The Psychological Corporation
Stella G, Pizzoli C e, Tressoldi PE: Peabody – Test di vocabolario recettivo. 2000, Torino: Omega
Rustioni D, Metz Lancaster M: Evaluation tests of linguistic understanding. 1994, Firenze, Italy: O.S. Giunti
Cornoldi C, Colpo G: MT tests of comprehension Firenze. 1995, Italy: O.S. Giunti
Sartori G, Job R, Tressoldi PE: Battery for the assessment of dyslexia and developmental spelling. 1995, Firenze, Italy: O.S. Giunti
Wechsler D: WISC-III: Wechsler intelligence scale for children. 1991, New York: The Psychological Corporation
van Daalen E, Kemner C, Verbeek NE, van der Zwaag B, Dijkhuizen T, Rump P, Houben R, Van't Slot R, de Jonge MV, Staal WG, Beemer FA, Vorstman JA, Burbach JP, van Amstel HK, Hochstenbach R, Brilstra EH, Poot M: Social Responsiveness Scale-aided analysis of the clinical impact of copy number variations in autism. Neurogenetics. 2011, 12 (4): 315-23. 10.1007/s10048-011-0297-2.
Poot M, Eleveld M MJ, van 't Slot R, Ploos van Amstel HK, Hochstenbach R: Recurrent copy number changes in mentally retarded children harbour genes involved in cellular localization and the glutamate receptor complex. Eur J Hum Genet. 2010, 18 (1): 39-46. 10.1038/ejhg.2009.120.
Acknowledgments
We wish to thank the speech therapists S. Artesani and A. Milishy for their valuable help.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors do not have any competing interest, financial or otherwise, to declare.
Authors’ contributions
RN, FB contributed to design the study, collected the literature data and performed the neuropsychiatric evaluation of the patient and the rehabilitation programme and wrote a part of the article MB contributed to design the study, collected the literature data and wrote a part of the article. PS contributed to the neuropsychiatric evaluation of the patient. NV contributed to design the study, performed genetic studies on the patient, collected the literature data and wrote a part of the article. S.R. performed arrayCGH. LD and FN contributed to design the study and critically read the manuscript. AS, AS performed the paediatric evaluation of the patient. All authors read and approved the final manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.
About this article
Cite this article
Nacinovich, R., Villa, N., Redaelli, S. et al. Interstitial 11q deletion: genomic characterization and neuropsychiatric follow up from early infancy to adolescence and literature review. BMC Res Notes 7, 248 (2014). https://doi.org/10.1186/1756-0500-7-248
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-0500-7-248