Abstract
Background
Tuberculosis (TB) remains a major global health problem. To investigate the genotypes of Mycobacterium tuberculosis (MTB) and the distribution of Beijing family strains, molecular epidemiology technologies have been used widely.
Methods
From June 2010 to June 2011, 55 M. tuberculosis isolates from patients with pulmonary TB were studied by Beijing family-specific PCR (detection of the deletion of region of difference 105 [RD105]), and mycobacterial interspersed repetitive units variable number tandem repeat (MIRU-VNTR) analysis. Twenty-four MIRU-VNTR loci defined the genotypes and clustering characteristics of the local strains. All strains were subjected to a drug susceptibility test (DST) by the proportion method on Lowenstein-Jensen (LJ) culture media.
Results
Fifty-five clinical isolates of MTB were collected. Beijing family strains represented 85.5% of the isolates studied. Using 24 loci MIRU-VNTR typing categorized the strains into eight gene groups, 46 genotypes, and seven clusters. 83.6% (46/55) of the isolates belonged to the largest gene group. Thirty-six isolates (65.5%) were susceptible, nineteen (34.5%) were resistant to at least one drug, seven (12.8%) were Multidrug-Resistant Tuberculosis (MDR TB), and two (3.6%) were extremely drug-resistant tuberculosis (XDR-TB).
Conclusion
The results showed there were obvious polymorphisms of VNTRs of MTB clinical strains. Beijing family strains of MTB were predominant in the Shihezi region of Xinjiang province. There was no correlation between the drug-resistance and Beijing family strains of MTB. It is necessary to strengthen the monitoring, treatment, and management of drug-resistance TB in Shihezi region, Xinjiang.
Similar content being viewed by others
Background
Tuberculosis (TB) is an infectious disease caused by the bacillus Mycobacterium tuberculosis (MTB). TB remains a major public health threat worldwide. China has occupied second place, behind India, among the top five high-burden countries for the last decade (http://www.who.int/tb/en). In 2010, there were 8.8 million (range, 8.5–9.2 million) incident cases of TB, and 1.1 million (range, 0.9–1.2 million) deaths from TB among HIV-negative people [1]. Genotyping methods have been extensively used to analyze the recent transmission dynamics of MTB. Different PCR-based genotyping approaches targeting the variable number of tandem repeats (VNTR) have been developed, based on the mycobacterial interspersed repetitive units (MIRU) [2, 3], which are considered a good alternative to the reference method and have proven to be faster and easier to perform. The discriminatory power of MIRU-VNTR analysis is related to the number of loci. MIRU-VNTR genotyping is performed by amplifying a panel of 12, 15, or 24 loci [4]. More recently, a set of 24 MIRU-VNTR loci was reported to have greater discriminatory power than the original 12 loci system and may exceed that of restriction fragment length polymorphism (RFLP) when combined with spoligotyping [5, 6]. The MIRU-VNTR method is a reliable and reproducible typing method with high discriminatory power for studying the MTB population structure in different countries [7–12].
The MTB Beijing family, first identified in 1995 in Beijing, China, is ubiquitously and significantly prevalent in certain world regions, e.g., East Asia [13]. Members of the MTB Beijing family are a major concern because of their high prevalence in tuberculosis patients and their high rate of multi-drug resistance [14]. Several studies have observed that the Beijing family MTB strains exhibits important pathogenic features that might be associated with drug resistant TB in China [15–17]. As the prevalence of drug resistant clones of MTB varies from one area to another, studies of the geographical distribution of resistant clones are useful for understanding the epidemiological characteristics of TB. Spoligotyping usually determines the genotype of a Beijing strain of MTB. However, this technique requires special equipment and is time-consuming. A previous study demonstrated a high the correlation between Spoligotyping and the RD105 deletion for the identification of the Beijing family genotype [17]. The simple and rapid new method, the RDl05 deletion test, was used to identify the Beijing family instead of Spoligotyping [18]. RD105 deletions have previously been reported to be Beijing family strains-specific. One large sequence polymorphism, the (LSP)-RD105 genomic deletion, was observed in all Beijing family strains and thus serves as a useful marker for the identification of this family of strains [19]. The RD105 deletion test was performed to identify Beijing family isolates in different countries [20].
Xinjiang is a province on the Northwestern coast of China. The incidence of TB in Xinjiang is estimated at 463 cases per 100,000 persons per year. The prevalence of drug-resistant TB in Xinjiang Province is higher than the average level in China. Xinjiang is a multiethnic area, being home to 55 ethnicities. The most numerous are the Uygur (46.06%), followed by Han (39.33%), and Kazakh (7.08%). In the northern regions of Xinjiang, most of the population is Han, but in the southern regions, about 85% of the population is Uygur, and only 8% of the population is Han. Shihezi region is in northern Xinjiang and has area of 456.8 square kilometers and a population of 630,000; 95% of the population is Han. In 2008, the network epidemic of Shihezi reported 991 tuberculosis cases and prevalence of 152.15/100,000. TB ranks as the second statutory infectious disease in Shihezi [21].
The main goal of this study was to genotype MTB strains circulating in the Shihezi region of Xinjiang Province using MIRU-VNTR-24 locus analysis and to understand the genetic diversity of Beijing and non-Beijing isolates by the RD105 deletion test. We also sought to the determine drug susceptibility patterns of the MTB isolates and whether the drug resistance of epidemic TB are directly related to the spread of Beijing family strains.
Methods
Mycobacterial specimens
This study included M. tuberculosis samples isolated between June 2010 and June 2011 from Shihezi in Xinjiang Province. Standard questionnaires were used to collect classical epidemiology data. Information was obtained on sex, age, place of birth, recent positive smear test, previous history of TB, and current address.
Strain isolation and drug susceptibility test
The sputum samples were cultured and isolated on Lowenstein-Jensen (LJ) culture media. Four first-line anti-TB drugs (isoniazid [INH], rifampicin [RFP], streptomycin [SM], and ethambutol [EMB]) and seven second-line anti-TB drugs (ofloxacin [Ofx], Capreomycin [Cm], Amikacin [Am], Kanamycin [KM], P-aminosalicylicacid [PAS], Ethionamide [Eto], and Cycloserine [Cs]) were incorporated into LJ medium, at the following concentrations: INH0, 2 μg/ml; RFP, 40.0 μg/ml; SM, 4.0 μg/ml; EMB, 2.0 μg/ml; Ofx, 2.0 μg/ml; Cm, 40.0 μg/ml; Km, 30.0 μg/ml; Am, 40.0 μg/ml; PAS, 1.0 μg/ml; Eto, 40.0 μg/ml; and Cs, 40.0 μg/m, and used to detect the drug-resistance of the MTB by the proportion method. Strain were scored as resistant to a specific drug, or were defined as sensitive thief their growth rate was < 1% compared to the control. Strain isolation, identification, and drug susceptibility tests (DST) were performed at the Ministry of Education Key Laboratory of Xinjiang Endemic and Ethnic Disease.
Genomic DNA extraction and molecular identification of M. tuberculosis isolates
Mycobacterial genomic DNA was extracted from mycobacterial colonies growing on LJ medium. Scraped colonies were dissolved in 200-300 μl of distilled water and inactivated at 85°C for 30 min, before being centrifuged at 8000 r/min for 5 min. The pellets were resuspended in 300 μl of TE (pH 8.3), boiled for 30 min, and centrifuged at 10,000 r/min for 5 min. Supernatants were collected and stored at −20°C until further use [22].
Molecular identification of the mycobacterial isolates was performed using PCR amplification of the 16 S rRNA gene and MTP40 gene [23]. The PCR mixture consisted of 0.2 μg DNA template, 3 μl buffer, 4 μl 10 mM deoxynucleoside triphosphates, 1 μl of each primer (10 pmol/μl), and 1 μl DNA Taq polymerase. The amplification cycle was 5 min at 95°C; followed by 30 cycles of 40 s at 95°C, 50 s at 65°C, and 40 s at 72°C; with a final 10 min at 72°C. PCR products were analyzed on a 2% agarose gel against a 100-bp DNA ladder.
Genotyping by MIRU-VNTR PCR
MIRU-VNTR genotyping was performed by amplifying the 24 MIRU-VNTR loci as described previously in a technical guide [24]. The 24 MIRU-VNTR genetic loci consisted of ten original MIRU-VNTR loci; six loci of exact tandem repeats (ETRs: ETR-A, -B, -C, -D, -E and –F), five Mtub loci (Mtub4, 21, 30, 38, and 39), and three Queen’s University of Belfast (QUBs) loci (QUB-11b, -26, and 4156c). Primers were as described by Supply et al. [25]. For each reaction, DNA from M. tuberculosis H37Rv was used as a positive control, and sterile water was used as a negative control. PCR products were electrophoretically separated on 2% agarose gels, using a 100-bp DNA ladder as size markers. From the gel images, the corresponding MIRU-VNTR bands were interpreted as copy numbers based on the reference table in the Supply 2005 protocol [24]. The copy number at each locus was calculated using the Quantity 1 gel imaging system.
Beijing Family strains analysis
The identification of Beijing Family strains was performed by detecting deletions in region of difference 105 (RD105) by PCR [18]. Each PCR mixture was prepared in a volume of 15 μl containing 50 ng of DNA, 1U of Taq polymerase, a 0.2 mM concentration of each deoxynucleoside triphosphate (dNTP), and 0.2 μM (each) primer. The amplification cycle was 5 min at 94°C; followed by 30 cycles of 30 s at 94°C, 30 s at 62°C, and 45 s at 72°C; with a final step for 10 min at 72°C. PCR products were electrophoretically separated on 2% agarose gels.
Results
Patient characteristics
Ninety sputum specimens from patients with pulmonary TB were collected from June 2010 to June 2011. All of the pulmonary TB patients were Han Chinese. Among 90 patients, 30 (33.3%) specimens were either culture negative or had culture contamination and were excluded. In addition, another five specimens (5.5%) were excluded because their cultures were mycobacteria other than TB (MOTT). Therefore, 55 culture positive specimens were used for this study.
Of the 55 patients, 20 were new TB cases and 35 were previously treated patients. The mean age of the patients was 47.21 yr (± 2.26). There were 38 males (69.1%) and 17 females (30.9%).
MIRU-VNTR genotyping
Fifty-five MTB isolates were genotyped and 46 different VNTR genotypes were detected. Thirty nine strains (70.9%) were unique and 16 strains (29.1%) could be grouped into seven clusters, each including 2–3 strains (Figure 1). Two main clusters which contained 3 (18.7%) and 3 (18.7%) strains showed 4 2 4 3 5 2 3 3 2 5 2 6 3 3 3 4 5 4 4 4 3 8 6 2 and 4 2 4 3 5 2 3 3 2 5 1 7 3 3 3 4 5 4 4 4 3 7 5 2 VNTR profiles, respectively. The isolates were divided into eight groups based on phylogenetic clustering and genotypic characteristics. Groups I to VIII contained 2, 2, 1,1,1,46,1 and 1 isolates, respectively (Table 1). All the clustered isolates were in group VI. In this study, H37Rv separated into a group (Figure 1).
Epidemic of Beijing family strains in Shihezi of Xinjiang Province
During the study period, 55 M. tuberculosis isolates were identified using molecular methods. We used the RD105 deletion test instead of spoligotyping, and found that 47 of the 55 isolates (85.5%) were of the Beijing family genotype, while eight (24.5%) were non-Beijing family strains.
Characteristics of the clustered isolates
Among the 47 Beijing family isolates, 45 (95.7%) were in group VI and the remaining two isolates were in groups V and VII. This suggested that the Beijing family isolates distributed mainly in group VI. Sixteen isolates (16/47) were clusters, and were all Beijing family isolates. In contrast, none of the eight non-Beijing family isolates were clustered (Table 2).
Drug susceptibility patterns of the MTB isolates
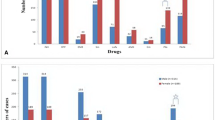
The 55 MTB strains isolated from the sputum samples of TB patients were subjected to a drug susceptibility test. Drug susceptibility with the four first-line anti-tuberculosis and the seven second-line anti-tuberculosis drugs was examined to determine the association between drug resistance patterns and genotype.
Thirty-six isolates (65.5%) were susceptible to all eleven drugs; 19 (34.5%) were resistant to at least one drug; seven (12.8%) were MDR-TB strains, which were resistant to at least INH and RIF, the two most powerful anti- tuberculosis drugs; two (3.6%) were XDR-TB, defined as MDR TB with further resistance for any quinolones and to >1 of the three classes of second-line drugs (Cm, Am, or Km)[26]. We examined the distribution of drug resistance between Beijing and non-Beijing strains. Among the seven MDR-TB isolates, five (10.6%, 5/47) isolates were Beijing family strains, and two (0.25%,2/8) were non-Beijing family strains. The rates of MDR-TB among Beijing and non-Beijing family strains were not statistically different (P = 0.386). Among the Beijing family strains, 36.2% (n = 17) were resistant to any drug; among the non-Beijing family strains, 2.5% (n = 2) were resistant to any drug (Table 2).
Of the 55 patients, 20 (36.4%) were new TB cases and 35 (63.6%) were previously treated patients. Among the four MDR-TB patients, all had been previously treated, while among the 51 patients with non-MDR-TB, 31 patients (60.8%) had been previously treated. However, there was no association of MDR-TB cases with either new cases or previously treated cases, as shown in Table 3.
Discussion
Recent advances in molecular technology, such as RD105 deletion and MIRU-VNTR typing, have provided powerful tools for analyzing MTB genotype and transmission patterns, and should prove invaluable for developing effective infection-control policies.
The RDl05 deletions, which can identify the Beijing Family strains, are very valuable molecular markers. In this study, we found that 85.5% of MTB isolates in the Shihezi region were Beijing family strains. In the previous study, the prevalence of Beijing family strains were different in various regions of China, e.g., 80% to 90% in Beijing, 67% in Ningxia, 89% in Shanghai, 70% in Zhejiang, 91.5% in Tianjin, 55.3% in Guangxi, 80.4% in Jiangsu, and 89.5% in Heilongjiang [12]. Thus, the Beijing family strains were prevalent in China. The association of drug resistance and the Beijing family strains has become a research hotspot. However, results obtained differ. These strains may have a particular propensity for acquiring drug resistance [27]. In our study, the statistical analysis showed that there was no difference between the Beijing and non-Beijing family strains in terms of their drug resistance patterns, indicating that the Beijing family was less likely to be associated with the high prevalence of drug resistance in Shihezi region.
In this study, general resistance to at least one drug and MDR were all higher than the resistance rate observed in 2008 [28]. The presence of two XDR-TB cases (3.6%) was another important discovery, and it is the first report of XDR-TB cases in Xinjiang. Worldwide, XDR-TB cases have been reported in 45 countries [29]. In China, a survey of resistance to tuberculosis showed that the rate of XDR-TB was 0.68% in 2007–2008 [30]. Therefore, we should pay more attention to MDR-TB and XDR-TB to control tuberculosis.
The MIRU-VNTR method has been used in molecular epidemiology studies, and it is adequate for tracking recent transmission and distinguishing relapses and reinfections [9]. In addition to its high discriminatory power, MIRU typing is simple to perform, has a high throughput, and is highly reproducible with a short turnaround time. MIRU types are represented in digital format; therefore, results from different laboratories can be easily compared. In this study, 55 isolates were classified into 46 types, eight gene groups, and seven clusters by MIRU-VNTR typing. The eight major gene groups, combined with flow epidemiological data, did not identify the means of mutual transmission and direct contact, because we only focused on strains collected in June 2010 to June 2011. Patients of the experimental investigation distributed in a different location of the transmission chain, and were insufficient to reflect the full spread relationship. At the same time, we were unable to collect the strains of previously treated patients; therefore, the infection pattern may be the recurrence of endogenous or recent infection. The analysis indicated that the Beijing family isolates were less likely to be part of a cluster. Future studies using more isolates are required to confirm this hypothesis.
Conclusion
This is the first report of the genotypes of MTB isolated from patients with pulmonary TB in the Shihezi region of Xinjiang Province, China. We defined 24 MIRU-VNTR loci for analyzing the strains. The 55 isolates show a high number (16/55) of clusters, and Beijing family strains (47/55) are prevalent in Shihezi region. The drug-resistance rate was high; in particular, two XDR-TB cases were found. It is necessary to strengthen the monitoring, treatment and management for drug-resistance TB. There was no correlation between drug-resistance and the Beijing family genotype.
References
WHO report, World Health Organisation: Global tuberculosis control. 2011, WHO report
Frothingham R, Meeker-O'Connell WA: Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology. 1998, 144 (Pt 5): 1189-1196.
Supply P, Mazars E, Lesjean S, Vincent V, Gicquel B, Locht C: Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol Microbiol. 2000, 36 (3): 762-771.
Alonso-Rodriguez N, Martinez-Lirola M, Herranz M, Sanchez-Benitez M, Barroso P, Bouza E, Garcia DVD: Evaluation of the new advanced 15-loci MIRU-VNTR genotyping tool in Mycobacterium tuberculosis molecular epidemiology studies. BMC Microbiol. 2008, 8: 34-10.1186/1471-2180-8-34.
Christianson S, Wolfe J, Orr P, Karlowsky J, Levett PN, Horsman GB, Thibert L, Tang P, Sharma MK: Evaluation of 24 locus MIRU-VNTR genotyping of Mycobacterium tuberculosis isolates in Canada. Tuberculosis (Edinb). 2010, 90 (1): 31-38. 10.1016/j.tube.2009.12.003.
Maes M, Kremer K, van Soolingen D, Takiff H, de Waard JH: 24-locus MIRU-VNTR genotyping is a useful tool to study the molecular epidemiology of tuberculosis among Warao Amerindians in Venezuela. Tuberculosis (Edinb). 2008, 88 (5): 490-494. 10.1016/j.tube.2008.04.003.
Sola C, Filliol I, Legrand E, Lesjean S, Locht C, Supply P, Rastogi N: Genotyping of the Mycobacterium tuberculosis complex using MIRUs: association with VNTR and spoligotyping for molecular epidemiology and evolutionary genetics. Infect Genet Evol. 2003, 3 (2): 125-133. 10.1016/S1567-1348(03)00011-X.
Supply P, Warren RM, Banuls AL, Lesjean S, Van Der Spuy GD, Lewis LA, Tibayrenc M, Van Helden PD, Locht C: Linkage disequilibrium between minisatellite loci supports clonal evolution of Mycobacterium tuberculosis in a high tuberculosis incidence area. Mol Microbiol. 2003, 47 (2): 529-538. 10.1046/j.1365-2958.2003.03315.x.
Supply P, Lesjean S, Savine E, Kremer K, van Soolingen D, Locht C: Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J Clin Microbiol. 2001, 39 (10): 3563-3571. 10.1128/JCM.39.10.3563-3571.2001.
Dickman KR, Nabyonga L, Kateete DP, Katabazi FA, Asiimwe BB, Mayanja HK, Okwera A, Whalen C, Joloba ML: Detection of multiple strains of Mycobacterium tuberculosis using MIRU-VNTR in patients with pulmonary tuberculosis in Kampala. Uganda. Bmc Infect Dis. 2010, 10: 349-
Rovina N, Karabela S, Constantoulakis P, Michou V, Konstantinou K, Sgountzos V, Roussos C, Poulakis N: MIRU-VNTR typing of drug-resistant tuberculosis isolates in Greece. Ther Adv Respir Dis. 2011, 5 (4): 229-236. 10.1177/1753465811402120.
Liu Q, Yang D, Xu W, Wang J, Lv B, Shao Y, Song H, Li G, Dong H, Wan K: Molecular typing of mycobacterium tuberculosis isolates circulating in Jiangsu Province. China. Bmc Infect Dis. 2011, 11: 288-10.1186/1471-2334-11-288.
van Soolingen D, Qian L, de Haas PE, Douglas JT, Traore H, Portaels F, Qing HZ, Enkhsaikan D, Nymadawa P, van Embden JD: Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J Clin Microbiol. 1995, 33 (12): 3234-3238.
Rivera-Ordaz A, Gonzaga-Bernachi J, Serafin-Lopez J, Hernandez-Pando R, Van Soolingen D, Estrada-Parra S, Estrada-Garcia I, Chacon-Salinas R: Mycobacterium Tuberculosis Beijing Genotype Induces Differential Cytokine Production by Peripheral Blood Mononuclear Cells of Healthy BCG Vaccinated Individuals. Immunol Invest. 2012, 41 (2): 144-156. 10.3109/08820139.2011.596604.
Hu Y, Hoffner S, Jiang W, Wang W, Xu B: Extensive transmission of isoniazid resistant M. tuberculosis and its association with increased multidrug-resistant TB in two rural counties of eastern China: a molecular epidemiological study. BMC Infect Dis. 2010, 10: 43-10.1186/1471-2334-10-43.
Ma X, Wang H, Deng Y, Liu Z, Xu Y, Pan X, Musser JM, Graviss EA: rpoB Gene mutations and molecular characterization of rifampin-resistant Mycobacterium tuberculosis isolates from Shandong Province, China. J Clin Microbiol. 2006, 44 (9): 3409-3412. 10.1128/JCM.00515-06.
Wang J, Liu Y, Zhang CL, Ji BY, Zhang LZ, Shao YZ, Jiang SL, Suzuki Y, Nakajima C, Fan CL: Genotypes and characteristics of clustering and drug susceptibility of Mycobacterium tuberculosis isolates collected in Heilongjiang Province, China. J Clin Microbiol. 2011, 49 (4): 1354-1362. 10.1128/JCM.02274-10.
Jinghua L, Pourel C, Hauck Y, Xiuqin Z, Zhiguang L, Kanglin W: A new method for the identification of the "Beijing family" strain of Mycobacterium tuberculosis. Chinese Journal of Microbiology and Immunology. 2008, 28 (2): 172-175.
Tsolaki AG, Gagneux S, Pym AS, Goguet DLSY, Kreiswirth BN, Van Soolingen D, Small PM: Genomic deletions classify the Beijing/W strains as a distinct genetic lineage of Mycobacterium tuberculosis. J Clin Microbiol. 2005, 43 (7): 3185-3191. 10.1128/JCM.43.7.3185-3191.2005.
Stavrum R, Valvatne H, Bo TH, Jonassen I, Hinds J, Butcher PD, Grewal HM: Genomic diversity among Beijing and non-Beijing Mycobacterium tuberculosis isolates from Myanmar. PLoS One. 2008, 3 (4): e1973-10.1371/journal.pone.0001973.
Yuan X: The impact of the implementation of the DOTS strategy to TB control in Shihezi region. Endemic Diseases Bulletin. 2010, 25 (3): 44-45. chinese title in the paper
Dou HY, Tseng FC, Lin CW, Chang JR, Sun JR, Tsai WS, Lee SY, Su IJ, Lu JJ: Molecular epidemiology and evolutionary genetics of Mycobacterium tuberculosis in Taipei. BMC Infect Dis. 2008, 8: 170-10.1186/1471-2334-8-170.
Huard RC, Lazzarini LC, Butler WR, van Soolingen D, Ho JL: PCR-based method to differentiate the subspecies of the Mycobacterium tuberculosis complex on the basis of genomic deletions. J Clin Microbiol. 2003, 41 (4): 1637-1650. 10.1128/JCM.41.4.1637-1650.2003.
Kanduma E, McHugh TD, Gillespie SH: Molecular methods for Mycobacterium tuberculosis strain typing: a users guide. J Appl Microbiol. 2003, 94 (5): 781-791. 10.1046/j.1365-2672.2003.01918.x.
Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rusch-Gerdes S, Willery E, Savine E, de Haas P, van Deutekom H, Roring S: Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol. 2006, 44 (12): 4498-4510. 10.1128/JCM.01392-06.
Banerjee R, Schecter GF, Flood J, Porco TC: Extensively drug-resistant tuberculosis: new strains, new challenges. Expert Rev Anti Infect Ther. 2008, 6 (5): 713-724. 10.1586/14787210.6.5.713.
Kremer K, Glynn JR, Lillebaek T, Niemann S, Kurepina NE, Kreiswirth BN, Bifani PJ, van Soolingen D: Definition of the Beijing/W lineage of Mycobacterium tuberculosis on the basis of genetic markers. J Clin Microbiol. 2004, 42 (9): 4040-4049. 10.1128/JCM.42.9.4040-4049.2004.
Wei J, Wei Z, Weidong W, Xiaoming G, Jing Y, Yinuer A, Nianqiang L, Peng C: The report of WHO drug resistance surveillance on tuberculosis, Xinjiang. The Journal Of The Chinese Antituberculosis Association. 2008, 30 (4): 307-310.
WHO/IUATLD: Anti-tuberculosis drug resistance in the world: The WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance. WHO Report NO.4. 2008
World Health Organization: Global tuberculosis control and patient care: a ministerial meeting of high M/XDR-TB burden countries. Beijing, China. 2009
Acknowledgements and funding
This study was supported by the “National Natural Science Foundation of China” (30960356) and the “Regional Science Foundation of China” (81160368).
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors’ contribution
LY designed and supervised experiments, and collected the sputa samples; JZ wrote the manuscript, collected the sputa samples, and cultured and identified all strains; JZ, LG Mi, and PZL performed the VNTR genotyping of MTB; YZ. Wang identified the Beijing family strains; HYL and YH performed the drug susceptibility tests; and BL analyzed the data. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zhang, J., Mi, L., Wang, Y. et al. Genotypes and drug susceptibility of Mycobacterium tuberculosis Isolates in Shihezi, Xinjiang Province, China. BMC Res Notes 5, 309 (2012). https://doi.org/10.1186/1756-0500-5-309
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-0500-5-309