Abstract
Background
To assess the relationship between serial serum leptin levels in patients with acute myocardial infarction (AMI) who received thrombolysis and the degree of coronary atherosclerosis, coronary reperfusion, echocardiographic findings, and clinical outcome. 51 consecutive patients presenting with AMI were studied. Clinical characteristics including age, sex, body mass index (BMI) and cardiovascular risk factors were recorded. Serial serum leptin levels at the time of admission and subsequently at 0, 6, 12, 24, 36, 60 hours afterwards were obtained. Coronary angiography was performed in 34 patients; the relation between serum leptin levels and evidence of coronary reperfusion as well as the extent of coronary atherosclerosis according to the coronary artery surgery study classification (CASS) were evaluated. Echocardiographic evaluation was performed in all patients. 36 matched patients were enrolled as control group who had serum leptin level 9.4 ± 6.5 ng/ml.
Results
The patients mean age was 50.5 ± 10.6 years. There were 47 males and 3 females. 37.1% were diabetics, 23.5% were hypertensive, 21.6% were dyslipidemic and 22.7% were obese (BMI ≥ 30). Leptin concentrations (ng/ml) increased and peaked at the 4th sample (36 hrs) after admission (mean ± SD) sample (1) =9.55 ± 7.4, sample (2) =12.9 ± 8.4, sample (3) =13.8 ± 10.4, sample (4) =18.9 ± 18.1, sample (5) =11.4 ± 6.5, sample (6) =10.8 ± 8.9 ng/ml. There was a significant correlation between serum leptin and BMI (r = 0.342; p = 0.03). Leptin levels correlated significantly to creatine kinase level on the second day (r = 0.43, p ≤ 0.01). Significant correlation of mean serum leptin with the ejection fraction (P < 0.05) was found. No difference in timing of peak serum leptin between patients who achieved coronary reperfusion vs. those who did not (p = 0.8). There was a trend for an increase in the mean serum leptin levels with increasing number of diseased vessels. There was no correlation between serum leptin levels and outcome neither during the hospitalization nor at 9 months follow up.
Conclusion
Serum leptin levels increase after myocardial infarction. Serum leptin level may be a predictor of the left ventricular ejection fraction and the degree of atherosclerosis but not of coronary reperfusion.
Similar content being viewed by others
Background
Leptin is the 16,000 Dalton protein product of the obesity gene (ob) [1]. Leptin has a role in the body weight maintenance in humans. It is released into the blood stream, where it binds to leptin-binding protein and is transported into the cerebrospinal fluid [2–5] and exerts its major effect on the hypothalamus. The role of leptin in coronary artery vasoreactivity has been raised in a study by Sundell J et al. [6] in obese and non-obese patients, suggesting that leptin might have a role in the regulation of the myocardial blood flow. A preliminary study in a small number of patients suggested that leptin levels might increase with acute myocardial infarction [7]. Leptin is found to have multiple roles in the cardiovascular system. A number of investigators suggested its role as a vasoactive substance [7]. There is also some evidence that leptin may have a role in obesity-related hypertension [8]. In a human study, a correlation was seen between the serum concentration of leptin and blood pressure among patients with essential hypertension [9]. Leptin also may have a prothrombotic effect [10]. This effect appears to be mediated through the platelet leptin receptor. In a previous study we suggested that TNF alpha may represent a modulator of leptin action in the hypothalamus, such finding may have implication in the setting of acute myocardial infarction [11].
The aims of the current study were to evaluate serial levels of serum leptin among patients presenting with acute myocardial infarction (AMI) and whether there is a correlation between leptin and coronary reperfusion as well as with angiographic and echocardiographic data.
Methods
Study design
This study was conducted at Hamad General Hospital. 51 patients who were admitted to the Coronary Care Unit with a diagnosis of acute coronary syndrome (ACS) were studied; after written informed consent for participation in the study was obtained from participants. The definition of ACS in this study is according to the definition for acute myocardial infarction by the joint committee of American College of Cardiology/European Society of Cardiology [12]. Patients with major co-morbid conditions including renal failure were excluded from the study. Hamad Medical Corporation institutional review board approved the study.
Baseline clinical characteristics including age, sex, cardiovascular risk factors, and complete cardiovascular physical examination findings were recorded. The mode of administered therapy was also recorded. 49 patients with ST elevation myocardial infarction (STEMI) were given thrombolytic therapy with either metalyse, or streptokinase [13]. Tow patients suffered non-ST elevation myocardial infarction (NSTEMI). None of these patients developed acute complication such as cardiogenic shock or acute homodynamic disturbance. Height, weight, body mass index, smoking habit, alcohol intake, diabetes mellitus, hypertension, lipid profile including serum cholesterol, high density and low density lipoprotein, and serum creatinine of patients were analyzed. Measurements of serum creatinine kinase, creatinine kinase-MB portion, troponin T levels were taken at the time of admission (0 time) and subsequently at 6, 12, 24, 36, 60 hours afterwards. Full echocardiographic studies were performed for all patients on the 2nd day of admission. Variables including left ventricular ejection fraction (LV EF%), left ventricular end-systolic dimension (LVESD), left ventricular end-diastolic dimension (LVEDD), left atrial size, right ventricular dimension (RVD) and right ventricular systolic pressure (RVSP) were all recorded. Coronary angiography was performed in 36 patients as a routine clinical check up. Extent of diseased vessels involved; whether single vessel, two -vessel, or three-vessel diseases, and whether re-canalization achieved (defined non-invasively as rapid resolution of ST segment elevation reperfusion arrhythmias and chest pain resolution after thrombolytic therapy or angiographically by the evidence of non-occluded culprit coronary artery) were recorded.
-
36
matched (age and BMI) stable cardiac patients with the same age and sex were taken as a control group and their data were collected from the cardiology out patient department.
Biochemical analyses
Blood samples were collected in plain tubes from each patient at the time of admission and then at 6, 12, 24, 36, 48, 60 and 84 hours. All samples were separated and analyzed immediately with the exception of serum leptin. Aliquots for serum leptin were stored at −80 °C until the collection was completed. Leptin concentrations were measured by ELISA method. Linco Research, Inc USA supplied the reagents. The measuring range was 0.5–100 ng/ml. According to manufacturer the assay has high correlation with their radioimmunoassay for human leptin (r = 0.967), lower detection limit is 0.5 ng/ml and the concentration variations (CVs) at concentrations of 3.2 ng/ml & 8.18 ng/ml were 5% & 3.0% respectively [14]. Total cholesterol (TC), Triglycerides (TG) and HDL-cholesterol (HDL-C) were determined enzymatically by Hitachi 917, Roche, Mannheim, Germany. LDL- cholesterol (LDL-C) was estimated among those with TG concentrations < 4.5 mmol/l as TC - HDL-C - Tg/2.2 [15]. Those with TG >4.5 mmol/l were measured directly by reagents from the same manufacturer [16]. Between-run imprecision (CVs) for TC, TG & measured LDL- C were 3.0%, 4.0% 2.0% and 2.0% respectively. Troponin T & CKMB were measured immunochemically by Elecys 2010, Roche, according to the manufacturer protocols. Imprecision, between run (CVs), for troponin & CKMB were 2% & 3% respectively [17].
Normal fasting level of serum leptin
Normal fasting ranges for serum leptin are directly correlated with the degree of adiposity, with BMI range 18–25; serum leptin level for men (3.8 ± 1.8 ng/ml), and for women (7.4 ± 3.7 ng/ml). Serum leptin level rises approximately 2.5 times faster in women per unit BMI as compared to man. Keeping in mind that a diurnal rhythm of serum leptin concentrations, the values being 20 to 40 percent higher in the middle of the night as compared with daytime [18, 19]. The peak shifts are in parallel with shifts in the timing of meals [20].
Coronary angiography and echocardiographic analysis
Coronary angiography was performed according to the standard Judkin technique femoral approach either on admission or during the recovery period. Several views of each coronary artery were analyzed. The severity of arterial stenosis (defined as maximal percent reduction in luminal diameter) was determined according to visual estimation using the coronary artery surgery study classification (CASS) study analysis. Significant coronary stenosis was defined as a 70% lumen narrowing or > than 50% lumen narrowing of the left main coronary artery. The extent of coronary artery disease was classified as 1, 2 or 3 vessels according to number of major coronary arteries with significant stenosis [21].
Transthoracic 2 dimensional echocardiography 3 megahertz, 5500 Hewlett Packard Sonos machine was used for the study. Images were captured in the 5 standard views, parasternal long axis views, short axis view and 4, 2 &3 chamber views according to criteria of American society of echocardiography. The measurement of left ventricular dimensions was performed from 2 dimensional targeted M-mode at end diastolic and end systolic dimensions and subsequently LV ejection fraction was calculated. Left atrial size at end systole was measured from the parasternal long axis view. Right ventricular dimensions were recorded from the parasternal long axis view. Conventional Doppler measurements were obtained using tricuspid inflow, mitral early and late diastolic inflow velocities, volumetric relaxation times were also recorded. Wall motion abnormalities were recorded using 16 segment models [22].
Statistical analysis
The data were analyzed by using the Statistical Packages for Social Sciences [SPSS] version 19 [23]. Data were expressed as mean and standard deviation (SD) unless otherwise stated; student t test was used to ascertain the significance of difference between mean values of two continuous variables and confirmed by non-parametric Mann Whitney test. Fisher exact and Chi square test were performed to test for difference in proportions of categorical variable between two and more groups. The Pearson’s correlation coefficient was used to evaluate the strength association between two variables. The level of P < 0.05 was considered as the cut off value for significance. Repeated measures ANOVA utilized to test differences in serial leptin measures.
Results
Baseline clinical characteristics of the studied patients shown in Table 1. The mean age of patients was 50.5 ± 10.6 years. There were 47 males and 4 females. 37.1% had diabetes mellitus, 23.5% had hypertension, 21.6% had high cholesterol, and 22.7% were obese (BMI ≥ 30). Thirty six matched (with age and BMI) stable cardiac patients were taken as a control group. The mean serum leptin level of the control group was 9.43 ± 6.5 ng/ml.
Table 2 shows echocardiographic and coronary angiographic characteristics compared with mean and peak serum leptin sample.
Figure 1 shows the mean values of serum leptin level during the course of AMI at different time points. Leptin concentrations (ng/ml) increased and peaked at fourth reading (36 hrs) after admission and gradually decreased thereafter (mean ± SD sample (1) =9.55 ± 7.4, sample (2) = 12.9 ± 8.4, sample (3) =13.8 ± 10.4, sample (4) =18.9 ± 18.1, sample (5) =11.4 ± 6.5, sample (6) =10.8 ± 8.9) ng/ml, P value 0.04 based on repeated measures ANOVA.
There was a significant correlation between serum leptin and BMI (r = 0.34; p = 0.03). Leptin levels also correlated significantly with serum creatine kinase level on the second day (r = 0.43, p ≤ 0.01). There was no difference in timing of peak serum levels between patients who had evidence of coronary reperfusion compared to those who did not achieve coronary reperfusion (p = 0.8), also there was no significant difference in mean serum leptin level in patients with anterior MI vs. inferior MI (mean ± SD = 11.71 ± 7, 9 vs.11.86 ± 6.6 ng/ml, p = 0.9) respectively. However, there was significant correlation with left ventricular ejection fraction, LVEF (r = −0.25, p = 0.01). No significant correlation between mean serum leptin and left atrial size (r = −0.09; p = 0.5), left ventricular systolic (r = 0.017, p = 0.9) or diastolic dimension (r = 0.1; p = 0.47) were found.
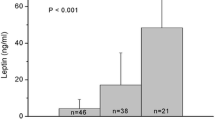
Figure 2 shows that mean serum leptin, which appeared to increase with the increase in the number of diseased vessels (mean, SVD = 9.2, 2VD = 12.0, 3VD = 12.9, p = 0.6), although this was not statistically significant.
Correlation between mean and peak leptin level and variables of interest showed significant correlation of mean serum leptin with the ejection fraction and body mass index (P < 0.05), and significant correlation of creatinine kinase with mean leptin level (P < 0.05) (Table 3).
Discussion
Studies on the role of serum leptin in coronary artery disease are scarce [24–34] (Table 4). To the best of our knowledge, this study is among few studies that analyzed the serial serum leptin levels in the setting of acute myocardial infarction and the first that evaluated its correlation with angiographic and echocardiographic findings in this high-risk group.
In the United States population, increased leptin concentrations was significantly associated with increased risk of myocardial infarction and stroke in men and women, independent of traditional cardiovascular risk factors and obesity status [36]. We have shown previously a significant correlation between serum leptin and hs-CRP in stable cardiac patients [37]. Study by Stangl et al. [38] concluded that patients with coronary artery disease exhibited higher serum leptin concentrations than controls matched for age, gender & BMI, suggesting that leptin could contribute to the development of cardiovascular disease, possibly via activation of the sympathetic nervous system. The Trp64Arg variant of the β-adrenoceptor did not influence serum leptin levels [38]. Leptin might be a marker of risk of coronary artery disease, at least in men, and contributes to the risk profile in subjects with insulin resistance. Leptin concentrations were significantly higher in diabetic and coronary artery disease patients than in controls. Body weight, serum triglyceride concentration and systolic blood pressure were all significantly related to the logarithm of the serum leptin concentration in stable coronary artery disease patients [39]. Wallace et al. [40] documented that leptin is an independent risk factor for coronary artery disease using data from the west Scotland coronary prevention study. It has been suggests that leptin might participate in the catabolic state leading to development of cardiac cachexia in the course of congestive heart failure [41].
The current study demonstrates elevation of serum leptin levels in the acute phase of myocardial infarction and it peaks at 36 hours after admission (doubled). Whether leptin is released from the myocytes or it acts only as an acute phase reactant protein after its release from adipose tissue, was the main issue of this project. The fact that there was no earlier peak in serum leptin in patients who had coronary reperfusion after thrombolytic therapy compared to those who don’t achieve reperfusion suggests that leptin is not released from the myocytes and is mainly an acute phase reactant similar to high sensitivity C- reactive protein. This finding is concordant to the study in Poland, which included 35 patients with AMI and showed that plasma leptin levels in diabetic patients were significantly higher in AMI than in the period of convalescence. These findings suggest that leptin may play an important role in the metabolic changes taking place during the first days of AMI [24].
Correlating mean and peak serum leptin levels; serum leptin is positively correlated with the extent of diseased coronary vessels (1-, 2-, and 3 -vessel disease) (Figure 2), although statistically not significant, expanding the study sample may be confirmatory of this finding. Furthermore no significant correlation with either evidence of coronary re-canalization or with the type of MI (whether anterior or inferior) was found. We observed a significant correlation between serum leptin and LVEF with no significant correlation with other echocardiographic findings. Significant correlations were found between high serum leptin level and BMI as was demonstrated by previous studies (Table 3), with no significant correlation to other cardiovascular risk factor; diabetes, hypertension, total cholesterol LDL, HDL or triglyceride in this patients’ population. In contrast to our study which has followed the leptin up to 60 hours after the onset of AMI, investigators from Turkey studied the influence of plasma leptin concentrations obtained at the time of admission and 6 hours afterwards in 41 AMI patients who were treated with thrombolytic therapy. The investigators found that failure of reperfusion therapy with streptokinase was significantly higher in patients with admission plasma leptin concentrations ≥14 ng/mL as compared to patients with admission plasma leptin concentrations <14 ng/m, i.e. hyperleptinemia decreased the chance of successful reperfusion . Left ventricular ejection fraction was slightly but significantly higher in patients with admission plasma leptin concentrations ≥14 ng/mL than in patients with admission plasma leptin concentrations <14 ng/m (p = 0.031) [25]. Another study involving 30 consecutive AMI patients with a similar profile to the current one, showed that leptin levels reached its peak on the second day of hospitalization, with a 2-fold increase from baseline level on admission (p < 0.02). On day 3, leptin levels declined, and were 46%, 9%, and 6% above baseline on days 3, 4 and 5, respectively, suggesting that leptin may have a role in the metabolic changes taking place during the first days after an AMI. [26].
The predictive power of leptin on cardiovascular disease was addressed in a report from the Quebec cardiovascular study[27], eighty-six patients who developed ischemic heart disease were compared with referent matched for a number of traditional cardiovascular risk factor including body mass index. Leptin did not emerge as a predictor in coronary artery disease. However, fundamental differences between the two studies, first and most importantly, patients in our present study were all proven first ever AMI cases according to newly defined criteria [12], whereas in Quebec, the study group constituted a mixture of stable and unstable angina [27]. Furthermore Fujimaki et al. [35] correlated serum leptin with other myocardial infarction markers and interleukin level in 15 aged-matched controls and found a significant negative correlation between these two markers. These studies again suggest that leptin may play an important role in the metabolic changes taking place during the first days of myocardial infarction.
Limitations of the study
Since this study included a small number of patients, the result should be interpreted cautiously.
Conclusion
Serum leptin acts as an acute phase reactant in AMI patients. Significant correlation was found in mean serum leptin level with BMI, CK, and LVEF. There was no significant difference in mean serum leptin level in patients with anterior vs. inferior infarction and statistically no significant correlations of serum leptin with severity of angiographic findings and other echocardiographic parameters.
Abbreviations
- BMI:
-
body mass index
- SVD:
-
single vessel disease
- 2VD:
-
two vessel disease
- 3VD:
-
three vessel disease
- LVEF:
-
left ventricular ejection fraction
- RVD:
-
right ventricular dimension
- RVSP:
-
right ventricular systolic pressure
- STEMI:
-
ST elevation myocardial infarction.
References
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM: Positional cloning of the mouse obese gene and its human homologue. Nature. 1994, 372: 425-10.1038/372425a0.
Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM: Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995, 269: 543-10.1126/science.7624777.
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F: Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995, 269: 540-10.1126/science.7624776.
Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P: Recombinant mouse ob protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995, 269: 546-10.1126/science.7624778.
Sharma K, Considine RV: The Ob protein (leptin) and the kidney. Kidney Int. 1998, 53: 1483-10.1046/j.1523-1755.1998.00929.x.
Sundell J, Huuppon R, Raitakari OT, Nuutila P, Knuuti J: High serum leptin is associated with attenuated coronary vasoreactivity. Obes Res. 2003, 11 (6): 776-782. 10.1038/oby.2003.108.
Matsuda K, Teragawa H, Fukuda Y, Nakagawa K, Higashi Y, Chayama K: Leptin causes nitric-oxide independent coronary artery vasodilation in humans. Hypertens Res. 2003 Feb, 26 (2): 147-152. 10.1291/hypres.26.147.
Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y: Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000, 105: 1243-10.1172/JCI8341.
Agata J, Masuda A, Takada M, Higashiura K, Murakami H, Miyazaki Y, Shimamoto K: High plasma immunoreactive leptin level in essential hypertension. Am J Hypertens. 1997, 10: 1171-10.1016/S0895-7061(97)00310-5.
Bodary PF, Westrick RJ, Wickenheiser KJ, Shen Y, Eitzman DT: Effect of leptin on arterial thrombosis following vascular injury in mice. JAMA. 2002, 287: 1706-10.1001/jama.287.13.1706.
Rizk NM, Stammsen D, Preibisch G, Eckel J: Leptin and tumor necrosis factor-alpha induce the tyrosine phosphorylation of signal transducer and activator of transcription proteins in the hypothalamus of normal rats in vivo. Endocrinology. 2001 Jul, 142 (7): 3027-3032. 10.1210/en.142.7.3027.
Alpert JS, Thygesen K, Antman E, Bassand JP: Myocardial infarction redefined –a consensus document of the Joint European Society of Cardiology/American College of cardiology Committee for redefinition of of myocardial infarction. J Am Coll Cardiol. 2000, 36 (3): 959-973. 10.1016/S0735-1097(00)00804-4.
Hadi HAR: Al Suwaidi J, Bener A, Al Binali H: Thrombolytic therapy use in acute myocardial infaction and outcome in Qatar, int J. Cardiol. 2005, 102: 249-254.
Ma Z, Gingerich RL, Santiago JV, Klein S, Smith CH, Landt M: Radioimmunoassay of Leptin in Human Plasma. Clin Chem. June 1996, 42: 942-946.
Fridewald WF, Levy RI, Frederickson DS: Estimation of LDLcholesterol concentration without use of the Preparative Ultra-centrifuge. Clin Chem. 1972, 18: 499-502.
Nauck M, Warnick GR, Rifai N: Methods for measurement of LDL-cholesterol: a critical assessment of direct measurement by homogeneous assays versus calculation. Clin Chem. 2002, 48: 236-254.
Panteghini M, Pagani F: Yeo KTJ, Apple FS, Christenson RH, Dati F, Mair J, Ravkilde J. Wu AH: Committee on Standardization of Markers of Cardiac Damage of the IFCC. Evaluation of imprecision for cardiac troponin assays at low-range concentrations. Clin Chem. 2004, 50: 327-332.
Boden G, Chen X, Kolaczynski JW, Polansky M: Effects of prolonged hyperinsulinemia on serum leptin in normal human subjects. J Clin Invest. 1997, 100: 1107-10.1172/JCI119621.
Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, Mulla A, Cearnal L, Veldhuis JD, Flier JS: Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proc Natl Acad Sci U S A. 1998, 95: 2541-10.1073/pnas.95.5.2541.
Schoeller DA, Cella LK, Sinha MK, Caro JF: Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1882, 1997: 100-
Rogers WJ, Coggin CJ, Gersh BJ, Fisher LD, Myers WO, Oberman A, Sheffield LT: Ten-year follow-up of quality of life in patients randomized to receive medical therapy or coronary artery bypass graft surgery: The Coronary Artery Surgical Study (CASS). Circulation. 1990, 82: 1647-10.1161/01.CIR.82.5.1647.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS: American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002, 105: 539-10.1161/hc0402.102975.
Norusis MJ: SPSS/PC + for windows. Base System and Advanced Statistical User’s Guide, Window Version. Chicago, Illinois. 1998, 12:
Krasnodebski P, Bak MI, Opolski G, Karnafel W: Leptin in acute myocardial infarction and period of convalescence in patients with type 2 diabetes mellitus. Kardiol Pol. 2010 Jun, 68 (6): 648-653.
Amasyali B, Aytemir K, Kose S, Kilic A, Abali G, Iyisoy A, Kursaklioglu H, Turan M, Bingol N, Isik E: Admission plasma leptin level strongly correlates with the success of thrombolytic therapy in patients with acute myocardial infarction. Angiology. 2006, 57 (6): 671-680.
Meisel SR, Ellis M, Pariente C, Pauzner H, Liebowitz M, David D, Shimon I: Serum leptin levels increase following acute myocardial infarction. Cardiology. 2001, 95 (4): 206-211. 10.1159/000047373.
Després JP, Lupien PJ, Moorjani S, Dagenais GR, Cantin B, Mauriege P, Lamarche B, Couillard C: Leptenemia is not a risk factor for ischemic heart disease in men. Prospective results from Quebec Cardiovascular study. Diabetic Care. 1998, 21: 782-786. 10.2337/diacare.21.5.782.
Yan GT, Xue H, Lin J, Hao XH, Zhang K, Wang LH: Correlation analysis of increase in serum level of leptin with that of C reactive protein, troponin T and endothelin in patients with acute myocardial infarction. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2005 Sep, 17 (9): 530-532.
Wallander M, Söderberg S, Norhammar A: Leptin: a predictor of abnormal glucose tolerance and prognosis in patients with myocardial infarction and without previously known Type 2 diabetes. Diabet Med. 2008 Aug, 25 (8): 949-955. 10.1111/j.1464-5491.2008.02509.x.
Taneli F, Yegane S, Ulman C, Tikiz H, Bilge AR, Ari Z, Uyanik BS: Increased serum leptin concentrations in patients with chronic stable angina pectoris and ST-elevated myocardial infarction. Angiology. 2006, 57 (3): 267-272. 10.1177/000331970605700302.
Piestrzeniewicz K, Luczak K, Komorowski J, Maciejewski M, Goch JH: The relationship between leptin and obesity and cardiovascular risk factors in men with acute myocardial infarction. Cardiol J. 2007, 14 (3): 252-259.
Selvakumar D, Selvakumar PV, George P, Jose VJ Mariappan P: Serum leptin levels in acute myocardial infarction. Indian Heart J. 2005, 57 (1): 39-43.
Söderberg S, Ahrén B, Jansson JH, Johnson O, Hallmans G, Asplund K, Olsson T: Leptin is associated with increased risk of myocardial infarction. J Intern Med. 1999 Oct, 246 (4): 409-418. 10.1046/j.1365-2796.1999.00571.x.
Stejskal D, Růzicka V, Bartek J, Horalík D: Leptinemia in persons with acute myocardial infarct. Vnitr Lek. 1998 Oct, 44 (10): 588-592.
Fujimaki S, Kanda T, Fujita K, Tamura J, Kobayashi I: The significance of measuring plasma leptin in acute myocardial infarction. J Int Med Res. 2001, 29 (2): 108-113.
Sierra-Johnson J, Romero-Corral A, Lopez-Jimenez F, Gami AS, Sert Kuniyoshi FH, Wolk R, Somers VK: Relation of increased leptin concentrations to history of myocardial infarction and stroke in the United States population. Am J Cardiol. 2007, 100 (2): 234-239. 10.1016/j.amjcard.2007.02.088.
Khafaji HAR Hadi, Bener A, Osman M, Al-marri A, Al Suwaidi J: The impact of diurnal fasting during Ramadan on the lipid profile, hs-CRP, and serum leptin in stable cardiac patients. Vascular Health and Risk Management. 2012, 8: 7-14.
Stangl K, Cascorbi I, Laule M, Stangl V, Vogt M, Ziemer S, Roots I, Wernecke K, Baumann G, Hauner H: Elevated serum leptin in patients with coronary artery disease: no association with the Trp64Arg polymorphism of the beta3-adrenergic receptor. Int J Obes Relat Metab Disord. 2000 Mar, 24 (3): 369-375. 10.1038/sj.ijo.0801159.
Al-Daghri N, Al-Rubean K, Bartlett WA, Al-Attas O, Jones AF, Kumar S: Serum leptin is elevated in Saudi Arabian patients with metabolic syndrome and coronary artery disease. Diabet Med. 2003 Oct, 20 (10): 832-837. 10.1046/j.1464-5491.2003.01044.x.
Wallace AM Mc Mahan AD, Packed CJ, Kelly A, Shepherd J, Gaw A, Sattar N: Plasma Leptin and the risk of cardiovascular disease in the wast of Scotland coronary prevention study. Circulation. 2001, 104: 3052-3056. 10.1161/hc5001.101061.
Schuler G, Moebius-Winkler S, Erbs S, Gielen S, Adams V, Schoene N, Linke A, Kratzsch J, Schule PC: Elevated serum levels of leptin and soluble leptin receptor in patients with advanced heart failure. EurJ Heart Fail. 2003, 33 (1):
Acknowledgements
This study was funded by the Medical Research Center of Hamad Medical Corporation, Doha, Qatar [Grant No 263]. The study was conducted in collaboration with biomedical department of the Qatar University, Doha, Qatar. We sincerely acknowledge: Ms. Nora Basem and Mrs. Wafa Khalil for their laboratory work and their technical support in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HARHK- participated in the design of the study, patients’ recruitment, writing, analyzing and reviewing the paper. AB- performed the statistical analysis. NR- participated in the design of the study and the performance of laboratory investigations. JA- participated in the design of the study and patients’ enrollment. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Khafaji, H.A.H., Bener, A.B., Rizk, N.M. et al. Elevated serum leptin levels in patients with acute myocardial infarction; correlation with coronary angiographic and echocardiographic findings. BMC Res Notes 5, 262 (2012). https://doi.org/10.1186/1756-0500-5-262
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-0500-5-262