Abstract
Biosynthesis of liquid fuels and biomass-based building block chemicals from microorganisms have been regarded as a competitive alternative route to traditional. Zymomonas mobilis possesses a number of desirable characteristics for its special Entner-Doudoroff pathway, which makes it an ideal platform for both metabolic engineering and commercial-scale production of desirable bio-products as the same as Escherichia coli and Saccharomyces cerevisiae based on consideration of future biomass biorefinery. Z. mobilis has been studied extensively on both fundamental and applied level, which will provide a basis for industrial biotechnology in the future. Furthermore, metabolic engineering of Z. mobilis for enhancing bio-ethanol production from biomass resources has been significantly promoted by different methods (i.e. mutagenesis, adaptive laboratory evolution, specific gene knock-out, and metabolic engineering). In addition, the feasibility of representative metabolites, i.e. sorbitol, bionic acid, levan, succinic acid, isobutanol, and isobutanol produced by Z. mobilis and the strategies for strain improvements are also discussed or highlighted in this paper. Moreover, this review will present some guidelines for future developments in the bio-based chemical production using Z. mobilis as a novel industrial platform for future biofineries.
Similar content being viewed by others
Introduction
There have been growing concerns about biosynthesis of fuels, desired chemicals and materials from renewable biomass resources for limited fossil resources and associated environmental issues in the past few decades [1, 2]. As model industrial or laboratory organisms, Escherichia coli and Saccharomyces cerevisiae were selected as important platforms for the purpose of desired biofuels and chemicals production via metabolic engineering [3–5]. Currently, strain optimization to utilize various feedstocks (for example, starch, sugarcane, agricultural residues, industrial waste, forest residues, energy crops, et cetera) [6, 7], desired products spectrum (for example, biofuels and building block chemicals), and higher yields, which have made great progress in the past decades and provided a basis for industrial applications [1–5].
As a candidate bio-ethanol producer, Zymomonas mobilis showed some advantages, for example, higher specific rate of sugar uptake, high ethanol yield, lower biomass production, non-requirement of controlled addition of oxygen during fermentation, et cetera [8–13]. Extensive fundamental studies on Z. mobilis over the last 30 years have also made this strain a promising ethanologenic organism for large-scale bio-ethanol production. On the other hand, extensive studies on different genetic techniques (including plasmid vector, expression system, transposon system, gene knockout, gene transformation, and gene function, et cetera) will help Z. mobilis are amenability to genetic improvement for industrial biotechnology [13]. Furthermore, strategies of strain improvement (such as conventional mutagenesis, transposon mutagenesis, adaptive laboratory evolution, and metabolic pathway engineering, et cetera), and different value-added bio-products have also been paid more and more attention in the past 20 years. Importantly, genomics and transcriptomic of Z. mobilis have also been developed since 2005, which will aid future metabolic engineering and synthetic biology in strain improvement for industrial applications [14]. Selected milestones in Z. mobilis research are summarized in Figure 1.
Currently, three subspecies (subsp.) of Z. mobilis have been found, including Z. mobilis subsp. mobilis, Z. mobilis subsp. pomaceae and Z. mobilis subsp. Francensis[15–19]. All strains have also been summarized in the Ph D thesis of So Lok-yan (University of Hong Kong) and other review articles [19]. Among these strains, ATCC 31821 (ZM4), ATCC 10988 (ZM1), ATCC29191 (ZM6), CP4, and NCIMB 11163 from Z. mobilis subsp. mobilis, ATCC 29192 from Z. mobilis subsp. pomaceae, which were well-charcterized by previous studies on the level of physiology, biochemical, fermentation, genetics, metabolism, and omics. These strains are regarded as a model organism in Z. mobilis research or industrial applications.
In general, Z. mobilis may play a critical role as a novel platform in industrial biotechnology for the development of a green replacement for petrochemical products. In this paper, we review some critical research progress on Z. mobilis for its use as a platform for the production of ethanol and other buck chemicals from biomass.
Review
Genetic background of Z. mobilis
Currently, general genetic tools have been developed in Z. mobilis since the 1980s, including native plasmids, broad host-range vectors or shuttle vectors, expression system, gene transfer, promoter, and reporter gene, as reviewed in other articles [8, 11, 13]. Specific gene knockout, genomics, and transcriptomics will be emphasised as below.
Specific gene knockout
The development of gene deletion approaches have been performed for gene function and there has also been greatly improved metabolic engineering of Z. mobilis. Currently, different methods, including insertional mutant, suicide plasmid-based mutant construction, site-specific FLP recombinase, fusion-PCR-based construction technique, and transposon mutagenesis, have been employed for inactivating specific genes of Z. mobilis. Up to date, many genes, such as pyruvate decarboxylase (pdc, ZMO1360), alcohol dehydrogenase (adhB, ZMO1596), lactate dehydrogenase (ldhA, ZMO1237), NADH dehydrogenase (ndh, ZMO1113), RNA-binding protein Hfq (hfq, ZMO0347), hydroxylamine reductase (nha A, ZMO0117), glucose-fructose oxidoreductase (gfo, ZMO0689), aldo/keto reductase (him A, ZMO0976), restriction-modification (R-M) systems-related gene (ZMO0028, ZMO1933, ZMO1934, ZMO1934, ZMO0575), cytochrome-related gene (cyt C, cyt B, ctb D, ZMO0957, ZMO1572) et cetera, which were selected as targets for improvement of some specific phenotype (summarized in Table 1).
Sequenced genome of different Z. mobilis strains
Genome sequencing technology provides opportunities for fundamental insights and facilitates strain development [35]. Seo et al. reported the first genome sequence of Z. mobilis ZM4 in 2005. The complete genome of Z. mobilis ZM4 contains a 2,056,416-bp circular chromosome and five circular plasmids [9]. The complete genome sequence of other Z. mobilis strains have also been reported since 2005 [36–41]. All strains contain a circular chromosome and types of plasimd. However, genome sizes are various among these strains, ranging from 2.01 to 2.22, with two to six plasimds existing (Table 2). Although the genome of seven strains has been sequenced by different organizations, the comparative genome analysis has not been reported in public.
Transcriptome or gene expression of Z. mobilis
With different genome projects of Z. mobilis performed, further comparative genomics or global expression analysis could provide some guidelines for strain improvement in the future. Currently, many researchers are also focusing on transcriptomic profiling of Z. mobilis to better understand the network of gene or metabolic regulation. Especially, DNA microarray techniques or DNA sequencing have been used to identify differential gene expression under nutrition limitation, environmental stress (that is, heat stress, ethanol, furfural, et cetera). To date, there are ten datasets (including some unpublished data) from Gene Expression Omnibus (GEO) database (Table 2). For example, transcriptomic profiling of ZM4 during aerobic and anaerobic fermentations have been investigated for the first time [42]. Transcriptomic profiling of ZM4 in response to ethanol and furfural stress were also performed by our laboratory [43, 44]. Integrated “omics” approach (that is transcriptomic, proteomic and metabolic) was also used for studing the molecular mechanisms of ethanol stress response in ZM4 for the first time [45]. Expression data for ZM4 growing in rich and minimal media, heat-shocked, or at high ethanol were also performed by Lawrence Berkeley Laboratory (unpublished data). Genome changes associated with Z. mobilis sodium acetate-tolerant mutant (AcR) was aslo reported by Yang et al. In this study, next-generation sequencing (NGS), comparative genomics, transcriptomics, and genetics were used to elucidate the molecular mechanism of AcR sodium acetate tolerance. Especially, a key gene, nha A (ZMO0119), which conferred sodium acetate (NaAc) tolerance in Z. mobilis[29]. ZM401 (a flocculent mutant strain of Z. mobilis) was also studied by using genome-wide transcriptomic technology, which provided a deep understanding for evidence related to phenotypic changes associated with its cell-cell attachment behavior. These expression data indicate that cellulose and synthesis flagella-related proteins synthesis play an important role in its special flocculent behavior in ZM401 [46]. These studies will provide insights into molecular response to environmental stress in Z. mobilis or help to construct more resistant strains for ethanol or other chemical production in the future. In conclusion, those transcriptomic profiling generated in these studies will likely serve as useful reference data for industrial strain development at the level of systems biology in the future.
Strain improvement for Z. mobilis
Strain improvement by conventional mutagenesis
Traditionally, strain improvement was achieved mainly by mutagenesis and selection, which are still very useful in Z. mobilis. Currently, different mutagenesis agents, including UV light, 1-methyl-3-nitro-1-nitrosoguanidine (NTG), caffeine, ethyl methane sulfonate (EMS), et cetera, were used for Z. mobilis phenotype improvement. Many mutants were obtained by these mutageneses, that is, auxotrophic, ethanol and salt-tolerant, acetaldehyde-tolerant, osmotolerant, thermotolerant, sucrose-hypertolerant, acid-tolerant, fructose-negative, glucose-negative, mannitol-utilizing, levan-producing, and antibiotic-sensitive strains, et cetera (as reviewed by other authors) [8]. Among these mutants, environmental stress-tolerant mutant, and antibiotic-sensitive strains have showed some potential in industrial applications. For example, the acetate-tolerant Z. mobilis mutant (AcR) was generated by chemical mutagenesis and selection in the presence of acetate [47], and used as a host for constructing of engineered tolerant Z. mobilis strain for bio-ethanol production, that is ZM4/AcR (pZB5) [48–50].
Strain improvement by transposon mutagenesis
Transposon mutagenesis has also provided an alternate mutational approach in Z. mobilis. Although different transposons, including Tn5 and Tn10[51], Tn951[52] and Tn1725[53], which are carried by broad host-range plasmids, have been successfully transferred into Z. mobilis, no transposition event have been found. Morever, Carey et al. first found that plasmid pGC91.14 (RP1::Tn951) was stable in Z. mobilis at 30°C, and the lac operon encoded by Tn 951 was expressed sucessfully in Z. mobilis[52]. Pappas et al. also compared of the stability of different transposable elements Tn5, Tn501 or mini Mu in Z. mobilis, and the plasmid pULB113 (RP4::mini Mu) exhibited higher stability than others. With the help of mini Mu transposon, a large number of independent and stable auxotrophic mutants with polyauxotrophs, cysteine, methionine and isoleucine requiring-isolates were obtained [54]. The study proved that transposon mutagenesis is an extremely powerful tool for mutant construction in Z. mobilis[54, 55]. For example, Tn5 transposon was also used for construction of recombinant strain for ethanol production [56]. Actually, there are some transposon elements in Z. mobilis strains. For example, IS5-like insertion sequence, designated ISZm1068, was firstly isolated from Z. mobilis CP4, which was kept active in E. coli and led to plasmid replicon fusions [57].
Strain improvement by adaptive laboratory evolution (ALE)
Adaptive laboratory evolution (ALE) has emerged as a valuable method in metabolic engineering for strain development and optimization [58–62], and has been used successfully in model organisms such as E. coli[63, 64] and S. cerevisiae[65–68]. Previous studies demonstrated that adaptation and metabolic engineering can be used synergistically for strain improvement. Recently, ALE strategy was also employed for Z. mobilis strain improvement. For example, an adaptive mutation procedure was developed for screening of acetic acid-tolerant Z. mobilis, and many adapted mutants obtained for further use in bio-ethanol production [69]. Agrawal et al. also used this method to select a highly efficient xylose-fermenting Z. mobilis strain A3 [70]. These two studies demonstrated that the ALE method might be used as a powerful metabolic engineering strategy for improving certain features of Z. mobilis in the future, for example, inhibitor tolerance or substrate utilization.
Increase in the substrate utilization range of Z. mobilis
Extensive studies or reviews on ethanol production from sugarcane, molasses, starch, and glucose by Z. mobilis have been performed by many authors [8, 10–13, 19, 71, 72]. Based on the consideration of some debates about food security [73], environmental degradation [74] and other issues, developing lignocellulosic feedstocks to substitute corn or sugarcane for bioenergy production will be an inevitable trend in the future [75]. Currently, recombinant Z. mobilis capable of simultaneous fermentation of pentose and hexose sugars from lignocellulosic hydrolysates to ethanol have been achieved since 1995. The brief research history is shown in Figure 2.
In 1995, Zhang et al. from the National Renewable Energy Laboratory (NREL) constructed a recombinant Z. mobilis CP4 (pZB5) strain by introducing two operons encoding xylose assimilation and pentose phosphate pathway enzymes from E. coli into Z. mobilis for the first time, which could ferment pentose sugar and allowing for growth on xylose with 86% ethanol yield [76]. Based on Zhang’s research, another arabinose-fermenting recombinant Z. mobilis CP4 (pZB206) strain was also constructed by introducing five arabinose metabolism- related genes from E. coli into Z. mobilis CP4 in 1996, which could ferment arabinose sugar and produced ethanol at 98% of theoretical yield [77]. For co-fermenting glucose, xylose, and arabinose to ethanol simultaneously, co-culture processes of Z. mobilis ATCC 39676 (pZB4L) and ATCC 39676 (pZB206) have been performed, which showed 72.5% of theoretical ethanol yield [78]. However, both xylose-fermenting strain and xylose had a significant effect on the performance of the arabinose utilization strain. Based on these considerations, Zhang et al. constructed a single Z. mobilis 206C (pZB301) in 1998, which could ferment mixture sugars to ethanol via 82 to 84% theoretical yield [79]. However, all recombinant strains were constructed by antibiotic-resistant plasmid; addition of antibiotics to maintain stablity for large-scale fermentations is highly undesirable. For enhancing its genetic stability, all seven genes necessary for pentose utilization were integrated into theZymomonas genome and a stable Z. mobilis AX101 strain obtained in 2002, which could ferment a hextose and pentose mixture via a preferential order [80].
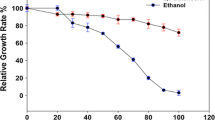
Although a strain capable of co-fermentation of all three sugars was achieved, all recombinant strains were sensitive to acetic acid stress. For example, nuclear magnetic resonance (NMR) studies found that acetic acid could inhibit efficiency of xylose utilization in Z. mobilis ZM4 (pZB5)[81]. Different strategies were developed to improve the tolerance of acetic acid and xylose utlization. For example, Lawford and Rousseau et al. developed a process via addition of extra glucose in acetic acid-containing media for improving fermentation performance of recombinant Zymomonas[82]. Recombinant plasmid pZB5 was also transferred into an acetic acid-tolerant strain (ZM4/AcR) [47], and a mutant recombinant Z. mobilis ZM4/AcR (pZB5) strain with increased acetate resistance was obtained [48]. Overexpression of xylulokinase in a xylose-metabolising recombinant strain was also performed, and resulted in another recombinant ZM4/AcR (pZB5, pJX1) [83]. The ALE strategy was also used for improving the tolerance of acetic acid [69] and efficiency of xylose utilization [70] in Z. mobilis as mentioned previously. An isolated mutant CP4 (pZB5) M1-2 strain could metabolize xylose more rapidly than glucose. Sequence data analysis revealed mutations in both the glucose facilitator (glf) and glucokinase (glk) genes [84]. Mohagheghi et al. developed a new integrant of ZM4(pZB5), and named itZ. mobilis 8b, and this can tolerate acetic acid up to 16 g l-1 and achieve 82 to 87% ethanol yields [49]. Another mutant of Z. mobilis strain 8b obtained through adaptation using 2-deoxyglucose has shown a higher rate of xylose utilization [85]. Specific gene inactivation was also performed for strain improvement, for example, a superior strain, ZM6014 △XR/pZMETX* obtained by inactivation of xylose reductase (XR, ZMO0976) [30, 86]. Another example is him A (ZMO0976) inactive by transposon mutagenesis (as also shown in Table 1) [24, 25]. In 2013, a cost-effective recombinant Z. mobilis HYMX was constructed by integrating seven genes (Pfu-sHSP, yfdZ, metB, xylA, xylB, tktA and talB) into the genome of Z. mobilis CP4 via Tn5 transposon, which showed tolerance tomultiple stresses, high yield and stable genetic characteristics [56].
Furthermore, fermentation characteristics of different recombinant strains were also analyzed in the past decade [30, 49, 56, 78–81, 83, 84, 86–89]. Importantly, fermentation performance of three best recombinant strains form different platforms used for cellulosic ethanol production, E. coli KO11, S. cerevisiae 424A (LNH-ST) and Z. mobilis AX101, which were compared with cellulosic material for the first time. Especially, Z. mobilis AX101 showed the highest rate of glucose consumption and lowest yield of byproducts [88]. These results also indicate that the metabolic pathway of E. coli KO11 and Z. mobilis AX101 are more effective in fermenting ethanol from the related yeast pathway of the consumed sugars [88]. However, utilization of xylose in lignocellulosic hydrolysate and growth robustness of recombinant Z. mobilis are also required to improve in the future. Moreover, different lignocellulosic feedstocks, such as agro-industrial wastes [90], sugarcane bagasse [91], oat hull [92], corn stover [49, 93], bamboo residues [94], and various hydrolysates produced by Arkenol Technology [50], have also been used for ethanol production by Z. mobilis. In general, these studies will provide a deep basis for the ethanol industry in the future.
Although different engineered Z. mobilis strains have also been successfully constructed by introducing desirable genes as previously mentioned, convertion of cellulosic biomass into ethanol directly is also a considerable task for ethanol production. Recently, there has been development of consolidated bioprocessing (CBP)- a combination of cellulase production, cellulose hydrolysis and fermentation into a single step, which is regarded as an alternative approach with outstanding potential [95, 96]. In 2010, two cellulolytic enzymes, E1 and GH12 from Acidothermus cellulolyticus were successfully expressed in Z. mobilis via a native secretion signal peptide [97]. Five cellulolytic enzymes from bacteria isolated from the gut of phytophagous insects were also transferred into Z. mobilis, and all the resulting recombinants fermented pretreated cellulosic feedstocks directly into ethanol [98]. In another study, six genes encoding cellulolytic enzymes (CenA, CenB, CenD, CbhA, CbhB, and Cex) from Cellulomonas fimi and other cellulolytic enzymes (cenA, bgl) from Ruminococcus albus were also introduced and co-expressed successfully in Zymobacter palmae, which enabled Z. palmae to efficiently ferment a water-soluble cellulosic polysaccharide to ethanol [99]. Although the recombinant Z. mobilis strains need to be improved further by simultaneous expression of additional cellulase genes, all these results also indicate that Z. mobilis could be serving as an important CBP platform organism.
Other value-added bio-products production by Z. mobilis
Sorbitol and bionic acid production
In 2013, the US Department of Energy (DOE) published 12 topvalue-added building-block chemicals from biomass [100]. Representative chemicals, including four carbon 1,4-diacids (succinic, fumaric, and malic), 2,5-furan dicarboxylic acid (FDCA), 3-Hydroxypropionic acid (3-HPA), aspartic acid, glutamic acid, glucaric acid, itaconic acid, levulinic acid, 3-Hydroxybutyrolactone, glycerol, sorbitol, xylitol/arabinitol. Sorbitol was identified as one of the top 12 building block chemicals by the US DOE [100], and could be produced by Z. mobilis.
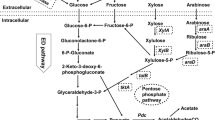
Actually, Barrow et al. found a phenomenon that ethanol yield was decreased when Z. mobilis grown on sucrose or mixtures of glucose plus fructose medium. Further study by NMR spectroscopy indicated that the reason for reduced ethanol yield was due to sorbitol formation from fructose [101]. Leigh et al. identified a proposed metabolic pathway for the production of sorbitol in Z. mobilis[102]. Zachariou and Scopes et al. demonstrated glucose-fructose oxidoreductase (GFOR) and glucono-σ-gluconase (GL) are responsible for sorbitol production, and gluconate intermediate could be converted to ethanol via the Entner-Doudoroff (ED) pathway [103]. These extensive studies demonstrated that Z. mobilis could produce sorbitol in a one-step reaction via GFOR, which is so far only known from this bacterium.
Based on these studies, many researchers developed different processes for producing sorbitol or gluconic acid production by Z. mobilis via whole cells, permeabilized cells or immobilized cells (as shown in Table 3). For example, Chun and Rogers et al. developed a simultaneous process for sorbitol and gluconic acid, 290 g/L of sorbitol and 283 g/L of gluconic acid were yielded from 60% total sugar solution (300 g L-1 glucose and 300 g L-1 fructose) after a 15-h reaction with Z. mobilis-permeabilized cells [104]. Rehr et al. found no gluconic acid formation when using glucose-grown cells for the conversion of equimolar fructose and glucose mixtures. However, nearly 295 g/L of sorbitol and gluconic acid were produced using cetyltrimethylammonium bromide (CTAB)-treated cells [105]. These results surported that gluconate intermediate converted to ethanol via the ED pathway [103, 106]. Silveira et al. found that the yield of sorbitol and gluconic acid increased with substrate concentration [107]. Cazetta et al. investigated sorbitol production from sugar cane molasses by Z. mobilis, which showed the best conditions for sorbitol production containing 300 g/L total reducing sugars (TRS) in the culture medium [108]. Actually, to improve the sorbitol yield, various cell permeabilization methods, that is toluene [104], dried Z. mobilis cells, CTAB [105], metal ions [109], which inhibited key enzymes of the ED pathway and led to decreased ethanol concentration.
Although Lactobacillus casei[110] and Lactobacillus plantarum[111] were also engineered for sorbitol production, sorbitol with a yield up to 0.65to 0.67 mol/mol glucose [111, 112], the conversion rate of sugar and yield of sorbitol are lower when compared to Z. mobilis. So, Z. mobilis showed some advantages of sorbitol production, including a one-step reaction via GFOR, higher conversion rate of sugar and yield, and higher value of byproduct. It may be used for sorbitol production in an industrial scale in the future.
However, activity of GFOR in wild-type Z. mobilis is very low and regulated by glucose concentration [103]. For further improvement of sorbitol production, overexpression of GFOR is an attractive strategy to improve its efficiency. As reported by Liu et al., an engineered strain harboring plasimd pHW20a-gfor, showed higher sorbitol yield than the wild strain [109]. On the other hand, although Z. mobilis could convert a mixture of glucose and fructose into sorbitol with high efficiency, the cost of the substrate may be very high. No natural feedstocks could meet the demand of high sugar-concentration. So, further research need to be carried out for searching for cheaper feedstocks or into the development of a novel process for conversion of lower sugar-concentration. Fortunately, the metabolic pathway of sorbitol and gluconic acid are clear [103, 113], and gene regulation of gfor has also been studied by many research groups [32, 114]. Loos et al. described a sorbitol-related protection mechanism of osmotic stress in concentrated sugar media [114]. Further research also indicates that sorbitol is required for cell growth and ethanol production under heat, ethanol, and osmotic stresses in Z. mobilis[32]. These clues will provide a chance for improving sorbitol and gluconic acid yield through metabolic engineering.
Furthermore, for determination of the substrate spectrum of GFOR, Satory et al. first reported that GFOR enzyme from Z. mobilis can oxidize different aldose sugars into corresponding aldonic acid when D-Fructose is used as the corresponding acceptor substrate. The conversion efficiency ranges from 9 to 84%, which shows a broad spectrum of substrates for the enzyme [115]. The study indicated that GFOR could be potentially used for other bionic acid production, that is, lactobionic acid (LBA), a lactose derivative that has many value-added applications in cosmetics, pharmaceutical or biomedicines, food, and chemical industries, as reviewed by Alonso et al.[113]. Lactose oxidation by GFOR was also performed by Satory et al., which showed a high productivity of 110 g/L-1/d-1 in a continuously stirred tank reactor (CSTR) after operating for 70 h [115]. Bioconversion of a mixture of fructose and lactose into sorbitol and LBA with immobilized cells of Z. mobilis in calcium-alginate has also been reported [116, 117]. Other bionic acids, such as maltobionic, xylonic acid, galactonic acid, arabinonic acid, mannonic acid and cellobionic acid, should also be performed in the future, which shows another important application for Z. mobilis (as shown in Figure 3).
Levan production by Z. mobilis
Levan is a fructose polymer with potential importance in food technology or medical applications [118]. Actually, Dawes and Ribbons et al. first found that reduction of ethanol yield has been attributed to levan formation when Z. mobilis is grown on sucrose medium [119]. Further research also verified that ethanol-yield reduction might be due to sorbitol and levan formation [101, 102, 120]. For example, Beker et al. developed a simultaneous sucrose bioconversion into ethanol and levan by Z. mobilis, and the levan yield of 0.22 g/g and the productivity of 3.2 g/L/h obtained [121]. Yoshida et al. and other researchers also found Z. mobilis could produce a high yield of levan when cultivated in sucrose medium [122–124]. Calazans et al. also found that levans produced by Z. mobilis strains have anti-tumor activities, and its molecular weight was also determined [125, 126]. Previous studies verified that intracellular sucrase (SacA), extracellular levansucrase (SacB) and extracellular sucrase (SacC) contribute to sucrose hydrolysis in Z. mobilis[127]. Based on its genetic and biochemical studies, Senthilkumar et al. constructed a SacC mutant via the insertional mutant method, and higher yield of levan was obtained [20]. To avoid unnecessary supplementation with vitamins and mineral salts, low-cost effective substrate needs be used for levan production in Z. mobilis[128]. Levan production in batch and continuous fermentation systems by Z. mobilis B-14023 was also investigated recently [129]. These extensive studies indicate that Z. mobils may be used for industrial levan production for some purposes.
Succinic acid production by Z. mobilis
Succinic acid was identified as one of the top 12 building-block chemicals by the US DOE[100]. Transparency Market Research also published a new report,Succinic Acid Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2012-2018, in October 2013, which predicted that its market will be expected to reach USD 836.2 million by 2018. Based on these considerations, biological production of succinic acid from abundant and available biomass has become a topic of worldwide interest. Currently, different natural succinate-producing or genetically modified strains, such as Actinobacillus succinogenes, Anaerobiospirillum succiniciproducens, Mannheimia succiniciproducens, Bacteroides fragilis, and Corynebacterium sp. have been used for bio-based succinic acid production from different feedstocks [130, 131]. Other strains, including E. coli[132, 133], and S. cerevisiae[134, 135] have also been engineered for succinic acid production. Although these strains showed some advantanges for succinic acid production, the process of fermentation is anaerobic and kinds of byproducts are formed. Recently, Lee et al. constructed a genome-scale metabolic model of Z. mobilis (ZmoMBEL601), which suggested a possible strategy for succinic acid production by disrupting pyruvate decarboxylase (pdc, ZMO1360) or alcohol dehydrogenase (adh B, ZMO1596) and D-lactate dehydrogenase (ldh A, ZMO1237) simultaneously [136]. Although this conclusion is based on the metabolic model, the higher yield of succinic acid will likely be achieved in the future. Actually, Seo et al. have constructed an engineered Z. mobilis for succinic acid production by redirecting metabolic pathways upon gene knockout of pdc and ldh A. The double gene-knockout strain ZM4 (△pdc△ldh A) has produced 1.46 mol succinate from 1 mol glucose, which showed 95% theoretical yield, and agrees well with the metabolic model ZmoMBEL601 [23]. Based on these studies, a suggested pathway for succinic acid may be proposed, as shown in Figure 4.
Metabolic pathways for the production of the high-value products by using Z. mobilis as platform. The solid lines indicate Z. mobilis native pathways and the dotted lines refer to the recombinant pathway obtained by metabolic engineering strategies. gfor, glucose-fructose oxidoreductase; ldh A, lactate dehydrogenase; pdc, pyruvate decarboxylase; gnl, glucono-σ-gluconase; adc, acetoacetate dehydrogenase; adh, secondary alcohol dehydrogenase; adh B, alcohol dehydrogenase; adh E, acetaldehyde/alcohol dehydrogenase; adh E2, secondary alcohol dehydrogenase; ato AD, acetyl-CoA:acetoacetyl-CoA transferase; ato B, acetyl-CoA acyltransferase; bcd, butyryl-CoA dehydrogenase; crt, crotonase; ctf AB, acetoacetyl-CoA transferase; etf BA, electrotransfer flavor protein; hbd, β-hydroxy butyryl-CoA dehydrogenase; thl, acetyl-CoA acyltransferase; kivd, ketoisovalerate decarboxylase.
Other studies in silico or stoichiometric analysis of the central metabolism of Z. mobilis are valuable, for instance, Widiastuti et al. have also confirmed the functional role of pdc and adh genes during ethanol production in Z. mobilis via a genome-scale metabolic network (i zm363) [137]. A medium-scale model based on stoichiometric analysis of central metabolism was also performed by Pentjuss et al.[138]. These studies will also help us to gain a deep understanding of its special physiological characteristics or re-direct its metabolic pathway for production of target products in the future.
Isobutanol production
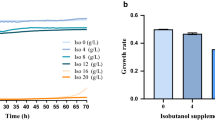
Isobutanol has also been paid more and more attention in recent years for its advantanges over bio-ethanol as a liquid fuel [2, 139]. Engineered strains for isobutanol production in E. coli[139–141], S. cerevisiae[142–144], Corynebacterium glutamicum[143, 145–147], Bacillus subtilis[148], and fungal-bacterial consortia [149], have been engineered or reviewed in previous studies. Recently, an engineered Z. mobilis strain was also constructed for isobutanol production via metabolic pathway engineering: 2-ketoisovalerate decarboxylase (kiv d) gene and alcohol dehydrogenase (adh A) gene from Lactococcus lactis were introduced into Z. mobilis ZM4, which led to isobutanol accumulation. Although the yield of isobutanol is very low, an engineered Z. mobilis is first used for producing the isobutanol. Higher yield may be obtained by disruption of key genes of the ED pathway (as shown in Figure 2) or addition of the extra biosynthesis pathway of alanine (for example, alaD, L-alanine dehydrogenase). Actually, alaD gene Bacillus sphaericus was also cloned and introduced into Z. mobilis, and 7.5 g/L alanine was excreted in the recombinant strain [150].
Other products
Isoprenoids represent another wide group of chemically active compounds, which could be produced by engineered microorganisms, and show a broad range of applications [151, 152]. Actually, Z. mobilis has the highest total hopanoid content (30 mg/g DCW, dry cell weight) among all bacteria, which leads to more tolerance by increasing the hopanoid content [153, 154]. Further research has also verified its biosynthesis pathway viathe methylerythritol phosphate (MEP) pathway [155]. Moreover, biosynthesis pathway of hopanoid lipids and its regulation have also been characterized on the genetic level, which formed a biosynthetic operon [43, 44, 156–158]. These results indicated that Z. mobilis has higher activity of the isoprenoid biosythensis pathway, which may be potentially used for isoprenoid compounds production and reflects a novel application for Z. mobilis. Actually, a group of plasmid-encoded carotene biosynthetic genes (crtB, crtE, crtI, crtY) have been introduced into Z. mobilis via conjugation, resulting in production of β-carotene [159]. Several genes from the thermallydimorphic fungus Penicillium marneffei with predicted terpene synthase function were also selected for functional analysis and evaluation of their potential for the bioproduction of isoprenoid compounds in Z. mobilis (as shown in the PhD thesis of So Lok-yan, University of Hong Kong). Although these studies represent preliminary work, with deeper understanding of its biosynthesis pathway, Z. mobilils shows great potential for isoprenoid compounds production in the future.
Conclusions
Based on the previous and our reviews, Z. mobilis is firstly being developed as an effective ethanologenic by engineering strain improvement, including utilization of xylose and arabinose in addition to glucose. Undoubtedly, Z. mobilis has showed desirable characteristics for its special metabolic pathway. The scientific and technological progress of Z. mobilis have also made a significant contribution to the bioethanol industry. Compared with E. coli, Z. mobilis has high restriction-modification enzyme activity, and cannot be contaminated by bacteriphages [13]. It is fairly osmo-tolerant and can hence tolerate very high sugar concentrations, which is an advatange in fermentation in a high-sugar medium. Its smaller genome and simple metabolic pathway, also lead to less byproducts formation. On the other hand, its desirable characteristics will also make it a novel platform for future biorefineries, which will make a significant contribution to green or sustainable chemistry (as shown in Figure 5).
Although extensive studies, such as general genetic tools, strategies of metabolic engineering, value-added bio-product production, genomic and transcriptomic, et cetera, have been developed in Z. mobilis since 1980s, non-commercialization of the Zymomonas process for ethanol production from sugar, starch-based or lignocellulosic biomass has developed successfully. Moreover, an increased range of higher-value product generation has also been restricted by its fundamental research. Especially, it is more difficult to engineereZ. mobilis than E. coli or yeast. Despite the extensive studies on general genetic tools and omics data available for Z. mobilis, it is necessary to further develop advanced technologies that can be used in metabolic engineering.
Therefore, to realize the industrial potential of Z. mobilis for future biorefineries, considerable efforts should be focused on the following points in the future: developing universal tools for deletion of several genes in one round, controlling metabolic flux and optimizing regulatory networks to improve the yield of desired products, and developing a highly express system, et cetera; these novel technologies are necessary for further strain improvement or redirection of the metabolic pathway for fuel and chemical production. Moreover, different systems of metabolic engineering approaches are becoming powerful tools in developing engineered E. coli or S. cerevisiae[3, 5], which should also be highlighted in engineered Z. mobilis strains. In particular, other biotechnological approaches, such as genome sequencing, functional genomics, genome engineering and omics will also provide a basis for pathway or genome reconstruction to improve its fitness and robustness for environmental stress [160, 161]. Representitive biotechnologies, such as CRISPR/Cas systems [162], site-specific recombinases [163, 164], genome shuffling [165], global transcription machinery engineering (gTME) [166], and Zinc-finger nucleases [167], which will also be used for enhancing cellular traits of Z. mobilils. Presumably, their potential will be further implemented with a promising future in developing or optimizing the metabolic pathway for the production of fuels as well as commodity and specialty chemicals.
Abbreviations
- AcR:
-
acetate-tolerant mutant
- ALE:
-
adaptive laboratory evolution
- bp:
-
base pairs
- CBP:
-
consolidated bioprocessing
- CSTR:
-
continuously stirred tank reactor
- CTAB:
-
cetyltrimethylammonium bromide
- US DOE:
-
US Department of Energy
- ED pathway:
-
Entner-Doudoroff pathway
- GFOR:
-
glucose-fructose oxidoreductase
- GL:
-
glucono-σ-lactonase
- gTME:
-
global transcription machinery engineering
- LBA:
-
lactobionic acid
- NGS:
-
next-generation sequencing
- NMR:
-
nuclear magnetic resonance
- subsp.:
-
subspecies.
References
Lee JW, Na D, Park JM, Lee J, Choi S, Lee SY: Systems metabolic engineering of microorganisms for natural and non-natural chemicals. Nat Chem Biol 2012, 8: 536-546.
Peralta-Yahya PP, Zhang F, Del Cardayre SB, Keasling JD: Microbial engineering for the production of advanced biofuels. Nature 2012, 488: 320-328.
Chen XZ, Zhou L, Kangming T, Kumar A, Singh S, Prior BA, Wang ZX: Metabolic engineering of Escherichia coli: a sustainable industrial platform for bio-based chemical production. Biotechnol Adv 2013, 31: 1200-1223.
Nielsen J, Larsson C, van Maris A, Pronk J: Metabolic engineering of yeast for production of fuels and chemicals. Curr Opin Biotechnol 2013, 24: 398-404.
Hong K-K, Nielsen J: Metabolic engineering of Saccharomyces cerevisiae : a key cell factory platform for future biorefineries. Cell Mol Life Sci 2012, 69: 2671-2690.
Bentsen NS, Felby C: Biomass for energy in the European Union-a review of bioenergy resource assessments. Biotechnol Biofuels 2012, 5: 1-10.
Zhou X, Wang F, Hu H, Yang L, Guo P, Xiao B: Assessment of sustainable biomass resource for energy use in China. Biomass Bioenergy 2011, 35: 1-11.
Panesar PS, Marwaha SS, Kennedy JF: Zymomonas mobilis : an alternative ethanol producer. J Chem Technol Biotechnol 2006, 81: 623-635.
Seo JS, Chong H, Park HS, Yoon KO, Jung C, Kim JJ, Hong JH, Kim H, Kim JH, Kil JI, Park CJ, Oh HM, Lee JS, Jin SJ, Um HW, Lee HJ, Oh SJ, Kim JY, Kang HL, Lee SY, Lee KJ, Kang HS: The genome sequence of the ethanologenic bacterium Zymomonas mobilis ZM4. Nat Biotechnol 2005, 23: 63-68.
Doelle H, Kirk L, Crittenden R, Toh H, Doelle M: Zymomonas mobilis : science and industrial application. Crit Rev Biotechnol 1993, 13: 57-98.
Sahm H, Bringer-Meyer S, Sprenger G: The Genus Zymomonas . Proc Natl Acad Sci U S A 2006, 5: 201-221.
Lin Y, Tanaka S: Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 2006, 69: 627-642.
Rogers PL, Jeon YJ, Lee KJ, Lawford HG: Zymomonas mobilis for fuel ethanol and higher value products. Adv Biochem Eng Biotechnol 2007, 108: 263-288.
Jang Y-S, Park JM, Choi S, Choi YJ, Seung DY, Cho JH, Lee SY: Engineering of microorganisms for the production of biofuels and perspectives based on systems metabolic engineering approaches. Biotechnol Adv 2012, 30: 989-1000.
Coton M, Laplace JM, Coton E: Zymomonas mobilis subspecies identification by amplified ribosomal DNA restriction analysis. Lett Appl Microbiol 2005, 40: 152-157.
Coton M, Laplace JM, Auffray Y, Coton E: “Framboisé” spoilage in French ciders: Zymomonas mobilis implication and characterization. LWT-Food Sci Technol 2006, 39: 972-979.
Coton M, Laplace J-M, Auffray Y, Coton E: Polyphasic study of Zymomonas mobilis strains revealing the existence of a novel subspecies Z. mobilis subsp. francensis subsp. nov., isolated from French cider. Int J Syst Evol Microbiol 2006, 56: 121-125.
Coton M, Laplace J, Auffray Y, Coton E: Duplex PCR method for rapid detection of Zymomonas mobilis in cider. J Inst Brew 2005, 111: 299-303.
Swings J, Deley J: Biology of Zymomonas . Bacterial Rev 1977, 41: 1-46.
Senthilkumar V, Rameshkumar N, Busby S, Gunasekaran P: Disruption of the Zymomonas mobilis extracellular sucrase gene ( Sac C) improves levan production. J Appl Microbiol 2004, 96: 671-676.
Kerr AL, Jeon YJ, Svenson CJ, Rogers PL, Neilan BA: DNA restriction-modification systems in the ethanologen, Zymomonas mobilis ZM4. Appl Microbiol Biotechnol 2011, 89: 761-769.
Wu B, He MX, Luo AJ, Zhang Y, Feng H, Hu QC, Zhang YZ: Construction and characterization of restriction-modification deficient mutants in Zymomonas mobilis ZM4. Chin J Appl Environ Biol 2013, 19(2):189-197.
Seo JS, Chong HY, Kim JH, Kim JY: Method for mass production of primary metabolites, strain for mass production of primary metabolites, and method for preparation thereof. US: Patent; 2007:20090162910 A1.
Viitanen PV, Tao L, Knoke K, Zhang Y, Caimi PG, Zhang M, Chou Y-c, Franden MA: Zymomonas with improved ethanol production in medium containing concentrated sugars and acetate. EP: Patent; 2012:2209899 B1.
Viitanen PV, Tao L, Knoke K, Zhang Y, Caimi PG, Zhang M, Chou Y, Franden MA: Process for the production of ethanol from a medium comprising xylose, employing a recombinant Zymomonas strain having a reduced himA expression. WO: Patent; 2009:2009058938.
Hayashi T, Furuta Y, Furukawa K: Respiration-deficient mutants of Zymomonas mobilis show improved growth and ethanol fermentation under aerobic and high temperature conditions. J Biosci Bioeng 2011, 111: 414-419.
Kalnenieks U, Galinina N, Strazdina I, Kravale Z, Pickford JL, Rutkis R, Poole RK: NADH dehydrogenase deficiency results in low respiration rate and improved aerobic growth of Zymomonas mobilis . Microbiology 2008, 154: 989-994.
Yang S, Pelletier DA, Lu TY, Brown SD: The Zymomonas mobilis regulator hfq contributes to tolerance against multiple lignocellulosic pretreatment inhibitors. BMC Microbiol 2010, 10: 135.
Yang S, Land ML, Klingeman DM, Pelletier DA, Lu TY, Martin SL, Guo HB, Smith JC, Brown SD: Paradigm for industrial strain improvement identifies sodium acetate tolerance loci in Zymomonas mobilis and Saccharomyces cerevisiae . Proc Natl Acad Sci U S A 2010, 107: 10395-10400.
Agrawal M, Wang Y, Chen RR: Engineering efficient xylose metabolism into an acetic acid-tolerant Zymomonas mobilis strain by introducing adaptation-induced mutations. Biotechnol Lett 2012, 34: 1825-1832.
Wang C, Liu C, Hong J, Zhang K, Ma Y, Zou S, Zhang M: Unmarked insertional inactivation in the gfo gene improves growth and ethanol production by Zymomonas mobilis ZM4 in sucrose without formation of sorbitol as a by-product, but yields opposite effects in high glucose. Biochem Eng J 2013, 72: 61-69.
Sootsuwan K, Thanonkeo P, Keeratirakha N, Thanonkeo S, Jaisil P, Yamada M: Sorbitol required for cell growth and ethanol production by Zymomonas mobilis under heat, ethanol, and osmotic stresses. Biotechnol Biofuels 2013, 6: 180.
Charoensuk K, Irie A, Lertwattanasakul N, Sootsuwan K, Thanonkeo P, Yamada M: Physiological importance of cytochrome c peroxidase in ethanologenic thermotolerant Zymomonas mobilis . J Mol Microbiol Biotechnol 2011, 20: 70-82.
Strazdina I, Kravale Z, Galinina N, Rutkis R, Poole R, Kalnenieks U: Electron transport and oxidative stress in Zymomonas mobilis respiratory mutants. Arch Microbiol 2012, 194: 461-471.
Jeffries TW: Ethanol fermentation on the move. Nat Biotechnol 2005, 23: 40-41.
Kouvelis VN, Saunders E, Brettin TS, Bruce D, Detter C, Han C, Typas MA, Pappas KM: Complete genome sequence of the ethanol producer Zymomonas mobilis NCIMB 11163. J Bacteriol 2009, 191: 7140-7141.
Desiniotis A, Kouvelis VN, Davenport K, Bruce D, Detter C, Tapia R, Han C, Goodwin LA, Woyke T, Kyrpides NC: Complete genome sequence of the ethanol-producing Zymomonas mobilis subsp. mobilis centrotype ATCC 29191. J Bacteriol 2012, 194: 5966-5967.
Kouvelis VN, Davenport KW, Brettin TS, Bruce D, Detter C, Han CS, Nolan M, Tapia R, Damoulaki A, Kyrpides NC, Typas MA, Pappas KM: Genome sequence of the ethanol-producing Zymomonas mobilis subsp. pomaceae lectotype ATCC 29192. J Bacteriol 2011, 193: 5049-5050.
Pappas KM, Kouvelis VN, Saunders E, Brettin TS, Bruce D, Detter C, Balakireva M, Han CS, Savvakis G, Kyrpides NC, Typas MA: Genome sequence of the ethanol-producing Zymomonas mobilis subsp. mobilis lectotype ATCC 10988. J Bacteriol 2011, 193: 5051-5052.
Zhao N, Bai Y, Zhao X-Q, Yang Z-Y, Bai F-W: Draft genome sequence of the flocculating Zymomonas mobilis Strain ZM401 (ATCC 31822). J Bacteriol 2012, 194: 7008-7009.
Kouvelis VN, Teshima H, Bruce D, Detter C, Tapia R, Han C, Tampakopoulou VO, Goodwin L, Woyke T, Kyrpides NC, Typas MA, Pappas KM: Finished genome of Zymomonas mobilis subsp. mobilis strain CP4, an applied ethanol producer. Genome Announce 2014, 2: e00813-e00845.
Yang S, Tschaplinski TJ, Engle NL, Carroll SL, Martin SL, Davison BH, Palumbo AV, Rodriguez M Jr, Brown SD: Transcriptomic and metabolomic profiling of Zymomonas mobilis during aerobic and anaerobic fermentations. BMC Genomics 2009, 10: 34.
He MX, Wu B, Shui ZX, Hu QC, Wang WG, Tan FR, Tang XY, Zhu QL, Pan K, Li Q, Su XH: Transcriptome profiling of Zymomonas mobilis under furfural stress. Appl Microbiol Biotechnol 2012, 95: 189-199.
He MX, Wu B, Shui ZX, Hu QC, Wang WG, Tan FR, Tang XY, Zhu QL, Pan K, Li Q, Su XH: Transcriptome profiling of Zymomonas mobilis under ethanol stress. Biotechnol Biofuels 2012, 5: 75.
Yang S, Pan C, Tschaplinski TJ, Hurst GB, Engle NL, Zhou W, Dam P, Xu Y, Rodriguez M Jr, Dice L: Systems biology analysis of Zymomonas mobilis ZM4 ethanol stress responses. PLoS One 2013, 8: e68886.
Jeon YJ, Xun Z, Su P, Rogers PL: Genome-wide transcriptomic analysis of a flocculent strain of Zymomonas mobilis . Appl Microbiol Biotechnol 2012, 93: 2513-2518.
Joachimsthal E, Haggett KD, Jang J-H, Rogers PL: A mutant of Zymomonas mobilis ZM4 capable of ethanol production from glucose in the presence of high acetate concentrations. Biotechnol Lett 1998, 20: 137-142.
Jeon YJ, Svenson CJ, Joachimsthal EL, Rogers PL: Kinetic analysis of ethanol production by an acetate-resistant strain of recombinant Zymomonas mobilis . Biotechnol Lett 2002, 24: 819-824.
Mohagheghi A, Dowe N, Schell D, Chou YC, Eddy C, Zhang M: Performance of a newly developed integrant of Zymomonas mobilis for ethanol production on corn stover hydrolysate. Biotechnol Lett 2004, 26: 321-325.
Yamada T, Fatigati M, Zhang M: Performance of immobilized Zymomonas mobilis 31821 (pZB5) on actual hydrolysates produced by arkenol technology. In Biotechnology for Fuels and Chemicals. Edited by: Finkelstein M, McMillan J, Davison B. Springer; Appl Biochem Biotechnol; 2002:98-100. 899-907
Skotnicki ML, Lee K, Tribe D, Rogers P: Genetic alteration of Zymomonas mobilis for ethanol production. In Genetic Engineering of Microorganisms for Chemicals. Edited by: Hollaende A, DeMoss RD, Kaplan S, Konisky J, Savage D, Wolfe RS. New York: Plenum Press; 1982:271-290.
Carey V, Walia S, Ingram L: Expression of a lactose transposon (Tn951) in Zymomonas mobilis . Appl Environ Microbiol 1983, 46: 1163-1168.
Buchholz SE, Eveleigh DE: Genetic modification of Zymomonas mobilis . Biotechnol Adv 1990, 8: 547-581.
Pappas KM, Galani I, Typas MA: Transposon mutagenesis and strain construction in Zymomonas mobilis . J Appl Microbiol 1997, 82: 379-388.
Pappas KM: Mini-Mu Transposon Mutagenesis of Ethanologenic Zymomonas mobilis . In Strain Engineering. Edited by: Williams JA. Springer; Methods Mol Biol; 2011:765. 419-434
Zhang X, Wang T, Zhou W, Jia X, Wang H: Use of a Tn5-based transposon system to create a cost-effective Zymomonas mobilis for ethanol production from lignocelluloses. Microb Cell Fact 2013, 12: 41.
Galeros M, Pappas K-M, Beletsiotis E, Typas MA: ISZm1068: an IS5-like insertion element from Zymomonas mobilis . Arch Microbiol 2001, 175: 323-333.
Chatterjee R, Yuan L: Directed evolution of metabolic pathways. Trends Biotechnol 2006, 24: 28-38.
Pál C, Papp B, Lercher MJ: Adaptive evolution of bacterial metabolic networks by horizontal gene transfer. Nat Genet 2005, 37: 1372-1375.
Portnoy VA, Bezdan D, Zengler K: Adaptive laboratory evolution—harnessing the power of biology for metabolic engineering. Curr Opin Biotechnol 2011, 22: 590-594.
Conrad TM, Lewis NE, Palsson BØ: Microbial laboratory evolution in the era of genome-scale science. Mol Syst Biol 2011, 7: 509.
Dragosits M, Mattanovich D: Adaptive laboratory evolution-principles and applications for biotechnology. Microb Cell Fact 2013, 12: 64.
Hua Q, Joyce AR, Palsson BØ, Fong SS: Metabolic characterization of Escherichia coli strains adapted to growth on lactate. Appl Environ Microbiol 2007, 73: 4639-4647.
Lee D-H, Palsson BØ: Adaptive evolution of Escherichia coli K-12 MG1655 during growth on a nonnative carbon source, L-1, 2-propanediol. Appl Environ Microbiol 2010, 76: 4158-4168.
Wallace-Salinas V, Gorwa-Grauslund MF: Adaptive evolution of an industrial strain of Saccharomyces cerevisiae for combined tolerance to inhibitors and temperature. Biotechnol Biofuels 2013, 6: 151.
Cakar ZP, Turanli‒Yildiz B, Alkim C, Yilmaz U: Evolutionary engineering of Saccharomyces cerevisiae for improved industrially important properties. FEMS Yeast Res 2012, 12: 171-182.
Demeke MM, Dietz H, Li Y, Foulquié-Moreno MR, Mutturi S, Deprez S, Den Abt T, Bonini BM, Liden G, Dumortier F: Development of a D-xylose fermenting and inhibitor tolerant industrial Saccharomyces cerevisiae strain with high performance in lignocellulose hydrolysates using metabolic and evolutionary engineering. Biotechnol Biofuels 2013, 6: 89.
Dhar R, Sägesser R, Weikert C, Yuan J, Wagner A: Adaptation of Saccharomyces cerevisiae to saline stress through laboratory evolution. J Evol Biol 2011, 24: 1135-1153.
Wang Y: Development of acetic-acid tolerant Zymomonas mobilis strains through adaptation. Master Thesis: Georgia Institute of Technology, Chemical Engineering 2008. URI: http://hdl.handle.net/1853/29747
Agrawal M, Mao Z, Chen RR: Adaptation yields a highly efficient xylose ‒ fermenting Zymomonas mobilis strain . Biotechnol Bioeng 2011, 108: 777-785.
He M-X, Feng H, Li Y, Bai F, Liu X, Zhang Y-Z: Direct production of ethanol from raw sweet potato starch using genetically engineered Zymomonas mobilis . Afr J Microbiol Res 2009, 3: 721-726.
He M-X, Li Y, Liu X, Bai F, Feng H, Zhang Y-Z: Ethanol production by mixed-cultures of Paenibacillus sp and Zymomonas mobilis using the raw starchy material from sweet potato. Ann Microbiol 2009, 59: 749-754.
Schmer KPV MR, Mitchell RB, Perrin RK: Net energy of cellulosic ethanol from switchgrass. Proc Natl Acad Sci U S A 2008, 105: 464-469.
Pimentel D, Patzek T: Ethanol production using corn, switchgrass, and wood; biodiesel production using soybean and sunflower. Nat Resour Res 2005, 14: 65-76.
Balat M, Balat H: Recent trends in global production and utilization of bio-ethanol fuel. Appl Energy 2009, 86: 2273-2282.
Zhang M, Eddy C, Deanda K, Finkelstein M, Picataggio S: Metabolic engineering of a pentose metabolism pathway in ethanologenic Zymomonas mobilis . Science 1995, 267: 240-243.
Deanda K, Zhang M, Eddy C, Picataggio S: Development of an arabinose-fermenting Zymomonas mobilis strain by metabolic pathway engineering. Appl Env Microbiol 1996, 62: 4465-4470.
Mohagheghi A, Evans K, Finkelstein M, Zhang M: Cofermentation of glucose, xylose, and arabinose by mixed cultures of two genetically engineered Zymomonas mobilis strains. Appl Biochem Biotechnol 1998, 70–72: 285-299.
Zhang M, Chou Y-C, Picataggio SK, Finkelstein M: Single Zymomonas mobilis strain for xylose and arabinose fermentation. US: Patent; 1998:5843760.
Mohagheghi A, Evans K, Chou Y-C, Zhang M: Cofermentation of glucose, xylose, and arabinose by genomic DNA-integrated xylose/arabinose fermenting strain of Zymomonas mobilis AX101. Appl Biochem Biotechnol 2002, 98–100: 885-898.
Kim IS, Barrow KD, Rogers PL: Nuclear magnetic resonance studies of acetic acid inhibition of rec Zymomonas mobilis ZM4 (pZB5). Appl Biochem Biotechnol 2000, 84: 357-370.
Lawford HG, Rousseau JD: Improving fermentation performance of recombinant Zymomonas in acetic acid-containing media. Appl Biochem Biotechnol 1998, 70–72: 161-172.
Jeon YJ, Svenson CJ, Rogers PL: Over-expression of xylulokinase in a xylose-metabolising recombinant strain of Zymomonas mobilis . FEMS Microbiol Lett 2005, 244: 85-92.
Supple SG, Joachimsthal EL, Dunn NW, Rogers PL: Isolation and preliminary characterization of a Zymomonas mobilis mutant with an altered preference for xylose and glucose utilization. Biotechnol Lett 2000, 22: 157-164.
Mohagheghi A, Linger J, Smith H, Yang S, Dowe N, Pienkos PT: Improving xylose utilization by recombinant Zymomonas mobilis strain 8b through adaptation using 2-deoxyglucose. Biotechnol Biofuels 2014, 7: 19.
Agrawal M, Chen RR: Discovery and characterization of a xylose reductase from Zymomonas mobilis ZM4. Biotechnol Lett 2011, 33: 2127-2133.
Kim IS, Barrow KD, Rogers PL: Kinetic and nuclear magnetic resonance studies of xylose metabolism by recombinant Zymomonas mobilis ZM4(pZB5). Appl Environ Microbiol 2000, 66: 186-193.
Lau MW, Gunawan C, Balan V, Dale BE: Comparing the fermentation performance ofEscherichia coliKO11, Saccharomyces cerevisiae424A(LNH-ST) and Zymomonas mobilis AX101 for cellulosic ethanol production. Biotechnol Biofuels 2010, 3: 11.
He MX, Feng H, Zhang YZ: Construction of a novel cell-surface display system for heterologous gene expression in Escherichia coli by using an outer membrane protein of Zymomonas mobilis as anchor motif. Biotechnol Lett 2008, 30: 2111-2117.
Ruanglek V, Maneewatthana D, Tripetchkul S: Evaluation of Thai agro-industrial wastes for bio-ethanol production by Zymomonas mobilis . Process Biochem 2006, 41: 1432-1437.
dos Santos DS, Camelo AC, Rodrigues KC, Carlos LC, Pereira N Jr: Ethanol production from sugarcane bagasse by Zymomonas mobilis using simultaneous saccharification and fermentation (SSF) process. Appl Biochem Biotechnol 2010, 161: 93-105.
Lawford HG, Rousseau JD, Tolan JS: Comparative ethanol productivities of different Zymomonas recombinants fermenting oat hull hydrolysate. Appl Biochem Biotechnol 2001, 91: 133-146.
Su R, Ma Y, Qi W, Zhang M, Wang F, Du R, Yang J, Zhang M, He Z: Ethanol production from high-solid SSCF of alkaline-pretreated corncob using recombinant Zymomonas mobilis CP4. Bioenergy Res 2013, 6: 292-299.
He MX, Li Q, Liu XY, Hu QC, Hu GQ, Pan K, Zhu QL, Wu J: Bio-ethanol production from bamboo residues with lignocellulose fractionation technology (LFT) and separate hydrolysis fermentation (SHF) by Zymomonas mobilis . Am J Biomass Bioenergy 2013, 1: 1-10.
Linger JG, Darzins A: Consolidated Bioprocessing. In Advanced Biofuels and Bioproducts. Edited by: Lee JW. Springer; 2013:267-280.
Lynd LR, Zyl WH, McBRIDE JE, Laser M: Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotechnol 2005, 16: 577-583.
Linger JG, Adney WS, Darzins A: Heterologous expression and extracellular secretion of cellulolytic enzymes by Zymomonas mobilis . Appl Environ Microbiol 2010, 76: 6360-6369.
Thirumalai Vasan P: Cloning of bacterial cellulose gene into Zymomonas mobilis for cellulosic ethanol production. PhD Thesis of Bharathidasan University 2012. URI: http://hdl.handle.net/10603/4784
Kojima M, Okamoto K, Yanase H: Direct ethanol production from cellulosic materials by Zymobacter palmae carrying Cellulomonas endoglucanase and Ruminococcus β-glucosidase genes. Appl Microbiol Biotechnol 2013, 97: 5137-5147.
Werpy T, Petersen G: Top Value Added Chemicals from Biomass. Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas. DOE Report by PNNL, NREL, EERE 2013. website: http://www.ascension-publishingcom/BIZ/HD49pdf
Barrow KD, Collins JG, Leight DA, Rogers PL, Warr RG: Sorbitol production by Zymomonas mobilis . Appl Microbiol Biotechnol 1984, 20: 225-232.
Leigh D, Scopes R, Rogers P: A proposed pathway for sorbitol production by Zymomonas mobilis . Appl Microbiol Biotechnol 1984, 20: 413-415.
Zachariou M, Scopes RK: Glucose-fructose oxidoreductase, a new enzyme isolated from Zymomonas mobilis that is responsible for sorbitol production. J Bacteriol 1986, 167: 863-869.
Chun U, Rogers P: The simultaneous production of sorbitol from fructose and gluconic acid from glucose using an oxidoreductase of Zymomonas mobilis . Appl Microbiol Biotechnol 1988, 29: 19-24.
Rehr B, Wilhelm C, Sahm H: Production of sorbitol and gluconic acid by permeabilized cells of Zymomonas mobilis . Appl Microbiol Biotechnol 1991, 35: 144-148.
Strohdeicher M, Schmitz B, Bringer-Meyer S, Sahm H: Formation and degradation of gluconate by Zymomonas mobilis . Appl Microbiol Biotechnol 1988, 27: 378-382.
Silveira MM, Wisbeck E, Lemmel C, Erzinger G, da Costa JP, Bertasso M, Jonas R: Bioconversion of glucose and fructose to sorbitol and gluconic acid by untreated cells of Zymomonas mobilis . J Biol Chem 1999, 75: 99-103.
Cazetta ML, Celligoi MAPC, Buzato JB, Scarmino IS, da Silva RSF: Optimization study for sorbitol production by Zymomonas mobilis in sugar cane molasses. Process Biochem 2005, 40: 747-751.
Liu C, Dong HW, Zhong J, Ryu DD, Bao J: Sorbitol production using recombinant Zymomonas mobilis strain. J Biotechnol 2010, 148: 105-112.
De Boeck R, Sarmiento-Rubiano LA, Nadal I, Monedero V, Pérez-Martínez G, Yebra MJ: Sorbitol production from lactose by engineered Lactobacillus casei deficient in sorbitol transport system and mannitol-1-phosphate dehydrogenase. Appl Microbiol Biotechnol 2010, 85: 1915-1922.
Ladero V, Ramos A, Wiersma A, Goffin P, Schanck A, Kleerebezem M, Hugenholtz J, Smid EJ, Hols P: High-level production of the low-calorie sugar sorbitol by Lactobacillus plantarum through metabolic engineering. Appl Environ Microbiol 2007, 73: 1864-1872.
Akinterinwa O, Khankal R, Cirino PC: Metabolic engineering for bioproduction of sugar alcohols. Curr Opin Biotechnol 2008, 19: 461-467.
Alonso S, Rendueles M, Díaz M: Bio-production of lactobionic acid: current status, applications and future prospects. Biotechnol Adv 2013, 31: 1275-1291.
Loos H, Krämer R, Sahm H, Sprenger GA: Sorbitol promotes growth of Zymomonas mobilis in environments with high concentrations of sugar: evidence for a physiological function of glucose-fructose oxidoreductase in osmoprotection. J Bacteriol 1994, 176: 7688-7693.
Satory M, Fürlinger M, Haltrich D, Kulbe K, Pittner F, Nidetzky B: Continuous enzymatic production of lactobionic acid using glucose-fructose oxidoreductase in an ultrafiltration membrane reactor. Biotechnol Lett 1997, 19: 1205-1208.
Pedruzzi I, da Silva EAB, Rodrigues AE: Production of lactobionic acid and sorbitol from lactose/fructose substrate using GFOR/GL enzymes from Zymomonas mobilis cells: a kinetic study. Enzyme Microb Technol 2011, 49: 183-191.
Malvessi E, Carra S, Pasquali FC, Kern DB, da Silveira MM, Ayub MAZ: Production of organic acids by periplasmic enzymes present in free and immobilized cells of Zymomonas mobilis . J Ind Microbiol Biotechnol 2013, 40: 1-10.
Bekers M, Laukevics J, Karsakevich A, Ventina E, Kaminska E, Upite D, Vı̄na I, Linde R, Scherbaka R: Levan-ethanol biosynthesis using Zymomonas mobilis cells immobilized by attachment and entrapment. Process Biochem 2001, 36: 979-986.
Dawes E, Ribbons D: Sucrose utilization by Zymomonas mobilis : formation of a levan. Biochem J 1966, 98: 804-812.
Viikari L: Formation of levan and sorbitol from sucrose by Zymomonas mobilis . Appl Microbiol Biotechnol 1984, 19: 252-255.
Beker M, Shvinka J, Pankova L, Laivenieks M, Mezhbarde I: A simultaneous sucrose bioconversion into ethanol and levan by Zymomonas mobilis . Appl Biochem Biotechnol 1990, 24: 265-274.
Yoshida Y, Suzuki R, Yagi Y: Production of levan by a Zymomonas sp . J Ferment Bioeng 1990, 70: 269-271.
Reiss M, Hartmeier W: Levan Production With a Flocculent Strain of Zymomonas Mobilis. 1990.
Ananthalakshmy VK, Gunasekaran P: Isolation and characterization of mutants from levan-producing Zymomonas mobilis . J Biosci Bioeng 1999, 87: 214-217.
Calazans G, Lopes C, Lima R, De Franc F: Antitumour activities of levans produced by Zymomonas mobilis strains. Biotechnol Lett 1997, 19: 19-21.
Calazans GM, Lima RC, de França FP, Lopes CE: Molecular weight and antitumour activity of Zymomonas mobilis levans. Int J Biol Macromol 2000, 27: 245-247.
Gunasekaran P, Mukundan G, Kannan R, Velmurugan S, Ait-Abdelkader N, Alvarez-Macarie E, Baratti J: The sacB and sacC genes encoding levansucrase and sucrase form a gene cluster in Zymomonas mobilis . Biotechnol Lett 1995, 17: 635-642.
de Oliveira MR, da Silva RSSF, Buzato JB, Celligoi MAPC: Study of levan production by Zymomonas mobilis using regional low-cost carbohydrate sources. Biochem Eng J 2007, 37: 177-183.
Silbir S, Dagbagli S, Yegin S, Baysal T, Goksungur Y: Levan production by Zymomonas mobilis in batch and continuous fermentation systems. Carbohydr Polym 2014, 99: 454-461.
Beauprez JJ, De Mey M, Soetaert WK: Microbial succinic acid production: natural versus metabolic engineered producers. Process Biochem 2010, 45: 1103-1114.
Cheng KK, Zhao XB, Zeng J, Zhang JA: Biotechnological production of succinic acid: current state and perspectives. Biofuels Bioprod Biorefin 2012, 6: 302-318.
Lee SJ, Lee D-Y, Kim TY, Kim BH, Lee J, Lee SY: Metabolic engineering of Escherichia coli for enhanced production of succinic acid, based on genome comparison and in silico gene knockout simulation. Appl Environ Microbiol 2005, 71: 7880-7887.
Jiang M, Liu SW, Ma J, Chen K, Yu L, Yue F, Xu B, Wei P: Effect of growth phase feeding strategies on succinate production by metabolically engineered Escherichia coli . Appl Environ Microbiol 2010, 76: 1298-1300.
Agren R, Otero JM, Nielsen J: Genome-scale modeling enables metabolic engineering of Saccharomyces cerevisiae for succinic acid production. J Ind Microbiol Biotechnol 2013, 40: 735-747.
Raab AM, Gebhardt G, Bolotina N, Weuster-Botz D, Lang C: Metabolic engineering of Saccharomyces cerevisiae for the biotechnological production of succinic acid. Metab Eng 2010, 12: 518-525.
Lee K, Park J, Kim T, Yun H, Lee S: The genome-scale metabolic network analysis of Zymomonas mobilis ZM4 explains physiological features and suggests ethanol and succinic acid production strategies. Microb Cell Fact 2010, 9: 94.
Widiastuti H, Kim JY, Selvarasu S, Karimi IA, Kim H, Seo JS, Lee DY: Genome-scale modeling and in silico analysis of ethanologenic bacteria Zymomonas mobilis . Biotechnol Bioeng 2011, 108: 655-665.
Pentjuss A, Odzina I, Kostromins A, Fell D, Stalidzans E, Kalnenieks U: Biotechnological potential of respiring Zymomonas mobilis : a stoichiometric analysis of its central metabolism. J Biotechnol 2013, 165: 1-10.
Atsumi S, Hanai T, Liao JC: Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 2008, 451: 86-89.
Atsumi S, Wu TY, Eckl EM, Hawkins SD, Buelter T, Liao JC: Engineering the isobutanol biosynthetic pathway in Escherichia coli by comparison of three aldehyde reductase/alcohol dehydrogenase genes. Appl Microbiol Biotechnol 2010, 85: 651-657.
Atsumi S, Liao JC: Metabolic engineering for advanced biofuels production from Escherichia coli . Curr Opin Biotechnol 2008, 19: 414-419.
Chen X, Nielsen KF, Borodina I, Kielland-Brandt MC, Karhumaa K: Increased isobutanol production in Saccharomyces cerevisiae by overexpression of genes in valine metabolism. Biotechnol Biofuels 2011, 4: 2089-2090.
Brat D, Boles E: Isobutanol production from D-ylose by recombinant Saccharomyces cerevisiae . FEMS Yeast Res 2013, 3: 241-244.
Branduardi P, Longo V, Berterame NM, Rossi G, Porro D: A novel pathway to produce butanol and isobutanol in Saccharomyces cerevisiae . Biotechnol Biofuels 2013, 6: 1-12.
Smith KM, Cho K-M, Liao JC: Engineering Corynebacterium glutamicum for isobutanol production. Appl Microbiol Biotechnol 2010, 87: 1045-1055.
Blombach B, Riester T, Wieschalka S, Ziert C, Youn J-W, Wendisch VF, Eikmanns BJ: Corynebacterium glutamicum tailored for efficient isobutanol production. Appl Environ Microbiol 2011, 77: 3300-3310.
Yamamoto S, Suda M, Niimi S, Inui M, Yukawa H: Strain optimization for efficient isobutanol production using Corynebacterium glutamicum under oxygen deprivation. Biotechnol Bioeng 2013, 110: 2938-2948.
Li SS, Wen JP, Jia XQ: Engineering Bacillus subtilis for isobutanol production by heterologous Ehrlich pathway construction and the biosynthetic 2-ketoisovalerate precursor pathway overexpression. Appl Microbiol Biotechnol 2011, 91: 577-589.
Minty JJ, Singer ME, Scholz SA, Bae C-H, Ahn J-H, Foster CE, Liao JC, Lin XN: Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc Natl Acad Sci U S A 2013, 110: 14592-14597.
Uhlenbusch I, Sahm H, Sprenger GA: Expression of an L-alanine dehydrogenase gene in Zymomonas mobilis and excretion of L-alanine. Appl Environ Microbiol 1991, 57: 1360-1366.
Keasling JD: Manufacturing molecules through metabolic engineering. Science 2010, 330: 1355-1358.
Maury J, Asadollahi M, Møller K, Clark A, Nielsen J: Microbial Isoprenoid Production: An Example of Green Chemistry through Metabolic Engineering. In Biotechnology for the Future, Volume 100. Edited by: Nielsen J. Berlin Heidelberg: Springer; 2005:19-51. Advances in Biochemical Engineering/Biotechnology
Hermans MA, Neuss B, Sahm H: Content and composition of hopanoids in Zymomonas mobilis under various growth conditions. J Bacteriol 1991, 173: 5592-5595.
Schmidt A, Bringer-Meyer S, Poralla K, Sahm H: Effect of alcohols and temperature on the hopanoid content of Zymomonas mobilis . Appl Microbiol Biotechnol 1986, 25: 32-36.
Charon L, Pale-Grosdemange C, Rohmer M: On the reduction steps in the mevalonate independent 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway for isoprenoid biosynthesis in the bacterium Zymomonas mobilis . Tetrahedron Lett 1999, 40: 7231-7234.
Perzl M, Reipen IG, Schmitz S, Poralla K, Sahm H, Sprenger GA, Elmar L: Cloning of conserved genes from Zymomonas mobilis and Bradyrhizobium japonicum that function in the biosynthesis of hopanoid lipids. Biochim Biophys Acta (BBA) - Lipids Lipid Metabol 1998, 1393: 108-118.
Grolle S, Bringer-Meyer S, Sahm H: Isolation of the dxr gene of Zymomonas mobilis and characterization of the 1-deoxy-D-xylulose 5-phosphate reductoisomerase. FEMS Microbiol Lett 2000, 191: 131-137.
Vincent SP, Sinay P, Rohmer M: Composite hopanoid biosynthesis in Zymomonas mobilis : N-acetyl-D-glucosamine as precursor for the cyclopentane ring linked to bacteriohopanetetrol. Chem Commun 2003, 6: 782-783.
Misawa N, Yamano S, Ikenaga H: Production of beta-carotene in Zymomonas mobilis and Agrobacterium tumefaciens by introduction of the biosynthesis genes from Erwinia uredovora. Appl Environ Microbiol 1991, 57: 1847-1849.
Wang HH, Isaacs FJ, Carr PA, Sun ZZ, Xu G, Forest CR, Church GM: Programming cells by multiplex genome engineering and accelerated evolution. Nature 2009, 460: 894-898.
Carr PA, Church GM: Genome engineering. Nat Biotechnol 2009, 27: 1151-1162.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA: Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339: 819-823.
Kolb AF: Genome engineering using site-specific recombinases. Cloning Stem Cells 2002, 4: 65-80.
Wirth D, Gama-Norton L, Riemer P, Sandhu U, Schucht R, Hauser H: Road to precision: recombinase-based targeting technologies for genome engineering. Curr Opin Biotechnol 2007, 18: 411-419.
Zhang Y-X, Perry K, Vinci VA, Powell K, Stemmer WP, del Cardayré SB: Genome shuffling leads to rapid phenotypic improvement in bacteria. Nature 2002, 415: 644-646.
Alper H, Stephanopoulos G: Global transcription machinery engineering: a new approach for improving cellular phenotype. Metab Eng 2007, 9: 258-267.
Carroll D: Genome engineering with zinc-finger nucleases. Genetics 2011, 188: 773-782.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 31000028), partially supported by the Open Funds of Key Laboratory of Microbial Resources Collection and Preservation (Ministry of Agriculture, MOA, 2014), Open Funds of State Key Laboratory of Microbial Technology (Shandong University, 2014), Open Funds of Xinjiang Production & Construction Corps Key Laboratory of Protection and Utilization of Biological Resources in Tarim Basin (Tarim University, BRZD1403), Special Fund for Agro-scientific Research in the Public Interest (201403019), and Sichuan Key Technology R&D Program (2009NZ00045), and the Sci-tech Fund Project of the Chinese Academy of Agricultural Sciences (2009, 2010, 2013, 2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
This review was conceived, researched and written by MXH. BW and ZYR participated in omic data collection and helped in manuscript editing. FRT summarized the section on strain improvement for Z. mobilis. JLW, ZXS, HQ, and QLZ summarized the section on succinic acid production by Z. mobilis and helped in management of references. LCD, XYT, and WGW participated in data collection and were involved in drafting the manuscript. KP and QCH participated in the discussion and helped in the draft manuscript editing. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
He, M.X., Wu, B., Qin, H. et al. Zymomonas mobilis: a novel platform for future biorefineries. Biotechnol Biofuels 7, 101 (2014). https://doi.org/10.1186/1754-6834-7-101
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1754-6834-7-101