Abstract
Background
Human solid tumors that are hard or firm on physical palpation are likely to be cancerous, a clinical maxim that has been successfully applied to cancer screening programs, such as breast self-examination. However, the biological relevance or prognostic significance of tumor hardness remains poorly understood. Here we present a fracture mechanics based in vivo approach for characterizing the fracture toughness of biological tissue of human thyroid gland tumors.
Methods
In a prospective study, 609 solid thyroid gland tumors were percutaneously probed using standard 25 gauge fine needles, their tissue toughness ranked on the basis of the nature and strength of the haptic force feedback cues, and subjected to standard fine needle biopsy. The tumors' toughness rankings and final cytological diagnoses were combined and analyzed. The interpreting cytopathologist was blinded to the tumors' toughness rankings.
Results
Our data showed that cancerous and noncancerous tumors displayed remarkable haptically distinguishable differences in their material toughness.
Conclusion
The qualitative method described here, though subject to some operator bias, identifies a previously unreported in vivo approach to classify fracture toughness of a solid tumor that can be correlated with malignancy, and paves the way for the development of a mechanical device that can accurately quantify the tissue toughness of a human tumor.
Similar content being viewed by others
Background
Human solid cancers typically are harder and firmer than surrounding normal tissue upon clinical palpation [1]. This characteristic has been linked to the presence of abundant collagen in the tumor stroma, commonly referred to as the desmoplastic reaction [2]. While the origin of cancer is genetically based, accumulating evidence now indicates that the tumor-associated desmoplastic stroma is critical to cancer's growth and progression [3]. For example, in vitro studies have shown that stromal rigidity or stiffness strongly influences normal cell motility and tissue form, and may even select a malignant phenotype [4, 5]. There is growing realization that therapeutic intervention aimed at normalizing the tumor's stromal compartment may halt or even reverse the course of cancer growth [3]. Thus, an understanding of the interaction between tumor cells and their mechanical microenvironment is seen as increasingly relevant to the study of tumor biology. However, how best to measure, interpret, and model the mechanical attributes of tumor stromal tissue in vivo remain unclear. A novel technique, termed elastography [6–8], has shown promise in this regard and involves in vivo assessment of local responses of the tissue to an applied load (i.e., mechanical indentation) by imaging the longitudinal or shear-strain components at different locations in the tissue. In the present study, we have taken a different approach to this problem and report a fracture mechanics based in vivo method for testing the fracture toughness of solid thyroid gland tumors by manual probing using hypodermic needles.
During routine sonographically-guided fine needle biopsy of solid tumors [nodules] of the human thyroid gland, it was serendipitously observed by one of the authors (NR) that the nature and strength of the haptic force feedback cues varied among tumors. To further investigate this phenomenon and to assess the relationship between tumor hardness and cytological diagnosis, a prospective clinical study was designed and implemented incorporating the principles of fracture mechanics [9] with haptic modality [10].
Fracture mechanics governs how materials [solid and semisolid] behave before and after the nucleation and growth of micro and macro-cracks, and describes how cracks initiate and propagate in materials. Fracture toughness characterizes the material's intrinsic penetration resistance [fracture energy] to crack initiation and propagation. In the present study, the manually instigated thrusting movement of the probing needle provided the necessary force for the initiation and propagation of fractures through solid tumor tissue. However, what causes the build-up of intratumoral resistance to needle penetration is unclear. Recent data [11] show that in addition to abundant collagen deposition, structural realignment of collagen fibers, especially around groups of proliferating cancer cells, is a prominent feature within the growing tumor. We hypothesize that as the needle tip penetrates the tumor core, irreversible mechanical damage to the collagen tissue ensues which results in rapid disassociation of collagen fibers into subfibers, fibrils and microfibrils. The fracture energy that is released during this process is potentially quantifiable and may signify a unique mechanical tumor marker.
In the present investigation, we have analyzed the nature of the penetration resistance (fracture energy) qualitatively by means of extended haptic perception [12]. Haptic perception entails an active exploration of an object over time and space by integrating the body's sensory and kinesthetic abilities. This is known as active touch [10]. Since it is dependent upon the exchange of mechanical force cues, a man-made inorganic tool, such as a blind person's cane or a dental probe, can effectively extend the perception beyond the body's physical boundary [12]. In the present study, the fine needle used as an embodiment of haptic tool effectively extended the perception from the fingertip to the needle tip.
Results
Table 1 shows the distribution of cancer among 609 thyroid tumors categorized into Groups 1 and 2 on the basis of the nature and strength of haptic force cues. Bayesian analysis of data [Table 2] shows dramatic differences in the material toughness between cancerous and noncancerous tumors; 64% of Group 1 tumors exhibited thyroid cancer, while 95% of Group 2 tumors displayed no histological evidence of cancer.
Discussion
The results described in this report, though subject to some operator bias, demonstrate the feasibility of an in vivo approach for characterizing the fracture toughness of a solid tumor that can be correlated with malignancy. However, these results need to be validated and verified by quantitative analysis. We anticipate our method to be a good starting point for the development of a mechanical device that can quantify the tumor's toughness in vivo using the fracture mechanics principles and fracture toughness measurements.
Conclusion
A qualitative in vivo method for characterizing a solid tumor's fracture toughness by haptic means is reported. These results when validated by quantitative measurement data are likely to provide a new framework for understanding the mechanical attributes of tumor microenvironment.
Methods
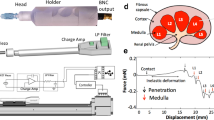
Solid tumors of the human thyroid gland [Fig. 1] were selected for testing because of their ready anatomic accessibility for direct clinical intervention; i.e., via fine-needle biopsy, and their high clinical prevalence in the United States population. The lead author (NR) was solely responsible for ranking the tissue hardness of all 609 thyroid gland tumors and subsequent fine needle biopsy. The tissue samples were processed on-site by a cytotechnologist and hand delivered to the cytopathologist for interpretation and final diagnosis. The interpreting cytopathologist was blinded to the tumors' hardness rankings.
A 3D rendered image of a right thyroid lobe solid tumor about to be pierced and probed manually with a fine needle. Haptic force feedback cues generated by the probing needle were used to characterize and classify a solid tumor into two groups. Tumors exhibiting haptic cues that evoked the perception of cutting through an unripe pear [hard and gritty] were placed in Group 1, while those with the perception of cutting through jelly [soft and spongy] in Group 2. The results were dramatic: 64% of Group 1 tumors were cancerous while 95% of Group 2 tumors were noncancerous.
The method, monitored by real-time sonographic image guidance [see Additional file 1] is described as follows: [a] after percutaneous introduction, the 5-cm long, 25-gauge fine needle is advanced through soft tissues of the neck, and its tip inserted into the tumor surface, [b] a fracture is initiated by the forward movement of the needle tip, [c] the fracture is propagated through the solid core by repeatedly advancing and withdrawing the needle, and [d] the tumor hardness is ranked on the basis of the nature and strength of the haptic force-feedback cues emanating from within the tumor due to tissue penetration and rupture induced by the rapidly moving needle tip.
Additional file 1: This movie clip demonstrates the technique of manual probing of the solid thyroid tumor with a fine needle. Note the apparent ease with which the needle travels through a non-cancerous tumor. In contrast, a cancerous tumor offers substantial resistance to needle insertion and penetration. Remarkable differences in haptic force cues were apparent between cancerous and benign tumors. (MOV 8 MB)
Ranked tumors were placed in one of two groups [Table 1]: Group 1 [N = 134]: tumors exhibiting penetration resistance with a distinctive force-feedback cue, as if cutting through an unripe pear; Group 2 [N = 475]: tumors exhibiting no resistance, as if cutting through jelly. The toughness rankings were then correlated to the final cytological diagnoses.
References
Lippman ME: Evaluation of Breast Masses in Men and Women. Harrison's Principles of Internal Medicine. Edited by: Fauci AS, Braunwald E, Kasper DL. 2008, New York: McGraw-Hill, Ch. 86. 17e:
Walker RA: The complexities of breast cancer desmoplasia. Breast Cancer Res. 2001, 3: 143-145. 10.1186/bcr287.
Bissell MJ, Radisky D: Putting tumours in context. Nat Rev Cancer. 2001, 1: 46-54. 10.1038/35094059.
Lo CM, Wang HB, Dembo M, Wang YL: Cell Movement Is Guided by the Rigidity of the Substrate. Biophys J. 2000, 79: 144-152.
Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM: Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005, 8: 241-254. 10.1016/j.ccr.2005.08.010.
Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X: Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrasonic Imaging. 1991, 13: 111-134. 10.1016/0161-7346(91)90079-W.
McKnight AL, Kugel JL, Rossman PJ, Manduca A, Hartmann LC, Ehman RL: MR elastography of breast cancer: preliminary results. AJR. 2002, 178: 1411-1417.
Lyshchik A, Higashi T, Asato R, et al: Thyroid gland tumor diagnosis at US elastography. Radiology. 2005, 237: 202-211. 10.1148/radiol.2363041248.
Sanford RJ: Principles of Fracture Mechanics. 2003, Prentice-Hall: Pearson Education Inc
Lederman SJ, Klatzky RL: Hand movements: a window into haptic object recognition. Cogn Psychol. 1987, 19: 342-368. 10.1016/0010-0285(87)90008-9.
Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ: Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Medicine. 2006, 4: 38-10.1186/1741-7015-4-38.
Burton G: Non-neural extensions of haptic sensitivity. Ecological Psychol. 1993, 5: 105-124. 10.1207/s15326969eco0502_1.
Acknowledgements
We thank Drs. S. Davis, V. Kamdar, D. Martinez, P. Cohan, C. Darwin, S. Ahmadi, J. Lin and A. Chen for patient referrals; Drs. J. Rao and S. Apple for reviewing the cytological slides; Drs. V. Dhir and A. Van Herle for reviewing the manuscript; sonographers and cytotechnologists for technical help; L. Chandler for data management; L. Goodman, L. Lauerman and B. Best Raga for editorial assistance; M. Scaramozzino [DreamLight.com] for 3D rendition of tumors, and C. Gray for movie file editing.
Institutional Review Board: This study was approved for data management and analysis. Certificate # G06-01-015-03; Expiring on October 1, 2009.
Funding support: Partial support for the study design and data management was provided to NR by the radiology exploratory research project grant # 4-401180-4Y-62259-100-DNP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
NR conceived of the study; substantially contributed to its design, acquisition and interpretation of data; wrote the paper and approved the overall manuscript. JWJ participated in revising the manuscript critically for important intellectual content. JWS performed the statistical analysis. SH carried out the cytological studies and interpreted the data in a double blind fashion. IC and MWY participated in drafting the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ragavendra, N., Ju, J., Sayre, J.W. et al. In vivo analysis of fracture toughness of thyroid gland tumors. J Biol Eng 2, 12 (2008). https://doi.org/10.1186/1754-1611-2-12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1754-1611-2-12