Abstract
Introduction
Nicolau syndrome, also known as livedo-like dermatitis or embolia cutis medicamentosa, is a rare complication following the intramuscular or intra-articular injection of various drugs.
Case presentation
In our case report we report the case of a 45-year-old Turkish woman who developed Nicolau syndrome after an intramuscular injection in her right gluteal region of single-dose diclofenac sodium to treat a headache. A culture taken from the ulcer showed growth of methicillin-sensitive Staphylococcus aureus on the 10th day. The secondary staphylococcal infection was treated effectively with intravascular ampicillin-sulbactam (4×1.5g/day). She was treated with surgical debridement, sterile dressings and analgesics. The ulcer healed completely within 12 weeks with scarring.
Conclusions
Although Nicolau syndrome develops very rarely, it is an important cause for morbidity. It is an iatrogenic condition, treated mostly by health care workers. Thus, although it appears to be a very simple procedure for a health care worker, care must be taken during intramuscular injections. Although diclofenac sodium is a widely used non-steroidal anti-inflammatory drug, Nicolau syndrome following intramuscular diclofenac sodium injection has rarely been reported in the published literature. The application of a cold compress was considered to be an aggravating factor in our patient. This case highlights the need for awareness about this condition and the need to exercise utmost care during the administration of any parenteral injections by health workers.
Similar content being viewed by others
Introduction
Embolia cutis medicamentosa, or Nicolau syndrome (NS), was first described in 1924 after an intragluteal injection of bismuth salts for the treatment of syphilis [1]. According to one theory, NS occurs when an intramuscular medication is inadvertently injected directly in an arterial lumen or wall, leading to vessel thrombosis and subcutaneous tissue and muscle necrosis [2]. The signs of skin discoloration are usually accompanied by severe pain and extensive inflammation [3]. Typically, necrosis develops following hyperemia, skin discoloration, livedoid dermatitis and hemorrhagic patch formation at the injection site [3]. Severe cases may take a rapid clinical course and lead to death [4].
We report a case of NS following an intramuscular injection of diclofenac sodium (Voltaren®).
Case presentation
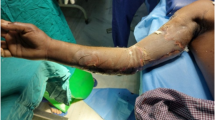
A 45-year-old Turkish woman presented to our clinic with skin discoloration in her right gluteal region, and a physical examination revealed a skin necrosis with a size of approximately 15×20cm. She had received an intramuscular (intragluteal) injection of diclofenac sodium (Voltaren®) for treatment of a headache prior to the onset of the skin necrosis. After receiving diclofenac sodium, she immediately experienced severe pain followed by blistering and ulceration. On the second day post-injection, her skin turned dark purple with a hemorrhagic patch. By the 10th day post-injection, the erythematic area had decreased, but most of the darkly colored skin had progressively turned black. Three weeks later, the black skin had changed into an eschar. There was no other drug intake or systemic illness.A cutaneous examination showed a large, tender, non-indurated ulcer with necrotic eschar covering almost the entire right gluteal region with minimal extension to the left (Figure 1). Other cutaneous and systemic examinations were normal. There was no regional or generalized lymphadenopathy.
A complete blood count, including bleeding and clotting time, and urine examinations were normal. Her chest X-ray, blood urea, serum creatinine, liver function tests and creatine kinase, were normal. The results of her Venereal Disease Research Laboratory (VDRL) and human immunodeficiency virus (HIV)-1 and HIV-2 tests were negative. A superficial ultrasonography showed diffuse edema, with sparing of the muscle and no fluid collections. The fluid found and specimens of infected deep soft tissues were sent for immediate Gram’s stain, culture and antibiotic sensitivity tests. The Gram’s stain revealed numerous polymorphonuclear cells. A culture from the ulcer showed growth of methicillin-sensitive Staphylococcus aureus. The secondary staphylococcal infection was treated effectively with intravascular ampicillin-sulbactam (4×1.5g/day) for 21 days. She was treated with surgical debridement, sterile dressings, and analgesics. The ulcer healed completely within 12 weeks with scarring.
Discussion
Firstly described by Freudenthal and Nicolau in 1924, typically NS presents with pallor, owing to a local reflex vasospasm, and pain, rapidly followed by erythema, hemorrhagic patch, blistering, and variable degree of necrosis [5]. NS has been reported with the administration of various other drugs such as penicillins, local anesthetics, corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs) in the literature [6].
According to Saputo and Bruni [7] the syndrome is more frequent in the pediatric population, mainly in children who are younger than 3 years, in which the phenomenon of artery embolism may be more likely to happen due to the smaller size of the vascular segments involved [7].
The pathogenesis of the disease is not known, but there have been several hypotheses. First, it is presumed that the sympathetic nerve is stimulated by pain from the intra-arterial or periarterial injection of drugs, causing vasospasms and leading to ischemia. Second, this is related to the pharmacologic properties of NSAIDs. NSAIDs inhibit prostaglandin synthesis by the inhibition of cyclooxygenase. Ischemic necrosis occurs after the vasospasms are induced by the drug’s suppression of prostaglandin. Third, the intra-arterially injected drug causes embolic occlusion. Nicolau histologically discovered bismuth salts in the peripheral arteries of his patient. Fourth, ischemic necrosis progresses from vascular rupture due to perivascular inflammation from a cytotoxic reaction to the drugs. Fifth, lipophilic drugs penetrate the blood vessels in a manner similar to that of fat embolism and induce physical occlusion [8].
The typical presentation is blanching and pain around the infection site soon after injection, followed by erythema, livedoid patch, hemorrhagic patch and finally necrosis [2]. Secondary infection might occur [2].
Diagnosis is mainly clinical; a skin biopsy shows necrotic changes caused by ischemia [9].
Additional treatment includes antibiotics, wound dressing, skin graft, and flap reconstruction; extensive scarring is usually inevitable [9]. Early treatment has been reported to avert necrosis of the skin [10]. Surgical debridement of the ulcer is of the utmost importance as it reduces infection and enhances wound healing [10]. Systemic antibiotics play a vital role in the management of this syndrome. In our case, the secondary staphylococcal infection was treated effectively with antibiotics. According to Uri and Arad [11], various studies reported the clinical improvement of patients that had being treated with anticoagulants (such as heparin), intravenous steroids (such as betamethasone, dexamethasone or intravenous methylprednisolones) and vasoactive therapy (such as pentoxifylline) [11]. According to Murthy et al. conservative treatment with debridement and pain control is the main therapy [12]. Late complications include contractures and deformities resulting from the scarring process that require corrective surgery [12]. There are neurological complications, usually transitional, in one-third of patients, most commonly hypoesthesia and paraplegia [12]. Studies are needed to determine the pathogenesis mechanisms and recommended treatment procedures of NS. However, the possibility of forming a large series does not seem to be likely. To the best of our knowledge, our patient is the 30th NS case associated with diclofenac sodium in the literature.
NS is an avoidable complication. Although the onset of NS cannot be predicted, in order to reduce the risk of tissue damage as much as possible, the injection is administered to the superolateral region of the gluteal muscle, for which an injection needle is used that is long enough to reach the muscle. The Z-track injection is a method of intramuscular injection into a large muscle using a needle and syringe and it can minimize or prevent NS [13]. Health care personnel should take these precautions. First, a long needle (long enough to reach the muscle) should be used. A 90-kg patient requires a 5 to 7.5cm (2- or 3-inch) needle and a 45-kg patient requires a 3.18 or 3.68cm (1.25- or 1.45-inch) needle. Second, the injection should be applied in the upper outer quadrant of the buttock. Third, aspirating the needle before injecting the medication should be performed to ensure that no blood vessel is hit. Fourth, the health care personnel should never inject more than 5ml of medication at a time when using the Z-track injection method. Finally, if more than one injection or a larger dose is required or ordered, different sites should be chosen [9, 13].
Conclusions
Health care personnel should be aware that NS, characterized by pain, skin discoloration, and necrosis, can be observed following an injection of diclofenac sodium, an NSAID. As the exact etiopathogenesis of this syndrome is not known, there is no standard guideline for its management. Systemic antibiotics, wound debridement in early stages, and corrective plastic surgery in late stages are the mainstay in management. Clinicians should be aware of this complication and use proper injection procedures.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
- HIV:
-
Human immunodeficiency virus
- NS:
-
Nicolau syndrome
- NSAID:
-
Non-steroidal anti-inflammatory drug.
References
Stiehl P, Wellsbach G, Schroter K: Nicolau Syndrome. Pathogenesis and clinical aspects of penicillin-induced arterial embolism. Schwelz Med Wochenschr. 1971, 101: 377-385.
Şenel E: Nicolau syndrome. A review of the literature. Clin Med Insights Dermatol. 2010, 1-4.
Hamilton B, Fowler P, Galloway H, Popovic N: Nicolau syndrome in an athlete following intra-muscular diclofenac injection. Acta Orthop Belg. 2008, 74: 860-864.
Rygnestad T, Kwam AM: Streptococcal myositis and tissue necrosis with intramuscular administration of diclofenac (Voltaren). Acta Anesthesiol Scand. 1995, 39: 1128-1130. 10.1111/j.1399-6576.1995.tb04243.x.
Guarneri C, Polimeni G: Nicolau syndrome following etanercept administration. Am J Clin Dermatol. 2010, 11 (Suppl 1): 51-52.
Şenel E: Nicolau syndrome as an avoidable complication. J Fam Commun Med. 2012, 19: 52-53. 10.4103/2230-8229.94017.
Saputo V, Bruni G: Nicolau syndrome from penicillin preparations: a review of the relevant literature in the search for potential risk factors. Pediatr Med Chir. 1998, 20: 105-123.
Faucher L, Marcoux D: What syndrome is this? Nicolau syndrome. Pediatr Dermatol. 1995, 12: 187-190. 10.1111/j.1525-1470.1995.tb00151.x.
Lie C, Leung F, Chow SP: Nicolau syndrome following intramuscular diclofenac administration: a case report. J Orthop Surg (Hong Kong). 2006, 14: 104-107.
Geukens J, Rabe E, Bieber T: Embolia cutis medicamentosa of the foot after sclerotherapy. Eur J Dermatol. 1999, 9: 132-133.
Uri O, Arad E: Skin necrosis after self-administered intramuscular diclofenac. J Plast Reconstr Aesthet Surg. 2009, 63 (1): 1-2.
Murthy SC, Sinddalingappo K, Suresh T: Nicolau’s syndrome following diclofenac administration: a report of two cases. Indian J Dermatol Venereol Leprol. 2007, 73: 429-431. 10.4103/0378-6323.37070.
Pullen RL: Administering medication by the Z-track method. Nursing. 2005, 35: 24-
Acknowledgements
The authors thank the patient for her cooperation and consent for permitting us to use her medical data and clinical photographs for this case report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
İK and FK were involved in the diagnosis, findings, and interpretation of the case. İK, AÖ and TD wrote the manuscript. İÇ was the mentor in all processes and determined the pattern of case presentation. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kılıç, İ., Kaya, F., Özdemir, A.T. et al. Nicolau syndrome due to diclofenac sodium (Voltaren®) injection: a case report. J Med Case Reports 8, 404 (2014). https://doi.org/10.1186/1752-1947-8-404
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1752-1947-8-404