Abstract
Introduction
Sunitinib, an oral multitargeted tyrosine kinase inhibitor, is widely used in the treatment of renal cell carcinoma and gastrointestinal stromal tumor and has had a variety of adverse events. However, sunitinib-related acute cholecystitis has been reported in only two patients with gastrointestinal stromal tumor and renal cell carcinoma (clear cell subtype).
Case presentation
A 75-year-old Japanese woman with a right sided abdominal swelling was referred to our hospital. Computed tomography (CT) showed a hypervascular bulky tumor in her right kidney, suggesting right renal cell carcinoma in clinical T4N0M0. Although sunitinib therapy was started as neoadjuvant chemotherapy, during the fourth week of the first cycle, she developed acute acalculous cholecystitis and disseminated intravascular coagulation associated with sunitinib. Sunitinib therapy was discontinued immediately and she recovered after subsequent treatment with antibiotics and gabexate mesilate followed by percutaneous cholecystostomy. Cholecystectomy and right radical nephrectomy were performed and pathological examination showed that her renal tumor was a chromophobe renal cell carcinoma (pT2) with necrosis. Inflammation and ischemia were observed in the gallbladder wall, which was compatible with acute acalculous cholecystitis. There has been no evidence of disease recurrence for more than six months.
Conclusion
We described the third case of sunitinib-related acute cholecystitis in a patient with chromophobe renal cell carcinoma. Attention is required to sunitinib-related acute cholecystitis which, while uncommon, could be life-threatening.
Similar content being viewed by others
Introduction
Sunitinib, an oral multitargeted tyrosine kinase inhibitor, is widely used in the treatment of metastatic renal cell carcinoma (RCC) and gastrointestinal stromal tumor (GIST) and has been administered in the perioperative period [1]. Although sunitinib has had a variety of adverse events, sunitinib-related acute cholecystitis has been reported in only two patients with GIST and RCC (clear cell subtype). We report a third case of sunitinib-related acute cholecystitis in a patient with chromophobe RCC who developed a serious condition.
Case presentation
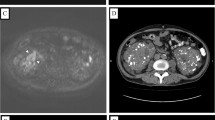
A 75-year-old Japanese woman with a right sided abdominal swelling was referred to our hospital. She had no history of medication or smoking and was a social drinker. Computed tomography (CT) showed a hypervascular bulky tumor in her right kidney with suspected liver invasion without distant metastasis (Figure 1), suggesting right RCC in clinical T4N0M0. For the purpose of downstaging of the tumor, sunitinib therapy (50 mg per day, four weeks on and two weeks off) was started in the neoadjuvant setting. During the fourth week of the first cycle, she experienced right upper quadrant pain with a positive Murphy's sign and abdominal fullness without fever. Laboratory tests revealed elevated levels of C-reactive protein, lactate dehydrogenase, and liver transaminases although total bilirubin, alkaline phosphatase, and amylase were at normal levels. She also had laboratory features of disseminated intravascular coagulation (DIC) including thrombocytopenia and disordered coagulation. Despite a normal gallbladder at the first visit (Figure 2), abdominal computed tomography (CT) revealed a tense and dilated gallbladder and thickening of the gallbladder wall without gallbladder stones or emphysematous change (Figure 3). Based on the diagnosis of acute acalculous cholecystitis associated with sunitinib, sunitinib therapy was discontinued immediately. She recovered in an intensive care unit after subsequent treatment with antibiotics and gabexate mesilate (FOY®) followed by percutaneous cholecystostomy.
After three months, a follow-up contrast-enhanced computed tomography (CT) revealed a marked shrinkage of the gallbladder and a 21% reduction in the size of the renal tumor with decreased enhancement of its center. Cholecystectomy and right radical open nephrectomy were performed. Adhesions thought to be due to cholecystitis made the operation difficult although common bile duct stenosis or retraction by the tumor that could lead to cholecystitis was not observed. Pathological examination showed that her renal tumor was chromophobe RCC (pT2) with necrosis occupying more than half of the tumor (Figure 4). Inflammation and ischemia were observed in the gallbladder wall which was compatible with acute acalculous cholecystitis (Figure 5). Computed tomography (CT) has revealed no evidence of disease recurrence for more than six months since the radical nephrectomy.
Discussion
We present the third case of sunitinib-related acute cholecystitis which developed in a patient with a chromophobe RCC. In the literature, sunitinib-related acute cholecystitis has been reported in only two other patients, one with GIST [2] and the other with RCC (clear cell subtype) [3]. We evaluated whether or not acute cholecystitis in our patient was caused by sunitinib with the use of the Naranjo scale [4] which assesses the probability of a drug-related adverse event [2, 3]. Its scale score for our patient was five (Table 1), indicating a probable association of acute cholecystitis with sunitinib. Sunitinib-related acute cholecystitis was also supported by the following findings: the symptoms improved with discontinuation of sunitinib; there were no risk factors including gallbladder stones, common bile duct stenosis and other hepatobiliary diseases that could cause acute cholecystitis and deranged liver function.
A common clinical feature of sunitinib-related acute cholecystitis in three cases including our patient and the previous two cases [2, 3] was acalculous cholecystitis while gallbladder stones have been found in 90% of patients with acute cholecystitis [5]. On the other hand, sunitinib causes vascular adverse events by vascular endothelial dysfunction [6, 7] resulting in myocardial ischemia [8] and proteinuria due to renal thrombotic microangiopathy [9]. Likewise, sunitinib-related acute cholecystitis might be caused by the antivascular effect of sunitinib rather than gallbladder stones although the detailed mechanism underlying this adverse event is unclear. As sunitinib is widely used in the treatment of RCC and GIST, we need to pay attention to sunitinib-related acute cholecystitis which, while uncommon, could be life-threatening.
Conclusion
We described the third case of sunitinib-related acute cholecystitis in a patient with chromophobe RCC. Attention needs to be given to sunitinib-related acute cholecystitis which could be life-threatening albeit uncommon.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
Thomas AA, Rini BI, Stephenson AJ, Garcia JA, Fergany A, Krishnamurthi V, Novick AC, Gill IS, Klein EA, Zhou M, Campbell SC: Surgical resection of renal cell carcinoma after targeted therapy. J Urol. 2009, 182: 881-886. 10.1016/j.juro.2009.05.014.
de Lima Lopes G, Rocha Lima CM: Emphysematous cholecystitis in a patient with gastrointestinal stromal tumor treated with sunitinib. Pharmacotherapy. 2007, 27: 775-777. 10.1592/phco.27.5.775.
Gomez-Abuin G, Karam AA, Mezzadri NA, Bas CA: Acalculous cholecystitis in a patient with metastatic renal cell carcinoma treated with sunitinib. Clin Genitourin Cancer. 2009, 7: 62-63. 10.3816/CGC.2009.n.011.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ: A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981, 30: 239-245. 10.1038/clpt.1981.154.
Barie PS, Fischer E: Acute acalculous cholecystitis. J Am Coll Surg. 1995, 180: 232-244.
Aparicio-Gallego G, Afonso-Afonso FJ, Leon-Mateos L, Firvida-Perez JL, Vazquez-Estevez S, Lazaro-Quintela M, Ramos-Vazquez M, Fernandez-Calvo O, Campos-Balea B, Anton-Aparicio LM: Molecular basis of hypertension side effects induced by sunitinib. Anticancer Drugs. 2011, 22: 1-8. 10.1097/CAD.0b013e3283403806.
Gotink KJ, Verheul HM: Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action?. Angiogenesis. 2010, 13: 1-14. 10.1007/s10456-009-9160-6.
Daher IN, Yeh ET: Vascular complications of selected cancer therapies. Nat Clin Pract Cardiovasc Med. 2008, 5: 797-805. 10.1038/ncpcardio1375.
Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008, 358: 1129-1136. 10.1056/NEJMoa0707330.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KN prepared the manuscript. KS and TM interpreted the patient data and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Nakano, K., Suzuki, K. & Morita, T. Life-threatening acute acalculous cholecystitis in a patient with renal cell carcinoma treated by sunitinib: a case report. J Med Case Reports 6, 69 (2012). https://doi.org/10.1186/1752-1947-6-69
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1752-1947-6-69