Abstract
Background
Mycobacterium chimaera is a recently described species within the Mycobacterium avium complex. Its pathogenicity in respiratory tract infection remains disputed. It has never been isolated during cystic fibrosis respiratory tract infection.
Case presentation
An 11-year-old boy of Asian ethnicity who was born on Réunion Island presented to our hospital with cystic fibrosis after a decline in his respiratory function over the course of seven years. We found that the decline in his respiratory function was correlated with the persistent presence of a Mycobacterium avium complex organism further identified as M. chimaera.
Conclusion
Using sequencing-based methods of identification, we observed that M. chimaera organisms contributed equally to respiratory tract infections in patients with cystic fibrosis when compared with M. avium subsp. hominissuis isolates. We believe that M. chimaera should be regarded as an emerging opportunistic respiratory pathogen in patients with cystic fibrosis, including young children, and that its detection warrants long-lasting appropriate anti-mycobacterial treatment to eradicate it.
Similar content being viewed by others
Introduction
Mycobacterium avium complex (MAC) organisms are opportunistic pathogens, and their isolation from the respiratory tract of cystic fibrosis (CF) patients correlates with declining respiratory function [1]. Six of nine MAC species have been described in the past five years [2–4], but the spectrum of MAC organisms infecting CF patients is not well established; only M. avium and Mycobacterium intracellulare have been reported in these patients [1, 5].
Our reference mycobacteriology laboratory routinely processes respiratory tract specimens collected from all CF patients who are followed by the Cystic Fibrosis Regional Reference Center in Marseilles, France. After Ziehl-Neelsen staining, cultures were routinely performed in BACTEC broth using the BACTEC 9000 MB system (BD Biosciences, Sparks, MD, USA), subcultures were grown on 5% sheep blood agar, and identification was done by partial rpoB-based sequencing [2]. Genotyping of the isolates was performed using multi-spacer sequence typing (MST) [6]. The minimum inhibitory concentrations were determined by 10-day incubation of a 1 McFarland mycobacterial suspension at 37°C on 5% sheep blood agar in the presence of the respective Etest strip (AB Biodisk, Solna, Sweden). The susceptibility to rifabutin was determined in BACTEC broth using the BACTEC 9000 MB system by incubating a 1 McFarland bacterial suspension with 1 μg/mL or 2 μg/mL rifabutin for five days in the presence of a growth control.
Over a six-year period, five (4.3%) of 115 children with CF had at least one respiratory tract specimen that was positive for MAC. M. avium subsp. hominissuis was found in two patients (with distinct MST48 and MST20 genotypes), Mycobacterium colombiense was found in one patient, and Mycobacterium chimaera was found in two additional patients (distinct MST33 and MST39 genotypes).
Case presentation
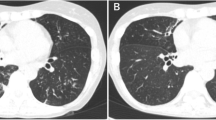
One of the latter five patients with CF described at the end of the Introduction was an Asian boy born on Réunion Island who exhibited a ΔF508 homozygous deletion in the CFTR gene, which is diagnostic of CF. He presented to our hospital when he was 11 years old because he had started complaining of hemoptoic cough. A physical examination revealed weight loss and wheezing. After his arrival in metropolitan France, a MAC organism further identified as genotype ST33 M. chimaera was isolated from his respiratory tract (Figure 1). This isolate was susceptible to clarithromycin (3 μg/mL), rifabutin (< 1 μg/mL), and ethambutol (2 μg/mL) and was resistant to ofloxacin (> 32 μg/mL), streptomycin (> 1024 μg/mL), doxycyclin (> 256 μg/mL), and rifampicin (> 32 μg/mL). The patient was given rifabutin and clarithromycin, but he interrupted this treatment after two weeks. Six months later his sputum yielded acid-fast bacilli that were identified as genotype MST33 M. chimaera after culture. The same antibiotic regimen prescribed for six months yielded negative mycobacterial cultures. Two years later a recurrent fever and cough correlated with MST33 M. chimaera- positive cultures. The patient once again interrupted his therapy of rifabutin combined with clarithromycin, this time after four months. Twenty-two months later the patient's condition severely worsened, as he had developed chronic respiratory insufficiency exacerbated by bilateral pneumothorax, which required non-invasive ventilatory support. From this period onward, insulin therapy became necessary to achieve glycemic control, and his nutritional condition justified the initiation of enteral feeding by percutaneous gastrostomy. The thoracic computed tomographic scan showed bronchiectasis, expanded areas of condensation, and mediastinal polyadenopathy. Acid-fast bacilli that were observed on sputum smears were cultured and revealed to be MST33 M. chimaera organisms. Rifabutin and clarithromycin were reintroduced, but sequential cultures remained positive. The patient was registered in the national list of lung transplant candidates.
Chronological representation of Mycobacterium chimaera respiratory tract infection in a CF patient. Arrows indicate requests for mycobacterial detection in the sputum. Dotted arrows indicate negative culture. Filled arrows indicate positive culture for M. chimaera. + indicates acid-fast bacilli stain seen upon direct microscopic examination of sputum samples. Antibiotic therapy is represented by black squares. Duration of treatment (in weeks) is indicated above the brackets.
During the whole treatment period, the microbiological investigations of the respiratory tract specimens also exhibited Pseudomonas aeruginosa, methicillin-susceptible Staphylococcus aureus, Stenotrophomonas maltophilia, and Aspergillus fumigatus.
Discussion
The prevalence of MAC organisms in patients with CF is highly variable, ranging from 9% in the US [1] to 1.5% in France, and it was evaluated at 1.8% in southeast France [5], where Marseilles is located, although our data yielded a prevalence of 4.3% there. In France and the US, M. avium has contributed to twice as many positive samples as M. intracellulare[5, 7]. However, the identification of MAC organisms has relied on hybridization probe-based methods, which has misidentified M. chimaera as M. intracellulare[3], thus preventing the detection of M. chimaera in patients with CF. Using sequence-based identification, we observed that M. chimaera organisms contribute equally to respiratory tract infections in patients with CF compared with M. avium subsp. hominissuis isolates.
The pathogenicity of M. chimaera remains controversial. It has been reported to cause respiratory infections in patients with pulmonary diseases other than CF, including chronic obstructive pulmonary disease, bronchiectasis, and cavitation [3]. Conversely, in two studies with an aggregate of 97 non-CF patients OK, only 3.3% infected with M. chimaera fulfilled the American Thoracic Society (ATS) criteria for MAC lung disease [8, 9]. The patient described in our case report eventually fulfilled the ATS criteria on the basis of positive cultures from at least two separate sputum samples. Moreover, his M. chimaera infection relapsed every time antibiotic treatment was interrupted, and this continuing infection correlated with a worsening condition of the patient in the presence of associated pathogens, which may have also contributed to this downward spiraling evolution [10].
Conclusion
We believe that M. chimaera should be regarded as an emerging opportunistic respiratory pathogen in patients with CF, including young children, and that its detection warrants long-lasting, appropriate antimycobacterial treatment to eradicate it.
Consent
Written informed consent was obtained from the patient's mother for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
- ATS:
-
American Thoracic Society
- CF:
-
cystic fibrosis
- MAC:
-
Mycobacterium avium complex
- MST:
-
multi-spacer sequence typing.
References
Olivier KN, Weber DJ, Lee JH, Handler A, Tudor G, Molina PL, Tomashefski J, Knowles MR, Nontuberculous Mycobacteria in Cystic Fibrosis Study Group: Nontuberculous mycobacteria. II: nested-cohort study of impact on cystic fibrosis lung disease. Am J Respir Crit Care Med. 2003, 167: 835-840. 10.1164/rccm.200207-679OC.
Ben Salah I, Adekambi T, Raoult D, Drancourt M: rpoB sequence-based identification of Mycobacterium avium complex species. Microbiology. 2008, 154: 3715-3723. 10.1099/mic.0.2008/020164-0.
Tortoli E, Rindi L, Garcia MJ, Chiaradonna P, Dei R, Garzelli C, Kroppenstedt RM, Lari N, Mattei R, Mariottini A, Mazzarelli G, Murcia MI, Nanetti A, Piccoli P, Scarparo C: Proposal to elevate the genetic variant MAC-A, included in the Mycobacterium avium complex, to species rank as Mycobacterium chimaera sp. nov. Int J Syst Evol Microbiol. 2004, 54: 1277-1285. 10.1099/ijs.0.02777-0.
Turenne CY, Wallace R, Behr MA: Mycobacterium avium in the postgenomic era. Clin Microbiol Rev. 2007, 20: 205-229. 10.1128/CMR.00036-06.
Roux AL, Catherinot E, Ripoll F, Soismier N, Macheras E, Ravilly S, Bellis G, Vibet MA, Le Roux E, Lemonnier L, Gutierrez C, Vincent V, Fauroux B, Rottman M, Guillemot D, Gaillard JL, Jean-Louis Herrmann for the OMA Group: Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in France. J Clin Microbiol. 2009, 47: 4124-4128. 10.1128/JCM.01257-09.
Cayrou C, Turenne C, Behr MA, Drancourt M: Genotyping of Mycobacterium avium complex organisms using multispacer sequence typing. Microbiology. 2010, 156: 687-694. 10.1099/mic.0.033522-0.
Olivier KN, Weber DJ, Wallace RJ, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schechter MS, Edwards LJ, Chakraborti S, Knowles MR, Nontuberculous Mycobacteria in Cystic Fibrosis Study Group: Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003, 167: 828-834. 10.1164/rccm.200207-678OC.
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America: An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007, 175: 367-416. 10.1164/rccm.200604-571ST.
Schweickert B, Goldenberg O, Richter E, Göbel UB, Petrich A, Buchholz P, Moter A: Occurrence and clinical relevance of Mycobacterium chimaera sp. nov., Germany. Emerg Infect Dis. 2008, 14: 1443-1446. 10.3201/eid1409.071032.
Bittar F, Richet H, Dubus JC, Reynaud-Gaubert M, Stremler N, Sarles J, Raoult D, Rolain JM: Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis patients. PLoS One. 2008, 3: e2908-10.1371/journal.pone.0002908.
Acknowledgements
This work was supported by Pôle des Maladies Infectieuses, Assistance Publique-Hôpitaux de Marseille, Marseille, France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SCB, JMR, JCD, and MD reviewed the clinical data and were major contributors to the writing of the manuscript. MD and NS were involved with patient management. SCB, JMR, and MD analyzed data and performed the laboratory analysis. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cohen-Bacrie, S., David, M., Stremler, N. et al. Mycobacterium chimaera pulmonary infection complicating cystic fibrosis: a case report. J Med Case Reports 5, 473 (2011). https://doi.org/10.1186/1752-1947-5-473
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1752-1947-5-473