Abstract
Background
Nickel ferrite, a kind of soft magnetic materials is one of the most attracting class of materials due to its interesting and important properties and has many technical applications, such as in catalysis, sensors and so on. In this paper the synthesis of NiFe2O4 nanoparticles by the hydrothermal method is reported and the inhibition of surfactant (Glycerol or Sodium dodecyl sulfate) on the particles growth is investigated.
Methods
For investigation of the inhibition effect of surfactant on NiFe2O4 particles growth, the samples were prepared in presence of Glycerol and Sodium dodecyl sulfate. The X-ray powder diffraction (XRD), transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FT-IR), vibrating sample magnetometer (VSM) and inductively coupled plasma atomic emission spectrometer (ICP-AES) techniques were used to characterize the samples.
Results
The results of XRD and ICP-AES show that the products were pure NiFe2O4 and also nanoparticles grow with increasing the temperature, while surfactant prevents the particle growth under the same condition. The average particle size was determined from the Scherrer's equation and TEM micrographs and found to be in the range of 50-60 nm that decreased up to 10-15 nm in presence of surfactant. The FT-IR results show two absorption bands near to 603 and 490 cm-1 for the tetrahedral and octahedral sites respectively. Furthermore, the saturated magnetization and coercivity of NiFe2O4 nanoparticles were in the range of 39.60 emu/g and 15.67 Qe that decreased for samples prepared in presence of surfactant. As well as, the nanoparticles exhibited a superparamagnetic behavior at room temperature.
Conclusions
Nanosized nickel ferrite particles were synthesized with and without surfactant assisted hydrothermal methods. The results show that with increasing of temperature, the crystallinity of nanoparticles is increased. In the presence of surfactants, the crystallinity of NiFe2O4 nanoparticles decreased in comparison with surfactant- free prepared samples. All of the nickel ferrite nanoparticles were superparamagnetic at room temperature.
Graphical abstract

Similar content being viewed by others
Introduction
Nanosized spinel ferrite particles, a kind of soft magnetic materials with structural formula of MFe2O4 (M = divalent metal ion, e.g. Mn, Mg, Zn, Ni, Co, Cu, etc.), are one of the most attracting class of materials due to their interesting and important properties such as low melting point, high specific heating, large expansion coefficient, low saturation magnetic moment and low magnetic transition temperature, etc.[1, 2]. Because of these properties, the spinel ferrites have many technical applications, such as in photoelectric devices [3] catalysis [4], sensors [5], nano devices [6], microwave devices [7, 8] and magnetic pigments [9].
Remarkable electrical and magnetic properties of ferrites depend upon the nature of the ions, their charges and their distribution among tetrahedral (A) and octahedral (B) sites [10]. Nickel ferrite is one of the versatile and technologically important soft ferrite materials because of its typical ferromagnetic properties, low conductivity and thus lower eddy current losses, high electrochemical stability, catalytic behavior, abundance in nature, etc[8]. This ferrite is an inverse spinel in which eight units of NiFe2O4 go into a unit cell of the spinel structure. Half of the ferric ions preferentially fill the tetrahedral sites (A-sites) and the others occupy the octahedral sites (B-sites) [11]. Thus the compound can be represented by the formula (Fe3+) A [Ni2+Fe3+]BO42- [12]. The synthesis of spinel ferrite nanoparticles has been intensively studied in the recent years and the principal role of the preparation conditions on the morphological and structural features of the ferrites is discussed [13–16]. Large-scale applications of ferrites with small particles and tailoring of specific properties have prompted the development of widely used chemical methods, including hydrothermal [10], sonochemical reactions [17], sol-gel methods[18], microwave plasma [19], co-precipitation [20], microemulsion methods [21], citrate precursor techniques [22] and mechanical alloying [23] for the fabrication of stoichiometric and chemically pure spinel ferrite nanoparticles. The hydrothermal route is one of the most commonly used techniques owing to its economics and high degree of compositional control. In addition, the hydrothermal synthesis does not require extremely high-processing temperature or sophisticated processing. For example, ferrites can be prepared via the hydrothermal route at a temperature of ~150°C, whereas the solid state method requires a temperature of 800°C [10].
In this work, nano crystalline nickel ferrite, NiFe2O4, was successfully prepared via reaction between metal chlorides in ethylacetate solution with and without surfactant assisted processes. Investigations on the particle size, morphology and magnetic properties of inverse spinel nickel ferrite in at different conditions are carried out by the XRD, FT-IR, TEM, ICP-AES and VSM techniques.
Results and discussion
FT-IR analysis
Figure 1(a-c) shows the FT- IR absorption spectra of nanocrystalline NiFe2O4 samples prepared without and with surfactant-assisted methods (Glyserole and Sodium dodecyl sulfate) which were recorded in the range of 400-4000 cm-1. On the bases of literature data, in the range of 1000-100 cm-1, the FT-IR bands of solids are usually assigned to vibration of ions in the crystal lattice [10, 24]. In all spinels and particularly in ferrites, two main broad metal oxygen bands are seen in the FT-IR spectra. Therefore the highest one, observed at ν1 = 603 cm-1 (Figure 1a), corresponds to intrinsic stretching vibrations of the metal at the tetrahedral site, (Mtetra-O), whereas the lowest band, that observed at ν2 = 490 cm-1 is assigned to octahedral metal stretching vibration (Mocta-O) [24]. Figure 1(b-c), shows a difference in the positions and area of ν1 and ν2 absorptions bands, that may be due to the changed conditions of formation of samples by surfactant assisted process. Because, the positions and intensities of the bands depends strongly on the methods and conditions of preparation [12, 25].
Structural analysis
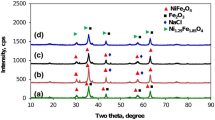
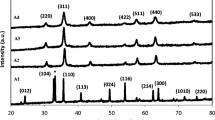
The X-ray diffraction patterns of the prepared samples are shown in Figure 2(a-g).
The samples (a) and (b) that prepared without surfactant at 45 and 80°C, consisted of amorphous solids which have not been detected by XRD technique. This may be related to the formation of amorphous state of nickel, iron oxides and or NiFe2O4 nanoparticles. Weak diffraction peaks in the samples (c) and (d), prepared without surfactant at 100 and 130°C, were attributed to the effect of increasing of temperature on the improvement of crystalline properties of amorphous nickel ferrite and also the conversion of some Ni and Fe oxides to produce nickel ferrite crystallites. Increasing of temperature up to 150°C led to the formation of well crystalline nickel ferrite (sample e). As Figure 2 shows, in the presence of surfactants (glycerol and sodium dodecyl sulfate) at 150°C in samples (f) and (g), crystallinity of NiFe2O4 nanoparticles was decreased in comparison with surfactant- free prepared samples [26].
In the case of sample (g), prepared by sodium dodecyl solfate assisted method, some impurities were observed. The XRD patterns of samples (c-g) exhibited the reflection plans (220), (311), (222), (400), (422), (511) and (440) that indicate the spinel cubic structure [10, 27–30]. The XRD patterns of the standard NiFe2O4 from JCPDS No. 10-325 has been presented in Figure 2. The average crystallite size was calculated from the most intense peak (311) using the Scherrer's formula:
where, D is the average crystalline size, k the Scherrer constant (0.89), λ the X-ray wavelength used, β the angular linewidth of half maximum intensity and θ is the Bragg's angle in degrees unit. The results are shown in Table 1.
Table 1 shows that with increase in the temperature from 100°C to 150°C in samples (c-e), the average size of NiFe2O4 nanoparticles also increases that can be attributed to the temperature assists crystal growth and/or the redistribution of cations among octahedral and tetrahedral sites [12]. Additionally, Figure 2 shows that in the presence of surfactant about samples (f and g), diffraction peak (311) is broad and therefore smaller NiFe2O4 nanoparticles are formed. This fact may be due to the role of surfactants in the decrease of the agglomeration of particles [31]. From the ICP-AES result, the atomic ratio of Ni- Fe is 0.49, which is close to that of NiFe2O4.
Morphology and microstructure
In order to investigate the morphology and particle size of products, the TEM images of samples (e-g), were obtained and are shown in Figure 3(a-c). From the TEM micrographs, it is clear that the nanoparticles obtained without surfactant are cubic-like but are not uniform (Figure 3a). On the other hand, in the presence of surfactant, the samples are sphere-like and uniform in both morphology and particle size (Figure 3b and 3c). Average grain-size obtained from TEM image of sample (e) is approximately 60 nm, which is in good agreement with the size determination by Scherrer equation from XRD patterns. In the cases of samples (f) and (g), the average size obtained were about 10-15 nm.
Magnetic properties
Figure 4(a and 4b) shows the hysteresis loops obtained from VSM measurements for surfactant- assisted prepared NiFe2O4 nano particles (samples f and g) at room temperature. The magnetic properties of the NiFe2O4 with an inverse spinel structure can be explained in terms of the cations distribution and magnetization originates from Fe3+ ions at both tetrahedral and octahedral sites and Ni2+ ions in octahedral sites [32, 33]. Hysteresis loops in Figure 4(a and 4b) are typical for soft magnetic materials and the "S" shape of the curves together with the negligible coercivity (Hc = 0.60 and 0.64Qe) indicate the presence of small magnetic particles exhibiting superparamagnetic behaviors [34]. In superparamagnetic materials, responsiveness to an applied magnetic field without retaining any magnetism after removal of the magnetic field is observed. This behavior is an important property for magnetic targeting carriers [35]. In fact, the difference between ferromagnetism and superparamagnetism fabricates in the particle size. Literature data imply that when the diameter of particles is less than 30 nm, the particles show the character of superparamagnetism [34, 36]. Figure 5 shows the magnetic hysteresis loop for NiFe2O4 sample (e), prepared without surfactant. The curve is "S" shape with low coercivity (15.67 Oe). This sample showed superparamagnetic behavior. The saturation magnetization (Ms) and the coercivity (Hc) values of products, are listed in Table 2.
It is obvious from Table 2 that: (a) the values of saturation magnetization for NiFe2O4 nanoparticles are significantly lower than the multidomain bulk particles (55 emu/g). Smit and Wijn have reported Ms equal to 50 emu/g for bulk nickel ferrite particles [37]. Nathani and Misra have measured Ms equal to 25 emu/g for NiFe2O4 nanoparticles with size 8 nm [38]. (b) The amounts of Ms and Hc for samples (g and f) are equal to 34.45-35.10 emu/g and 0.60-0.64Oe respectively, which increase to 39.60 emu/g and 15.67 Qe for sample (e). In fact, the magnetic behavior of nickel ferrite nanoparticles is very sensitive to the crystallinity and particle size. The increase in saturation magnetization was most likely attributed to the increasing of crystallinity and particle size of the samples [39] and can be explained on the basis of changes in exchange interactions between tetrahedral and octahedral sub-lattices[40]. In case of nickel ferrite, any configuration of Ni2+ and Fe3+ ions in both octahedral and tetrahedral sites, tends to increase the net magnetization per formula unit [12]. On the other hand, variation of coercivity with particle size can be explained on the basis of domain structure, critical diameter, strains, magneto crystalline anisotropy and shape anisotropy of crystal [39]. Therefore the magnetic behavior of nano-size nickel ferrites can be a collective effect of these interactions [41].
Conclusions
Nanosized nickel ferrite particles were synthesized with and without surfactant assisted hydrothermal methods. The FT-IR spectra showed two characteristic metal oxygen vibrational bands. The average particle size of samples was in the range of 12-53 nm, as revealed by XRD and TEM techniques. The temperature rise up to 150°C led to the increasing of crystallinity of nanoparticles. In the presence of surfactants, the crystallinity of NiFe2O4 nanoparticles decreased in comparison with surfactant- free prepared samples. All of the nickel ferrite nanoparticles were superparamagnetic at room temperature. The saturation magnetization and coercivity values were found to be low, which attributed to the various parameters such as crystallinity and particle size. The saturation magnetization and coercivity were reduced with decreasing of crystallinity and particle size of nanoparticles.
Experimental
Materials
NiCl2.6H2O, FeCl3.6H2O, NaOH, Triethyl amine, Ethyl acetate, Glycerol and Sodium dodecyl sulfate were purchased from Merck chemical company and were used as received without further purification. The deionized water used in all experiments, had a conductivity of less than 10-6 Scm-1.
Synthesis
A 0.4 M (25 mL) solution of iron chloride (FeCl3.6H2O) and a 0.2 M (25 mL) solution of nickel chloride (NiCl2.6H2O) in double distilled deionized water were mixed with vigorous stirring. 5 M triethyl amine in ethylacetate solution was used to adjust the pH at 10. The mixture was heated at different temperatures (45, 80, 100, 130 and 150°C) for 18 h. Hydrothermal synthesis at 100, 130 and 150°C, were carried out in a Teflon-lined autoclave reactor. The black precipitate was filtered off, washed with deionized water and dried in a vacuum oven at 70°C for 3 h. Also we prepared NiFe2O4 nanoparticles via surfactant assisted process. 0.078 M of surfactant (Glycerol or Sodium dodecyl sulfate) was dissolved in 35 mL deionized water and was added to the solution of salts under vigorous stirring. Adjustment of pH and other processes were performed as above at 150°C.
Measurements
Infrared spectra were recorded in the range of 400-4000 cm-1 with a Bruker vector 22 FT-IR spectrometer from samples in KBr pellets. The structural characterization was performed using Brucker AXS (model D8 Advance) X-ray powder diffractometer (Cu Kα radiation source, λ = 0.154 nm) with the Bragg angle ranging from 10-70° generated at 40 kV and 35 mA at room temperature. Transmission electron microscopy (TEM) analysis was performed using Cambridge, stereo scan 360, 1990, 100 kV accelerating voltage. The magnetic measurements were carried out at room temperature by using a vibrating sample magnetometer, model VSM, BHV-55, Riken Japan with a magnetic field up to 8 kQe. Inductively coupled plasma atomic emission spectrometer (ICP-AES) was carried out on a Thermo Fisher Scientific ICP-IRIS Advantage.
References
Xu Q, Wei Y, Liu Y, Ji X, Yang L, Gu M: Preparation of Mg/Fe spinel ferrite nanoparticles from Mg/Fe-LDH microcrystallites under mild conditions. Solid State Sci. 2009, 11 (2): 472-478. 10.1016/j.solidstatesciences.2008.07.004.
Tian MB: Magnetic Material. 2001, Beijing: Tsinghua University Press
Hu J, Li L-s, Yang W, Manna L, Wang L-w, Alivisatos AP: Linearly Polarized Emission from Colloidal Semiconductor Quantum Rods. Science. 2001, 292 (5524): 2060-2063. 10.1126/science.1060810.
Sloczynski J, Janas J, Machej T, Rynkowski J, Stoch J: Catalytic activity of chromium spinels in SCR of NO with NH3. Appl Catal B. 2000, 24 (1): 45-60. 10.1016/S0926-3373(99)00093-4.
Pena MA, Fierro JLG: Chemical Structures and Performance of Perovskite Oxides. Chem Rev. 2001, 101 (7): 1981-2018. 10.1021/cr980129f.
Ajayan PM, Redlich P, Ru"hle M: Structure of carbon nanotube-based nanocomposites. J Micro. 1997, 185 (2): 275-282. 10.1046/j.1365-2818.1997.1670730.x.
Baykal A, Kasapoglun , Durmus Z, Kavas H, Toprak MS, Koseoglu Y: CTAB-Assisted Hydrothermal Synthesis and Magnetic Characterization of NiXCo1-xFe2O4 Nanoparticles (x = 0.0, 0.6, 1.0). Turk J Chem. 2009, 33: 33-45.
Gunjakar JL, More AM, Gurav KV, Lokhande CD: Chemical synthesis of spinel nickel ferrite (NiFe2O4) nano-sheets. Appl Surf Sci. 2008, 254 (18): 5844-5848. 10.1016/j.apsusc.2008.03.065.
Wang X, Yang G, Zhang Z, Yan L, Meng J: Synthesis of strong-magnetic nanosized black pigment ZnxFe3-xO4. Dyes Pigm. 2007, 74 (2): 269-272. 10.1016/j.dyepig.2006.02.004.
Baykal Al, Kasapoglu N, Koseoglu Yk, Toprak MS, Bayrakdar H: CTAB-assisted hydrothermal synthesis of NiFe2O4 and its magnetic characterization. J Alloys Compd. 2008, 464 (1-2): 514-518. 10.1016/j.jallcom.2007.10.041.
Goldman A: Modern Ferrite Technology. 1993, New York: Marcel Dekker
Alarifi A, Deraz NM, Shaban S: Structural, morphological and magnetic properties of NiFe2O4 nano-particles. J Alloys Compd. 2009, 486 (1-2): 501-506. 10.1016/j.jallcom.2009.06.192.
Manova E, Tsoncheva T, Paneva D, Mitov I, Tenchev K, Petrov L: Mechanochemically synthesized nano-dimensional iron-cobalt spinel oxides as catalysts for methanol decomposition. Appl Catal A. 2004, 277 (1-2): 119-127. 10.1016/j.apcata.2004.09.002.
Ferreira TAS, Waerenborgh JC, Mendonsa MHRM, Nunes MR, Costa FM: Structural and morphological characterization of FeCo2O4 and CoFe2O4 spinels prepared by a coprecipitation method. Solid State Sci. 2003, 5 (2): 383-392. 10.1016/S1293-2558(03)00011-6.
De Guire M: The cooling rate dependence of cation distributions in CoFe2O4. J Appl Phys. 1989, 65 (8): 3167-3172. 10.1063/1.342667.
Li S: Cobalt-ferrite nanoparticles: Structure, cation distributions, and magnetic properties. J Appl Phys. 2000, 87 (9): 6223-6225. 10.1063/1.372661.
Shafi KVPM, Gedanken A, Prozorov R, Balogh J: Sonochemical Preparation and Size-Dependent Properties of Nanostructured CoFe2O4 Particles. Chem Mater. 1998, 10 (11): 3445-3450. 10.1021/cm980182k.
Kim C: Growth of ultrafine Co-Mn ferrite and magnetic properties by a sol-gel method. J Appl Phys. 1999, 85 (8): 5223-5225. 10.1063/1.369950.
Hochepied JF, Bonville P, Pileni MP: Nonstoichiometric Zinc Ferrite Nanocrystals: Syntheses and Unusual Magnetic Properties. J Phys Chem. 2000, B 104 (5): 905-912.
Kim YI, Kim D, Lee CS: Synthesis and characterization of CoFe2O4 magnetic nanoparticles prepared by temperature-controlled coprecipitation method. Phys B(Amestherdam, Neth). 2003, 337 (1-4): 42-51.
Feltin N, Pileni MP: New Technique for Synthesizing Iron Ferrite Magnetic Nanosized Particles. Langmuir. 1997, 13 (15): 3927-3933. 10.1021/la960854q.
Prasad S, Gajbhiye NS: Magnetic studies of nanosized nickel ferrite particles synthesized by the citrate precursor technique. J Alloys Compd. 1998, 265 (1-2): 87-92. 10.1016/S0925-8388(97)00431-3.
Shi Y, Ding J, Liu X, Wang J: NiFe2O4 ultrafine particles prepared by co-precipitation/mechanical alloying. J Magn Magn Mater. 1999, 205 (2-3): 249-254. 10.1016/S0304-8853(99)00504-1.
Salavati-Niasari M, Davar F, Mahmoudi T: A simple route to synthesize nanocrystalline nickel ferrite (NiFe2O4) in the presence of octanoic acid as a surfactant. Polyhedron. 2009, 28 (8): 1455-1458. 10.1016/j.poly.2009.03.020.
Deraz NM: Production and characterization of pure and doped copper ferrite nanoparticles. J Anal Appl Pyrolysis. 2008, 82 (2): 212-222. 10.1016/j.jaap.2008.03.009.
Maaz K, Karim S, Mumtaz A, Hasanain SK, Liu J, Duan JL: Synthesis and magnetic characterization of nickel ferrite nanoparticles prepared by co-precipitation route. J Magn Magn Mater. 2009, 321 (12): 1838-1842. 10.1016/j.jmmm.2008.11.098.
El-Sayed AM: Influence of zinc content on some properties of Ni-Zn ferrites. Ceram Int. 2002, 28 (4): 363-367. 10.1016/S0272-8842(01)00103-1.
Fu Y-P, Pan K-Y, Lin C-H: Microwave-induced combustion synthesis of Ni0.25Cu0.25Zn0.5 ferrite powders and their characterizations. Mater Lett. 2002, 57 (2): 291-296. 10.1016/S0167-577X(02)00780-2.
Kasapoglu N, Birsöz B, Baykal A, Köseoglu Y, Toprak M: Synthesis and magnetic properties of octahedral ferrite Ni χ Co1- χFe2O4 nanocrystals. Cent Eur J Chem. 2007, 5 (2): 570-580. 10.2478/s11532-007-0005-0.
Rashad MM, Elsayed EM, Moharam MM, Abou-Shahba RM, Saba AE: Structure and magnetic properties of NixZn1 - xFe2O4 nanoparticles prepared through co-precipitation method. J Alloys Compd. 2009, 486 (1-2): 759-767. 10.1016/j.jallcom.2009.07.051.
Kavas H, Kasapoglu N, Baykal A, Kaseoglu Y: Characterization of NiFe2O4 nanoparticles synthesized by various methods. Chem Papers. 2009, 63 (4): 450-455. 10.2478/s11696-009-0034-6.
Nathani H, Gubbala S, Misra RDK: Magnetic behavior of nanocrystalline nickel ferrite: Part I. The effect of surface roughness. Mater Sci Eng B. 2005, 121 (1-2): 126-136. 10.1016/j.mseb.2005.03.016.
Kodama RH, Berkowitz AE, McNiff JEJ, Foner S: Surface Spin Disorder in NiFe2O4 Nanoparticles. Phys Rev Lett. 1996, 77 (2): 394-397. 10.1103/PhysRevLett.77.394.
Manova E, Tsoncheva T, Estournes C, Paneva D, Tenchev K, Mitov I, Petrov L: Nanosized iron and iron-cobalt spinel oxides as catalysts for methanol decomposition. Appl Catal A. 2006, 300 (2): 170-180. 10.1016/j.apcata.2005.11.005.
Li G-y, Jiang Y-r, Huang K-l, Ding P, Chen J: Preparation and properties of magnetic Fe3O4-chitosan nanoparticles. J Alloys Compd. 2008, 466 (1-2): 451-456. 10.1016/j.jallcom.2007.11.100.
Zhi J, Wang Y, Lu Y, Ma J, Luo G: In situ preparation of magnetic chitosan/Fe3O4 composite nanoparticles in tiny pools of water-in-oil microemulsion. Reac Funct Polym. 2006, 66 (12): 1552-1558. 10.1016/j.reactfunctpolym.2006.05.006.
Smit J, Wijn HPJ: Ferrite. 1959, London: Cleaver-Hume Press
Nathani H, Misra RDK: Surface effects on the magnetic behavior of nanocrystalline nickel ferrites and nickel ferrite-polymer nanocomposites. Mater Sci Eng B. 2004, 113 (3): 228-235.
Nawale AB, Kanhe NS, Patil KR, Bhoraskar SV, Mathe VL, Das AK: Magnetic properties of thermal plasma synthesized nanocrystalline nickel ferrite (NiFe2O4). J Alloys Compd. 2011, 509 (12): 4404-4413. 10.1016/j.jallcom.2011.01.057.
Pradeep A, Priyadharsini P, Chandrasekaran G: Production of single phase nano size NiFe2O4 particles using sol-gel auto combustion route by optimizing the preparation conditions. Mater Chem Phys. 2008, 112 (2): 572-576. 10.1016/j.matchemphys.2008.05.090.
Gabal MA, Al Angari YM, Kadi MW: Structural and magnetic properties of nanocrystalline Ni1-xCuxFe2O4 prepared through oxalates precursors. Polyhedron. 2011, 30 (6): 1185-1190. 10.1016/j.poly.2011.01.032.
Acknowledgements
The author thanks the Payame Noor University, Tabriz center for the financial support of project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KN made a significant contribution to Survey results and data and their analysis and revising the manuscript for intellectual content. RZ participated in the synthesis of samples and collection of data and experimental work and analysis. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Nejati, K., Zabihi, R. Preparation and magnetic properties of nano size nickel ferrite particles using hydrothermal method. Chemistry Central Journal 6, 23 (2012). https://doi.org/10.1186/1752-153X-6-23
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1752-153X-6-23